Bipolar disorder is a severe psychiatric disorder with peak onset in late adolescence and early adulthood.Reference Merikangas, Jin, He, Kessler, Lee and Sampson 1 Bipolar disorder is ranked among the top 10 medical illnesses associated with the most disability worldwide.Reference Lopez, Mathers, Ezzati, Jamison and Murray 2 Difficulty in accurate diagnosis early in the course of illness contributes to this substantial illness burden and, in turn, worsened prognosis because of delayed and potentially harmful treatment.Reference Hirschfeld, Lewis and Vornik 3 Given the high heritability, family history is the most robust risk factor for predicting bipolar disorder and identifies a high-risk population,Reference Goodwin and Jamison 4 informative for development of clinical monitoring and early intervention strategies, and research into antecedent and prodromal features. Among individuals at confirmed genetic risk, bipolar disorder typically debuts as a depressive episode, preceding the first manic episode by an average of 4 years.Reference Angst and Gamma 5 , Reference Duffy, Horrocks, Doucette, Keown-Stoneman, McCloskey and Grof 6 However, there is substantial variability in clinical course between individuals. International research efforts have focused on identifying antecedent clinical precursors to bipolar disorder to refine risk prediction, improve early detection and prevent progression of the impairing disease.Reference Duffy 7 However, at present, it is challenging to predict among those at confirmed familial risk who will transition to full-blown bipolar disorder and related major mood disorders.Reference Duffy 8
Evidence from the Flourish Canadian high-risk offspring cohort study demonstrated that non-specific psychiatric disorders precede the onset of bipolar disorder in a substantial proportion of high-risk offspring, including sleep, anxiety and minor mood disorders.Reference Duffy, Horrocks, Doucette, Keown-Stoneman, McCloskey and Grof 6 , Reference Duffy, Alda, Crawford, Milin and Grof 9 Similar findings have been reported in other independent international high-risk cohort studies.Reference Mesman, Nolen, Reichart, Wals and Hillegers 10 –Reference Nurnberger, McInnis, Reich, Kastelic, Wilcox and Glowinski 12 Antecedent symptom-level psychopathology may also be evident and informative in the prediction of bipolar disorder and related major mood disorder onset. Other high-risk and clinical studies have reported associations between sub-threshold hypomanic symptoms (not meeting full DSM-IV criteria) and subsequent diagnosable episodes of (hypo)mania in offspring of a parent with bipolar disorder.Reference Axelson, Goldstein, Goldstein, Monk, Yu and Hickey 13 –Reference Findling, Youngstrom, McNamara, Stansbrey, Demeter and Bedoya 17 This finding requires replication based on prospective observations and further investigation. For example, it remains unclear if sub-threshold hypomanic symptoms are more common in offspring at confirmed familial risk compared with those not at familial risk and if they have the same clinical importance in these different populations. Although hypomanic symptoms are commonly reported in general and clinical populations, not all individuals reporting these symptoms develop bipolar disorder.Reference Lewinsohn, Klein and Seeley 18 – Reference Hazell, Carr, Lewin and Sly 20 Some studies using clinical and community populations report that sub-threshold hypomanic symptoms are precursors to full onset bipolar disorder in adults and predict conversion from unipolar major depressive disorder (MDD) to bipolar disorder,Reference Fiedorowicz, Endicott, Leon, Solomon, Keller and Coryell 21 , Reference Faedda, Marangoni, Serra, Salvatore, Sani and Vázquez 22 whereas other studies report no progression to bipolar disorder in children and adolescents,Reference Hazell, Carr, Lewin and Sly 20 or progression only when in conjunction with other clinical predictors,Reference Faedda, Marangoni, Serra, Salvatore, Sani and Vázquez 22 or when particularly persistent over adolescence.Reference Tijssen, van Os, Wittchen, Lieb, Beesdo and Mengelers 23
Taken together, sub-threshold hypomanic symptoms measured by several informants or clinical assessment may be useful in the prediction of bipolar disorder onset or conversion to bipolar disorder among individuals with other risk factors such as a confirmed familial risk. This study sought to determine whether self-reported hypomanic symptoms measured by the Hypomania Checklist-32 Revised (HCL-32) or clinically significant sub-threshold hypomanic symptoms (CSHS) diagnosed by a clinician are associated with the onset of bipolar disorder. In particular, our study objectives were to determine (1) if HCL-32 scores (self-rated) are correlated with clinician determined CSHS; (2) if hypomanic symptoms (self-rated and clinician determined) differentiate high-risk offspring of parents with bipolar disorder (high risk) from offspring of well parents (controls) and within those at high risk, differentiate subgroups based on the lithium response of the parent; and (3) within high risk, determine the predictive utility and timing of sub-threshold hypomanic symptoms in the onset of major mood disorders.
