The possibility of a link between bipolar disorder and positive attributes such as intelligence, productivity, sociability and creativity has been discussed since antiquity Reference Haldane1 and has been the subject of a number of contemporary reports. Reference Richards, Kinney, Lunde, Benet and Merzel2–Reference Murray and Johnson5 Biographical studies may suggest a relationship between bipolar disorder and exceptional intellectual and/or creative ability but are to some extent limited by non-systematic diagnoses and the potential for recall and selection biases. Reference Andreasen6–Reference Ludwig9
More recently, Swedish population registries have identified that, compared with the general population, individuals with bipolar disorder, as well as their healthy siblings, are more likely to be employed in the creative professions Reference Kyaga, Lichtenstein, Boman, Hultman, Långström and Landén10 and that traits associated with bipolar disorder may be linked to leadership qualities. Reference Kyaga, Lichtenstein, Boman and Landén11 Higier and colleagues Reference Higier, Jimenez, Hultman, Borg, Roman and Kizling12 assessed a large population sample of probands with bipolar disorder – along with their discordant (non-bipolar) co-twins and controls – on measures of temperament and cognitive function. Relative to controls, bipolar co-twins had elevated scores on ‘positive temperament’ and superior performance on tests of verbal learning and fluency, raising the possibility that sociability and verbal proficiency might represent adaptive endophenotypic traits which have contributed to the persistence of bipolar disorder across generations. Reference Higier, Jimenez, Hultman, Borg, Roman and Kizling12
Another line of evidence about a potential link between intellectual ability and risk of bipolar disorder comes from the Dunedin birth cohort, which found that lower childhood IQ was associated with later development of several psychiatric disorders, including schizophrenia spectrum disorder, depression and anxiety disorder, but that higher childhood IQ was associated with mania in adulthood. Reference Koenen, Moffitt, Roberts, Martin, Kubzansky and Harrington13 The authors of this study were cautious about this finding because only eight individuals developed mania, but they suggested a need for replication in other large-scale prospective cohorts.
MacCabe and colleagues Reference MacCabe, Lambe, Cnattingius, Sham, David and Reichenberg14 investigated this further by linking Swedish educational and hospital admissions data to test the association between scholastic achievement at age 16 years and subsequent risk of schizophrenia and bipolar disorder. They found that, as expected, poor scholastic achievement was associated with schizophrenia in males and females but that male students with excellent school performance (relative to students with average grades), as well as those with very poor performance, had an increased risk of admission for bipolar disorder. Reference MacCabe, Lambe, Cnattingius, Sham, David and Reichenberg14 Although Zammit and colleagues Reference Zammit, Allebeck, David, Dalman, Hemmingsson and Lundberg15 did not find an association between IQ at age 18 years and subsequent risk of bipolar disorder within the 1969 Swedish conscription cohort (n=50 087), a more recent and much larger cohort of Swedish military conscripts (n=1 049 607), assessed between the years 1969 and 1994, has identified high IQ as a possible risk factor for bipolar disorder in men, albeit only for the minority who had a ‘pure’ (non-comorbid) form of the disorder. Reference Gale, Batty, McIntosh, Porteous, Deary and Rasmussen16 It is notable that this latter study also found that men with the highest scores on verbal or technical abilities (in contrast to spatial and logical ability) were at highest risk of non-comorbid bipolar disorder. Reference Gale, Batty, McIntosh, Porteous, Deary and Rasmussen16
All of the studies above involved samples where index patients were identified as having a clinical diagnosis of bipolar disorder. In the present study, we take a dimensional approach by assessing the relationship between childhood IQ scores at age 8 years (including both verbal IQ (VIQ) and performance IQ (PIQ) subgroups) and the subsequent development of lifetime manic features (assessed at age 22 years) within a large UK birth cohort, while taking account of a range of potential confounding factors. To our knowledge, a dimensional approach has not been used in previous studies but has the advantage that (as a continuous measure) it is statistically more powerful and, further, does not make any assumptions about the correct threshold score for defining lifetime hypomania. We have also used a score derived from those 28 items within the Hypomania Checklist-32 (HCL-32) Reference Angst, Adolfsson, Benazzi, Gamma, Hantouche and Meyer17 which we previously identified as constituting a linear score. Reference Court, Forty, Jones, Gordon-Smith, Jones and Craddock18 Given the convergence of evidence summarised above, we hypothesised that high IQ in general, and high VIQ in particular, may be markers for the later development of manic features in young adulthood.
Method
This study received ethical approval from the Avon Longitudinal Study of Parents and Children (ALSPAC) Law and Ethics Committee and local research ethics committees. All participants provided written informed consent.
Description of ALSPAC cohort and study sample
The ALSPAC (www.bristol.ac.uk/alspac) is a UK birth cohort, from the geographical area of Avon in Southwest England, recruited between April 1991 and December 1992, with 15 445 participants. Reference Court, Forty, Jones, Gordon-Smith, Jones and Craddock18 Parents provided extensive information at baseline on their own health, demographics and lifestyle and have completed regular postal questionnaires about their child's health and development from birth. From age 7 years, the children attended a number of assessment clinics during which they took part in face-to-face interviews. The study website contains details of all the data that are available through a fully searchable data dictionary (www.bris.ac.uk/alspac/researchers/data-access/data-dictionary/). Recruitment of our final study sample for this study (n=1881) is illustrated in Fig. 1. From the original ALSPAC cohort, 9359 young people who were still contactable were invited to complete the ‘Your Life Now (21+)’ questionnaire, which included the HCL-32 questions. In the first letter, log-in details to complete the HCL-32 online were provided and in a follow-up letter both log-in details and a paper HCL-32 were provided.
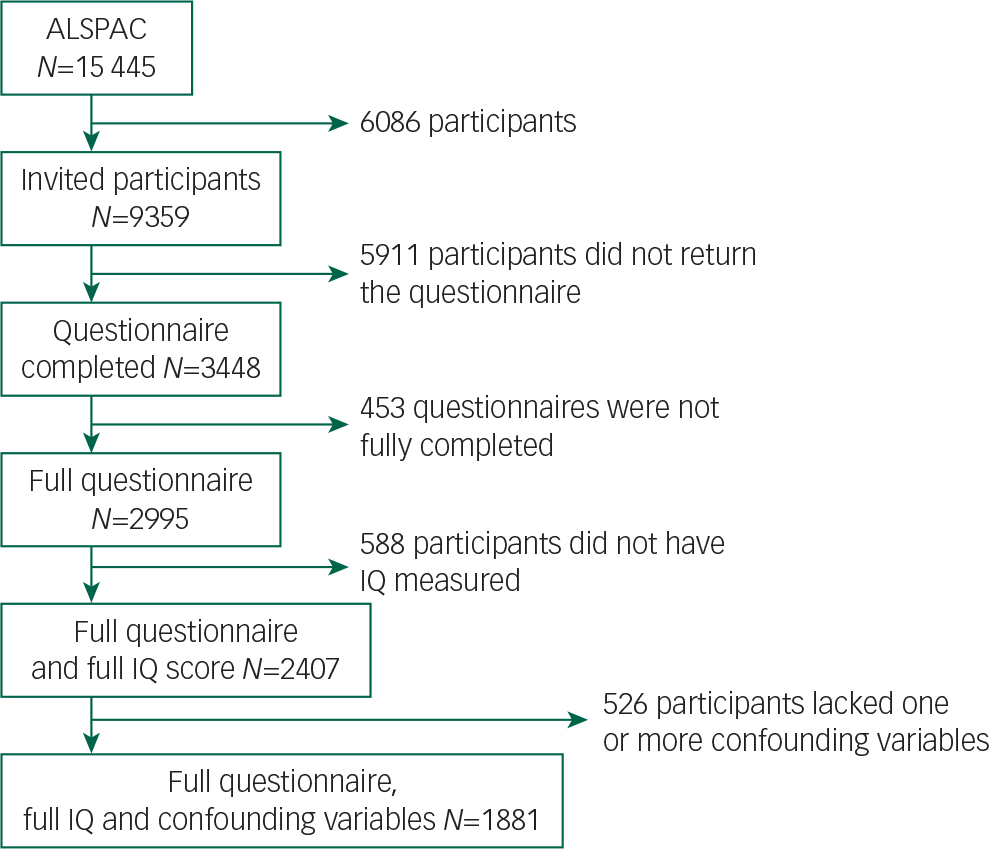
Fig. 1 Flow diagram of sample recruitment.
Assessment of childhood IQ at age 8 years
At age 8 years, the cohort was assessed on the short form of the Wechsler Intelligence Scale for Children-III (WISC-III; alternate items used for all subtests except the coding subtest), which provided a score for full-scale IQ (FSIQ), as well as scores for VIQ and PIQ. Reference Wechsler20 These scores were the primary exposure variables of interest for our study and were categorised into ‘extremely low IQ’ (>70), ‘borderline IQ’ (70–79), ‘low average IQ’ (80–89), ‘average IQ’ (90–109), ‘high average IQ’ (110–119), ‘superior IQ’ (120–129) and ‘very superior IQ’ (>130). We used these IQ categories (rather than raw IQ scores) because this most accurately reflects how these scores are used within educational and clinical settings, and within many previous studies of childhood IQ. This approach should make the interpretation of our findings more meaningful to clinicians.