Method
Recruitment
Participants were high-risk and control offspring enrolled in the Flourish Canadian high-risk offspring cohort study. Recruitment and procedures for this dynamic cohort study are described in detail elsewhere.Reference Duffy, Horrocks, Doucette, Keown-Stoneman, McCloskey and Grof 6 , Reference Duffy, Alda, Hajek, Sherry and Grof 24 Briefly, high-risk offspring were recruited, starting in 1996, from large multi-generational families identified through an adult patient with confirmed bipolar disorder I.Reference Duffy, Grof, Grof, Zvolsky and Alda 25 Probands and affected first-degree relatives with bipolar disorder I, bipolar disorder II or in a subset recurrent MDD who had children were approached for recruitment into the high-risk study given the evidence that recurrent mood disorders in relatives of patients with bipolar disorder I reflects the bipolar disorder diathesis.Reference Blacker, Lavori, Faraone and Tsuang 26 Lithium response of probands was determined based on a validated scale and previously described clinical criteria.Reference Manchia, Adli, Akula, Ardau, Aubry and Backlund 27 The other parent was confirmed to have no major DSM-IV psychiatric disorders at baseline to control for assortative mating. Control offspring were recruited from schools in Ottawa from parents confirmed to have no major DSM-IV psychiatric disorders at baseline. All diagnoses in both parents were confirmed by Schedule for Affective Disorders – Present and Lifetime Version (SADS-PL),Reference Endicott and Spitzer 28 semi-structured interviews by psychiatrists specialising in mood disorders, and were verified by blind consensus review with at least two additional psychiatrists.
Procedure
Consenting high-risk and control offspring were clinically assessed annually by a child and adolescent psychiatrist specialising in mood disorders using the Kiddie-SADS-PLReference Axelson, Birmaher, Brent, Wassick, Hoover and Bridge 29 semi-structured interview. As part of the semi-structured interview, sub-threshold hypomanic symptoms were systematically documented. The operationalised criteria to confirm CSHS included (1) participant endorsed a minimum of three DSM-IV hypomanic symptoms, but did not meet full DSM-IV criteria for hypomania or bipolar disorder not otherwise specified episode based on duration or severity; (2) symptoms represented a clear change from normal functioning, endorsed by self and others that knew the person well; and (3) no evidence of major impairment. All diagnoses (including CSHS) in high-risk and control offspring were confirmed by a blind consensus review with at least two additional psychiatrists. As part of the high-risk study, during each annual research assessment remitted or well offspring completed the HCL-32.Reference Angst, Adolfsson, Benazzi, Gamma, Hantouche and Meyer 30 Parents completed the Hollingshead socio-economic status (SES) scale,Reference Hollingshead 31 which is a validated measure of SES comprising a composite score of both working spouses highest achieved education and occupation. This research was approved by the local Independent Research Ethics Board in Ottawa, Ontario.
HCL-32 first revision
The HCL-32Reference Angst, Adolfsson, Benazzi, Gamma, Hantouche and Meyer 30 is a 32-item self-report questionnaire originally designed to assess hypomanic symptoms among patients with MDD. A score of 14 or more is considered a clinically significant level of bipolar symptoms and cut-offs of >12 and >3 have been established for active or elated and irritable or risk-taking subscales, respectively.Reference Angst, Adolfsson, Benazzi, Gamma, Hantouche and Meyer 30 The HCL-32 has been well validated in clinical populations,Reference Angst, Adolfsson, Benazzi, Gamma, Hantouche and Meyer 30 , Reference Meyer, Schrader, Ridley and Lex 32 with coefficients alpha ranging between 0.8 and 0.9.Reference Meyer, Schrader, Ridley and Lex 32 For this analysis, owing to the age cut-off of the HCL-32, only high-risk and control offspring aged 15 years and over were included.
Data analysis
This study involved repeated measures within individuals and interdependency among individuals because of more than one sibling in a family. Linear mixed-effect models (LMEMs) and shared frailty models were used to account for these design aspects. In particular, analyses pertaining to repeated HCL-32 continuous scores involved LMEMs. All LMEMs were adjusted for baseline age, HCL-32 assessment time and gender. A normally distributed random intercept for each individual (or each family, if multiple observations per family) was added to each LMEM which takes into account the interdependence among observations. The random intercept and residuals of fit must follow an approximately normal distribution, or the validity of the results may be compromised. Generalised linear mixed models with the logit link function were used wherever fundamental model assumptions for LMEM were not satisfied and thus the outcome had to be dichotomised (i.e. when examining HCL-32 scores using established cut-offs).
Shared frailty models, adjusted for gender were used to determine the association between CSHS and time to MDD and bipolar disorder onset. All cases in which a major depressive episode or activated episode (hypomanic, manic or mixed) occurred before the onset of CSHS or HCL-32 completion date were omitted from the analysis to ensure temporality. For individuals without any major depressive or activated episode, the observation period was censored at their age at last assessment; also age from date of birth was used as the beginning of observation period as this study uses an open, dynamic design, where participants enter the study at different times and ages. Hazard ratios and their corresponding 95% confidence intervals (CI) are reported and reflect, for example, the increase in hazard of first-onset episode of major depression or activated episode given prior CSHS compared to the absence of these symptoms. Owing to the age cut-off (≥15 years) and our dynamic study design, participants completing the HCL-32 were a subset of the entire sample; therefore, all models pertaining to the HCL-32 are of a different and smaller sample size compared with models pertaining to CSHS.