Lifetime features of bipolar disorder assessed in young adulthood
At age 22–23 years, the cohort was invited to complete the HCL-32. Reference Angst, Adolfsson, Benazzi, Gamma, Hantouche and Meyer17 The HCL-32 is a self-rating questionnaire that assesses lifetime history of manic symptoms. It includes detailed assessments of bipolar mood, energy and activity levels (in total 32 items). This instrument has been used extensively, validated in a number of large-scale studies, and in clinical settings is recognised to be a clinically useful screening instrument for hypomania and bipolar disorder type II. Reference Meyer, Hammelstein, Nilsson, Skeppar, Adolfsson and Angst21–Reference Carta, Hardoy, Cadeddu, Murru, Campus and Morosini26
We recently completed a Rasch analysis for unidimensionality of the HCL-32 manic symptoms within a sample of 389 individuals with DSM-IV bipolar disorder from the Bipolar Disorder Research Network. Reference Angst, Adolfsson, Benazzi, Gamma, Hantouche and Meyer17 This analysis identified that four items (item 14 ‘I wear more colourful and more extravagant clothes/make-up’; item 29 ‘I drink more coffee’; item 30 ‘I smoke more cigarettes’; and item 32 ‘I take more drugs’) should be eliminated to create a linear scale for lifetime bipolarity from the remaining 28 items, with raw scores converted to a score from 0 to 100 (Data supplement, Fig. DS1). Reference Angst, Adolfsson, Benazzi, Gamma, Hantouche and Meyer17 This score for bipolarity, from here on referred to as ‘lifetime manic features’, is our primary outcome of interest, namely a dimensional measure of propensity to bipolar disorder assessed at age 22–23 years.
Confounding variables
Based on knowledge of social and demographic influences on IQ and previous research on the relationship between cognitive/intellectual ability and bipolar disorder, Reference Kyaga, Lichtenstein, Boman, Hultman, Långström and Landén10,Reference Higier, Jimenez, Hultman, Borg, Roman and Kizling12,Reference MacCabe, Lambe, Cnattingius, Sham, David and Reichenberg14,Reference Gale, Batty, McIntosh, Porteous, Deary and Rasmussen16 we identified, a priori, a list of childhood and maternal factors which might confound the association between childhood IQ and bipolar features in young adulthood. These factors included gender (male/female), ethnicity (White/Black and minority ethnic), handedness (left/right-handed at age 10 years), maternal social class at recruitment (I–VI), maternal age at birth (categorised as either above or below 40 years), maternal history of depression (yes/no) and maternal educational level at recruitment (educated to degree level or not). We also adjusted analyses for online versus postal completion of the HCL-32 questionnaire.
Statistical analyses
We transformed the raw 28-item manic features score into a score between 0 and 100 using the key described in our previous paper. Reference Court, Forty, Jones, Gordon-Smith, Jones and Craddock18 A kernel density plot of the transformed score showed that it was normally distributed, so ordinary least squares were used as our modelling technique. Missing data on socioeconomic and demographic characteristics were imputed using imputation through chained equations within the ‘ice’ module for Stata Reference Royston27 and a total of 100 imputed data-sets were created. We did not impute the hypomania score. We tested for interactions with gender using likelihood ratio tests and compared Akaike information criterion (AIC) statistics for the regression model with VIQ and the regression model with PIQ. All analyses were carried out using Stata v13.1. 28
Results
Our final study sample was made up of 1881 individuals (Fig. 1 and Table 1). The FSIQ, VIQ and PIQ scores assessed at age 8 years were normally distributed (Table 2), as were the lifetime manic features scores (Data supplement, Fig. DS2). There was evidence of differences in terms of baseline characteristics between the study sample and the wider ALSPAC cohort (Table 1). The final study sample had a greater proportion of females (62.5% v. 46.6%, P>0.001), more participants of White ethnicity (96.9% v. 94.6%, P>0.001), higher maternal social class (P>0.001), higher maternal education level (P>0.001) and lower rates of maternal depression (5.0% v. 9.7%, P>0.001). They also had higher IQ assessed at age 8 years (mean FSIQ 109 (95% CI 99–120) v. 102 (95% CI 91–113), P>0.001; Table 2). There were no differences between the study sample and the broader ALSPAC cohort in terms of maternal age or handedness (Table 1).