Results
Descriptive characteristics
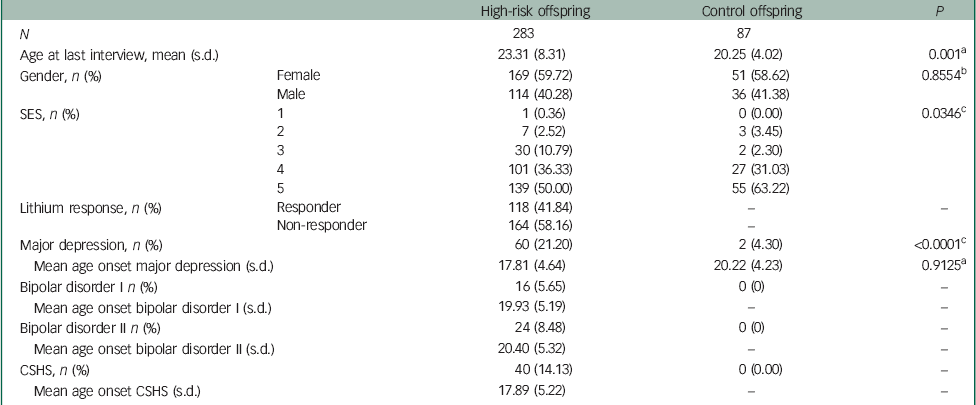
A total of 283 high-risk and 87 control offspring were prospectively assessed as part of the Canadian Flourish high-risk offspring cohort study, and of these, 156 high-risk and 45 control offspring completed the HCL-32 at least once. In the entire sample, the mean age at last interview was 23.3 years [standard deviation (s.d.)=8.3] in high-risk and 20.5 (s.d.=4.0) in control offspring. Approximately 60.0% of high-risk and control offspring were female, and control offspring had slightly higher proportions in upper class SES Hollingshead groups compared with high-risk offspring (Table 1). Mean follow-up time in years in high-risk offspring was 6.9 (s.d.=5.4, median 7.0, range 0.0–19.8) and in control offspring was 5.5 (s.d.=3.3, median 5.0, range 0.0–10.8). The pattern of differences in demographic variables remained similar among the subset of offspring completing the HCL-32 (156 high-risk and 45 control offspring) (data not shown). At the time of this analysis, high-risk and control offspring completed up to four repeated HCL-32 measures during annual clinical visits. The mean age in years when completing the baseline HCL-32 in high-risk offspring was 24.8 (s.d.=6.2) and in control offspring was 21.0 (s.d.=2.4).
Table 1 Demographic and clinical characteristics of the sample
| High-risk offspring | Control offspring | P | ||
|---|---|---|---|---|
| N | 283 | 87 | ||
| Age at last interview, mean (s.d.) | 23.31 (8.31) | 20.25 (4.02) | 0.001 a | |
| Gender, n (%) | Female | 169 (59.72) | 51 (58.62) | 0.8554 b |
| Male | 114 (40.28) | 36 (41.38) | ||
| SES, n (%) | 1 | 1 (0.36) | 0 (0.00) | 0.0346 c |
| 2 | 7 (2.52) | 3 (3.45) | ||
| 3 | 30 (10.79) | 2 (2.30) | ||
| 4 | 101 (36.33) | 27 (31.03) | ||
| 5 | 139 (50.00) | 55 (63.22) | ||
| Lithium response, n (%) | Responder | 118 (41.84) | – | – |
| Non-responder | 164 (58.16) | – | ||
| Major depression, n (%) | 60 (21.20) | 2 (4.30) | <0.0001 c | |
| Mean age onset major depression (s.d.) | 17.81 (4.64) | 20.22 (4.23) | 0.9125 a | |
| Bipolar disorder I n (%) | 16 (5.65) | 0 (0) | – | |
| Mean age onset bipolar disorder I (s.d.) | 19.93 (5.19) | – | – | |
| Bipolar disorder II n (%) | 24 (8.48) | 0 (0) | – | |
| Mean age onset bipolar disorder II (s.d.) | 20.40 (5.32) | – | – | |
| CSHS, n (%) | 40 (14.13) | 0 (0.00) | – | |
| Mean age onset CSHS (s.d.) | 17.89 (5.22) | – | – | |
CSHS, clinical significant sub-threshold hypomanic symptoms; s.d., standard deviation; SES, socio-economic status.
a T-test.
b Chi-square test.
c Fisher's exact test.
At the time of the last observation for this analysis, 21.0% (n=60) of high-risk offspring had a hierarchical lifetime diagnosis of MDD, 8.5% (n=24) bipolar disorder II and 5.6% (n=16) bipolar disorder I. Twenty-one per cent (n=59) of the high-risk offspring had a lifetime history of substance use disorder (SUD), 29% (n=82) anxiety disorder and 19% (n=55) sleep disorder at last observation with 63, 57 and 47% of these cases being comorbid with a mood disorder lifetime diagnosis, respectively. At last observation, the control offspring had low rates of major mood disorders as described in detail elsewhere.Reference Duffy, Horrocks, Doucette, Keown-Stoneman, McCloskey and Grof 6 Only two control offspring had a diagnosis of MDD (4.3%), whereas the remainder of the control offspring were unaffected for any lifetime major mood disorder (Table 1). Clinically significant sub-threshold hypomanic symptoms (CSHS) were present in 14% (40) of high-risk offspring compared with 0% of control offspring, and most of these cases (n=32, 80%) were among high-risk offspring with a diagnosed major mood disorder by last observation. The median age at onset of CSHS in high-risk offspring was 16.4 years (range=6.0–30.7). Among those high-risk offspring with CSHS (n=40) and who developed a major mood disorder, the median ages at onset for these hierarchical lifetime diagnoses were 16.8 years for MDD, 18.4 years for bipolar disorder I and 21.0 years for bipolar disorder II (Fig. 1).