Table 1 Baseline demographic characteristics
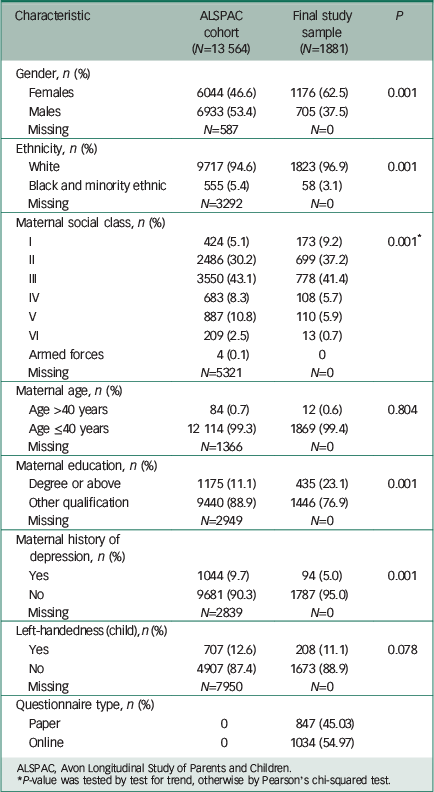
| Characteristic | ALSPAC cohort (N=13 564) |
Final study sample (N=1881) |
P |
|---|---|---|---|
| Gender, n (%) | |||
| Females | 6044 (46.6) | 1176 (62.5) | 0.001 |
| Males | 6933 (53.4) | 705 (37.5) | |
| Missing | N=587 | N=0 | |
| Ethnicity, n (%) | |||
| White | 9717 (94.6) | 1823 (96.9) | 0.001 |
| Black and minority ethnic | 555 (5.4) | 58 (3.1) | |
| Missing | N=3292 | N=0 | |
| Maternal social class, n (%) | |||
| I | 424 (5.1) | 173 (9.2) | 0.001 * |
| II | 2486 (30.2) | 699 (37.2) | |
| III | 3550 (43.1) | 778 (41.4) | |
| IV | 683 (8.3) | 108 (5.7) | |
| V | 887 (10.8) | 110 (5.9) | |
| VI | 209 (2.5) | 13 (0.7) | |
| Armed forces | 4 (0.1) | 0 | |
| Missing | N=5321 | N=0 | |
| Maternal age, n (%) | |||
| Age >40 years | 84 (0.7) | 12 (0.6) | 0.804 |
| Age ≤40 years | 12 114 (99.3) | 1869 (99.4) | |
| Missing | N=1366 | N=0 | |
| Maternal education, n (%) | |||
| Degree or above | 1175 (11.1) | 435 (23.1) | 0.001 |
| Other qualification | 9440 (88.9) | 1446 (76.9) | |
| Missing | N=2949 | N=0 | |
| Maternal history of depression, n (%) | |||
| Yes | 1044 (9.7) | 94 (5.0) | 0.001 |
| No | 9681 (90.3) | 1787 (95.0) | |
| Missing | N=2839 | N=0 | |
| Left-handedness (child), n (%) | |||
| Yes | 707 (12.6) | 208 (11.1) | 0.078 |
| No | 4907 (87.4) | 1673 (88.9) | |
| Missing | N=7950 | N=0 | |
| Questionnaire type, n (%) | |||
| Paper | 0 | 847 (45.03) | |
| Online | 0 | 1034 (54.97) | |
ALSPAC, Avon Longitudinal Study of Parents and Children.
* P-value was tested by test for trend, otherwise by Pearson's chi-squared test.