Fig. 1 Median ages of onset of clinically significant sub-threshold hypomanic symptoms (CSHS), major depressive disorder (MDD) and bipolar disorder I and II in the 40 high-risk offspring with CSHS and subsequent onset of MDD, bipolar disorder I or bipolar disorder II.
HCL-32 scores in high-risk and control offspring
Adjusted HCL-32 total scores were significantly higher in control compared with high-risk offspring (P=0.0165). Active or elated and irritable or risk-taking subscale scores were analysed using established cut points (>12 and >3) as the residuals were not normally distributed. Control offspring met criteria for active or elation significantly more than high-risk offspring (P=0.0176), whereas irritability or risk taking was not significantly different between the groups (P=0.9087) (Table 2). We included a variable indicating the presence of diagnosable (hypo)mania before completing the baseline HCL-32 measure in high-risk offspring into each model with HCL-32 total scores, active or elated and irritable or risk-taking subscale cut-offs as the outcome, and after the inclusion of this variable, the group differences in HCL-32 total scores and the active or elated subscale became non-significant (beta (b)=1.5, standard error (s.e.)=1.3, P=0.2312) and (b=1.0, s.e.=0.8, P=0.2265), respectively. Irritability or risk taking remained similar between the groups after including prior diagnosable (hypo)mania to the mixed model (b=0.2, s.e.=0.4, P=0.6827).
Table 2 Differences in HCL-32 scores over time between high-risk and control offspring adjusted for gender, time and baseline age

| High-risk mean (s.d.) | Control mean (s.d.) | beta a | s.e. | P | |
|---|---|---|---|---|---|
| HCL-total | 15.65 (6.06) | 17.52 (5.53) | 2.342 b | 0.968 | 0.0165 |
| HCL-active or elated | 11.29 (3.58) | 12.64 (3.35) | 0.915 c | 0.379 | 0.0176 |
| HCL-irritability or risk taking | 1.83 (2.16) | 1.94 (1.83) | 0.053 c | 0.465 | 0.9087 |
HCL, Hypomania Checklist; s.d., standard deviation.
a Each row reflects a separate adjusted model.
b Linear mixed model.
c Generalised linear mixed model.
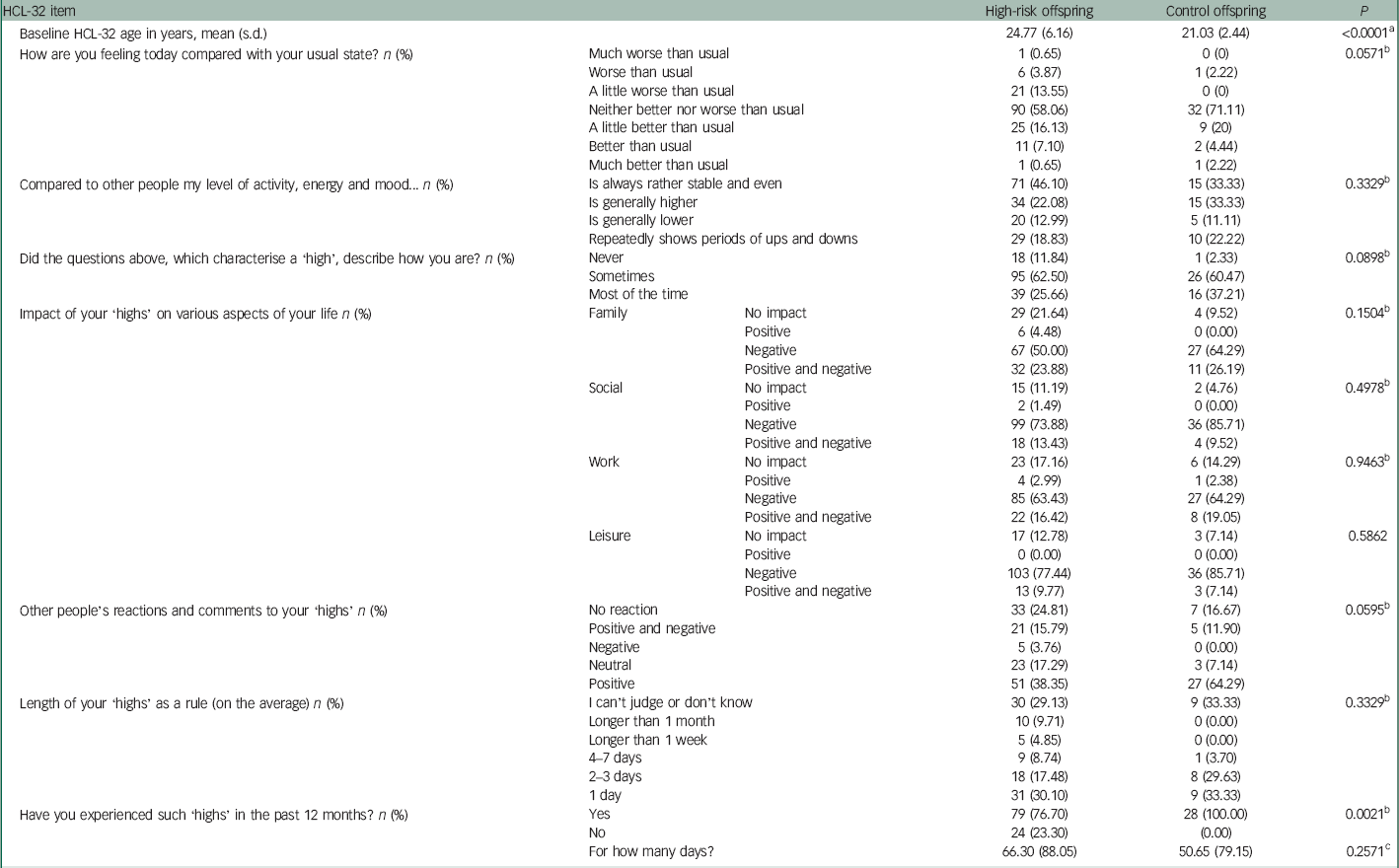
There were significant differences in the contextual questions from the HCL-32. Namely, 100% of control offspring endorsed that they experienced a high (as defined by the items from the prior question in the measure) in the past 12 months compared with 77% of high-risk offspring (P=0.0021). Other's reactions and comments to the offspring self-reported highs were marginally different between high-risk and control offspring (P=0.0595), where a large proportion of control offspring endorsed positive reactions to their highs from other people (64% compared with 38% of high-risk offspring). No control offspring endorsed negative reactions compared with 4% of high-risk offspring (Table 3).
Table 3 HCL-32 contextual items in high-risk and control offspring
| HCL-32 item | High-risk offspring | Control offspring | P | ||
|---|---|---|---|---|---|
| Baseline HCL-32 age in years, mean (s.d.) | 24.77 (6.16) | 21.03 (2.44) | <0.0001 a | ||
| How are you feeling today compared with your usual state? n (%) | Much worse than usual | 1 (0.65) | 0 (0) | 0.0571 b | |
| Worse than usual | 6 (3.87) | 1 (2.22) | |||
| A little worse than usual | 21 (13.55) | 0 (0) | |||
| Neither better nor worse than usual | 90 (58.06) | 32 (71.11) | |||
| A little better than usual | 25 (16.13) | 9 (20) | |||
| Better than usual | 11 (7.10) | 2 (4.44) | |||
| Much better than usual | 1 (0.65) | 1 (2.22) | |||
| Compared to other people my level of activity, energy and mood… n (%) | Is always rather stable and even | 71 (46.10) | 15 (33.33) | 0.3329 b | |
| Is generally higher | 34 (22.08) | 15 (33.33) | |||
| Is generally lower | 20 (12.99) | 5 (11.11) | |||
| Repeatedly shows periods of ups and downs | 29 (18.83) | 10 (22.22) | |||
| Did the questions above, which characterise a ‘high’, describe how you are? n (%) | Never | 18 (11.84) | 1 (2.33) | 0.0898 b | |
| Sometimes | 95 (62.50) | 26 (60.47) | |||
| Most of the time | 39 (25.66) | 16 (37.21) | |||
| Impact of your ‘highs’ on various aspects of your life n (%) | Family | No impact | 29 (21.64) | 4 (9.52) | 0.1504 b |
| Positive | 6 (4.48) | 0 (0.00) | |||
| Negative | 67 (50.00) | 27 (64.29) | |||
| Positive and negative | 32 (23.88) | 11 (26.19) | |||
| Social | No impact | 15 (11.19) | 2 (4.76) | 0.4978 b | |
| Positive | 2 (1.49) | 0 (0.00) | |||
| Negative | 99 (73.88) | 36 (85.71) | |||
| Positive and negative | 18 (13.43) | 4 (9.52) | |||
| Work | No impact | 23 (17.16) | 6 (14.29) | 0.9463 b | |
| Positive | 4 (2.99) | 1 (2.38) | |||
| Negative | 85 (63.43) | 27 (64.29) | |||
| Positive and negative | 22 (16.42) | 8 (19.05) | |||
| Leisure | No impact | 17 (12.78) | 3 (7.14) | 0.5862 | |
| Positive | 0 (0.00) | 0 (0.00) | |||
| Negative | 103 (77.44) | 36 (85.71) | |||
| Positive and negative | 13 (9.77) | 3 (7.14) | |||
| Other people's reactions and comments to your ‘highs’ n (%) | No reaction | 33 (24.81) | 7 (16.67) | 0.0595 b | |
| Positive and negative | 21 (15.79) | 5 (11.90) | |||
| Negative | 5 (3.76) | 0 (0.00) | |||
| Neutral | 23 (17.29) | 3 (7.14) | |||
| Positive | 51 (38.35) | 27 (64.29) | |||
| Length of your ‘highs’ as a rule (on the average) n (%) | I can't judge or don't know | 30 (29.13) | 9 (33.33) | 0.3329 b | |
| Longer than 1 month | 10 (9.71) | 0 (0.00) | |||
| Longer than 1 week | 5 (4.85) | 0 (0.00) | |||
| 4–7 days | 9 (8.74) | 1 (3.70) | |||
| 2–3 days | 18 (17.48) | 8 (29.63) | |||
| 1 day | 31 (30.10) | 9 (33.33) | |||
| Have you experienced such ‘highs’ in the past 12 months? n (%) | Yes | 79 (76.70) | 28 (100.00) | 0.0021 b | |
| No | 24 (23.30) | (0.00) | |||
| For how many days? | 66.30 (88.05) | 50.65 (79.15) | 0.2571 c | ||
HCL-32, Hypomania Checklist-32; s.d., standard deviation.
a Kruskal–Wallis test.
b Fisher's exact test.
c T-test.
The presence of CSHS was positively and significantly associated with HCL-32 baseline scores (b=3.7, s.e.=0.8, P=0.0000). High-risk offspring with prior CSHS reported HCL-32 total scores that were 3.7 units higher than those without CSHS. In high-risk offspring, proband lithium response was not significantly associated with repeated HCL-32 total scores (b=0.5, s.e.=1.2, P=0.6907), active or elated (b=0.2, s.e.=0.40, P=0.5168) or irritable or risk-taking subscale scores (b=0.1, s.e.=0.4, P=0.6993) after adjusting for gender, time and baseline age. Proband lithium response was also not significantly associated with CSHS in high-risk offspring (b=−0.1, s.e.=0.5, P=0.8956).
Hypomanic symptoms and the onset of MDD, bipolar disorder II and bipolar disorder I
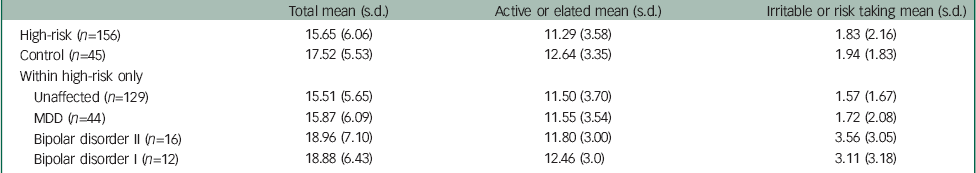
Given the design of this dynamic cohort study (recruiting offspring from ages 5 to 25 years as they became available), there were not enough cases of diagnosable major mood disorders that occurred after the completion of the HCL-32 measure; therefore, the numbers were too low to prospectively estimate the association between HCL-32 scores and subsequent onset of major mood disorder. Mean scores in remitted or well offspring, high-risk offspring with a lifetime diagnosis of mood disorder and control offspring are presented in Table 4.
Table 4 HCL-32 total and subscale scores in high-risk and control offspring
| Total mean (s.d.) | Active or elated mean (s.d.) | Irritable or risk taking mean (s.d.) | |
|---|---|---|---|
| High-risk (n=156) | 15.65 (6.06) | 11.29 (3.58) | 1.83 (2.16) |
| Control (n=45) | 17.52 (5.53) | 12.64 (3.35) | 1.94 (1.83) |
| Within high-risk only | |||
| Unaffected (n=129) | 15.51 (5.65) | 11.50 (3.70) | 1.57 (1.67) |
| MDD (n=44) | 15.87 (6.09) | 11.55 (3.54) | 1.72 (2.08) |
| Bipolar disorder II (n=16) | 18.96 (7.10) | 11.80 (3.00) | 3.56 (3.05) |
| Bipolar disorder I (n=12) | 18.88 (6.43) | 12.46 (3.0) | 3.11 (3.18) |
MDD, lifetime hierarchical diagnosis of major depression; s.d., standard deviation.
Although CSHS were not significantly associated with the subsequent lifetime diagnosis of MDD (hazard ratio=1.1, P=0.8647, 95% CI=0.4, 2.6), when separating out lifetime recurrent MDD from lifetime single episode MDD, CSHS were significantly associated with the development of lifetime recurrent MDD (hazard ratio 5.1, P=0.0002, 95% CI=2.1, 12.4) (Fig. 2). Clinically significant hypomanic symptoms were indicative of risk of the subsequent development of bipolar disorder II, however, this was not statistically significant (hazard ratio 2.0, P=0.2146, 95% CI=0.7, 5.7).
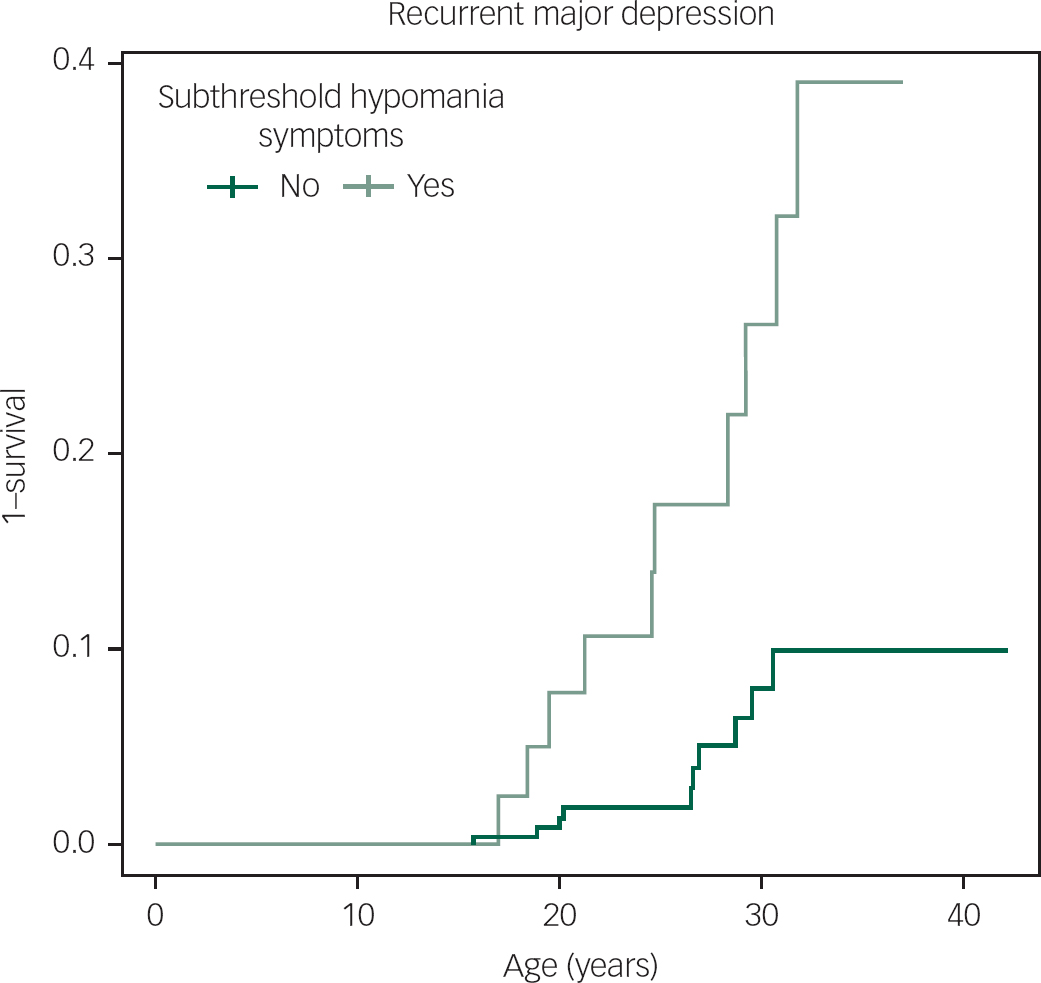
Fig. 2 The hazard of recurrent major depression in high-risk offspring with and without prior clinically significant sub-threshold hypomanic symptoms.
Discussion
The main findings from this prospective cohort study of offspring of parents with bipolar disorder compared with offspring of well parents include (1) clinically determined but not self-reported hypomanic symptoms differentiated high-risk from control offspring; (2) within high-risk offspring, there is evidence of concordance of clinically determined and self-report measures of hypomanic symptoms; and (3) hypomanic symptoms that are assessed to be of clinical importance in young people at familial risk predict onset of recurrent MDD – often heralding the onset of bipolar disorder in this population.
Self-reported hypomanic symptoms in the control offspring appeared to have a different meaning than in the high-risk offspring. This was evidenced by the paradoxical finding of higher total and active or elated HCL-32 scores in control compared with high-risk offspring, with 100% of control offspring endorsing experiencing such highs, despite 0% of clinically determined cases of bipolar disorder or CSHS and a high proportion of control offspring endorsing positive responses by other towards their reported highs. Moreover, when accounting for a prior diagnosable hypo(manic) episode in high-risk offspring, HCL-32 scores were no longer different between high-risk and control offspring and were strongly associated with prior CSHS, suggesting that self-reported hypomanic symptoms may be more clinically meaningful in offspring at confirmed familial high risk. Other studies have reported similar, contradictory findings pertaining to the HCL-32 in general population and control samples, where scores are surprisingly high (meeting the established cut-offs for possible bipolarity)Reference Richardson and Garavan 33 and sometimes higher than clinical samples of patients with bipolar disorder I and bipolar disorder II.Reference Lee, Oh, Lee, Kim, Kim and Hong 34
There are a few possible explanations for this finding. First, the high-risk offspring in this sample were in clinical remission when completing self-reported measures, and therefore, their responses may look similar to control offspring responses. Second, given that the majority of high-risk offspring were exposed to some form of their parents’ mood disorderReference Goodday, Levy, Flowerdew, Horrocks, Grof and Ellenbogen 35 and 18% experienced (hypo)mania themselves, the high-risk offspring may have a different perspective or point of comparison influencing their ranking of hypo(manic) symptoms compared with control offspring. That is, the two populations may be measuring different constructs (i.e. normal mood fluctuation v. psychopathology). General population and control samples may exhibit different baselines for ‘highs’ described in the HCL-32. Despite endorsing higher HCL-32 total and active or elated scores, only one control offspring reported that their highs were equal to or longer than 4 days and no control offspring endorsed negative reactions from other people to these highs, with most reactions reported as positive. Third, other unaccounted for variables may influence HCL-32 scores and reflect hypomanic-like symptoms (particularly related to the active or elated subscale). For example, one studyReference Brand, Luethi, von Planta, Hatzinger and Holsboer-Trachsler 36 found that intense romantic love in adolescents was associated with higher HCL-32 scores compared with adolescents not endorsing this experience.
These possible explanations of the paradoxical finding of higher HCL-32 total and active or elated subscale scores in control offspring compared with high-risk offspring do not discredit the measure's utility in clinical or high-risk populations, but warrants discretion of its use in the general population of young people, where high scores may not necessarily indicate clinically significant hypomanic symptoms, but rather a different conceptualisation of the construct of ‘high mood’. These findings are specific to the HCL-32 within this study cohort and cannot extend to other measures of hypomanic symptoms (e.g. Mood Disorders Questionnaire); however, replication of these findings using other commonly used measures in patient populations could be insightful into their utility in non-clinical populations.
Although the earliest DSM-IV diagnosable hypomanic episode in this cohort so far has been at age 12.5 years, this analysis showed that CSHS are evident in high-risk children as early as 6 years of age. There are no reported cases of CSHS in our control sample. In high-risk offspring, CSHS strongly increased the hazard of developing recurrent MDD and were indicative of risk for bipolar disorder II onset – but failed to reach significance in the latter case likely due to low power. Interestingly, prior CSHS were only significantly associated with recurrent MDD, but not when cases of MDD single episode were added to the group. Moreover, CSHS manifested before the development of major mood disorders in high-risk offspring, just before the median age at onset of MDD and approximately 2 and 4.5 years before the onset of meeting lifetime diagnostic criteria for bipolar disorder I and bipolar disorder II, respectively. Taken together, this finding lends supporting evidence that CSHS predict the development of bipolar disorder in high-risk offspring, as MDD is often the index mood episode of bipolar disorder, and recurrence of mood episodes has been characterised as the hallmark of bipolar disorder.Reference Angst and Sellaro 37 These findings are in agreement with other high-risk studiesReference Axelson, Goldstein, Goldstein, Monk, Yu and Hickey 13 , Reference Egeland, Endicott, Hostetter, Allen, Pauls and Shaw 15 and underscore the potential clinical utility of using CSHS to help predict among individuals at genetic risk, who will transition to the early stages and full onset bipolar disorder.
The following study limitations should be considered when interpreting these findings. We did not have enough cases of major mood disorder occurring before completion of the baseline HCL-32 measure, therefore were unable to estimate the association between self-reported hypomanic symptoms and first onset MDD, bipolar disorder I or bipolar disorder II. Similarly, there were not enough cases of bipolar disorder I occurring before CSHS and the number of cases of bipolar disorder II and MDD were low, limiting statistical power. The models pertaining to HCL-32 scores were in a smaller subset of the entire cohort owing to the age cut-off from the measure (15+). These missing data were not due to refusal to complete the measure, or drop out, and the subset cohort was not significantly different on key characteristics reported in Table 1 compared with the entire cohort making the likelihood of a selection bias low. However, some of these models had low power, impacting the variance, potentially making estimates appear less significant. There may have been other potential confounders not accounted for, particularly in models pertaining to self-reported hypomanic symptoms, such as positive life experiences, which may have influenced the findings. In this high-risk cohort, and others, anxiety and sleep disorders tend to onset before mood disorder and may represent an early manifestation of the bipolar disorder illness,Reference Duffy, Horrocks, Doucette, Keown-Stoneman, McCloskey and Grof 6 however SUDs are likely an effect modifier, where, the association between CSHS and mood disorder may be magnified among those with SUD. Unfortunately, we did not have enough cases of mood disorder to test this. Future research should explore how psychiatric comorbidity influences these associations. Some risk of retrospective recall bias is inevitable in naturalistic cohort studies using an open, dynamic design, where in some cases the outcome occurred before baseline. However, in this analysis, any instance of the outcome occurring before the completion of the HCL-32 or before CSHS were omitted from the analysis to ensure temporality, therefore, using these baselines, all data used in this analysis were prospectively captured.
These results have implications for the potential clinical utility of identifying CSHS in high-risk offspring early in development to help identify an ultra-high-risk population suitable for close monitoring and early intervention. These findings also underscore the importance of using several criteria to inform a bipolar disorder diagnosis including a detailed family history and clinical assessment with reports from multiple informants and cautions against the use of reliance on self-reported symptom level data in isolation as a predictive tool.
Acknowledgements
This work would not be possible without the continued support from the participating research families.
Funding
This research was supported by a Canadian Institute for Health Research Operating Grant (MOP 102761).









eLetters
No eLetters have been published for this article.