Table 2 Childhood IQ at 8 years
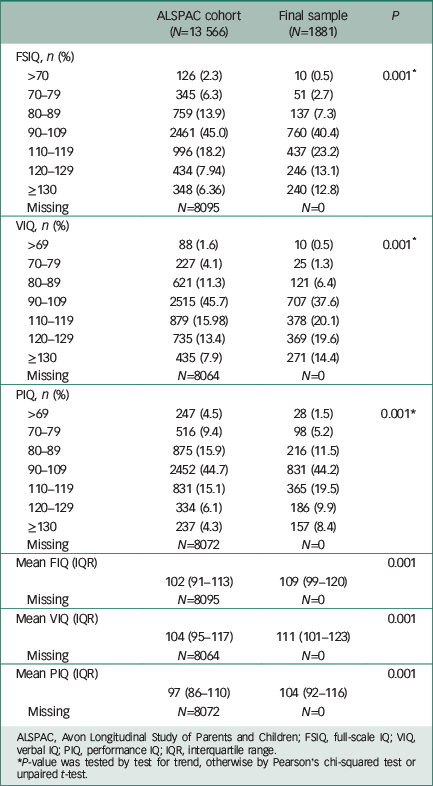
| ALSPAC cohort (N=13 566) |
Final sample (N=1881) |
P | |
|---|---|---|---|
| FSIQ, n (%) | |||
| >70 | 126 (2.3) | 10 (0.5) | 0.001 * |
| 70–79 | 345 (6.3) | 51 (2.7) | |
| 80–89 | 759 (13.9) | 137 (7.3) | |
| 90–109 | 2461 (45.0) | 760 (40.4) | |
| 110–119 | 996 (18.2) | 437 (23.2) | |
| 120–129 | 434 (7.94) | 246 (13.1) | |
| ≥130 | 348 (6.36) | 240 (12.8) | |
| Missing | N=8095 | N=0 | |
| VIQ, n (%) | |||
| >69 | 88 (1.6) | 10 (0.5) | 0.001 * |
| 70–79 | 227 (4.1) | 25 (1.3) | |
| 80–89 | 621 (11.3) | 121 (6.4) | |
| 90–109 | 2515 (45.7) | 707 (37.6) | |
| 110–119 | 879 (15.98) | 378 (20.1) | |
| 120–129 | 735 (13.4) | 369 (19.6) | |
| ≥130 | 435 (7.9) | 271 (14.4) | |
| Missing | N=8064 | N=0 | |
| PIQ, n (%) | |||
| >69 | 247 (4.5) | 28 (1.5) | 0.001* |
| 70–79 | 516 (9.4) | 98 (5.2) | |
| 80–89 | 875 (15.9) | 216 (11.5) | |
| 90–109 | 2452 (44.7) | 831 (44.2) | |
| 110–119 | 831 (15.1) | 365 (19.5) | |
| 120–129 | 334 (6.1) | 186 (9.9) | |
| ≥130 | 237 (4.3) | 157 (8.4) | |
| Missing | N=8072 | N=0 | |
| Mean FIQ (IQR) | 0.001 | ||
| 102 (91–113) | 109 (99–120) | ||
| Missing | N=8095 | N=0 | |
| Mean VIQ (IQR) | 0.001 | ||
| 104 (95–117) | 111 (101–123) | ||
| Missing | N=8064 | N=0 | |
| Mean PIQ (IQR) | 0.001 | ||
| 97 (86–110) | 104 (92–116) | ||
| Missing | N=8072 | N=0 | |
ALSPAC, Avon Longitudinal Study of Parents and Children; FSIQ, full-scale IQ; VIQ, verbal IQ; PIQ, performance IQ; IQR, interquartile range.
* P-value was tested by test for trend, otherwise by Pearson's chi-squared test or unpaired t-test.
Relationship between childhood IQ and lifetime manic features in young adulthood
We found a positive association between FSIQ at age 8 years and lifetime manic features at age 22–23 years (Pearson's correlation coefficient 0.159 (95% CI 0.120–0.198), P>0.001). Individuals in the lowest decile of manic features had a mean FSIQ which was almost 10 points lower than those in the highest decile of manic features: mean FSIQ 100.71 (95% CI 98.74–102.6) v. 110.14 (95% CI 107.79–112.50), P>0.001 (Table 3). Mean VIQ and mean PIQ scores were also significantly lower within the lowest decile of manic features compared with the highest decile (P>0.001; Table 3).
Table 3 Mean IQ scores by lowest and highest deciles of manic features scores

| Lowest deciles of manic features | Highest deciles of manic features | ||||
|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | P | |
| Full-scale IQ | 100.71 | (98.74–102.68) | 110.14 | (107.79–112.50) | >0.001 |
| Verbal IQ | 4.27 | (4.11–4.43) | 4.96 | (4.77–5.15) | >0.001 |
| Performance IQ | 3.95 | (3.79–4.11) | 4.49 | (4.30–4.68) | >0.001 |
The association between IQ and manic features was present for FSIQ, VIQ and PIQ but was strongest for VIQ (Fig. 2). The ‘extremely low’ VIQ group (VIQ >70) scored on average 7.1 points less on the manic features scale than the normal range VIQ group (VIQ >90–109) (P=0.044) and the ‘very superior’ VIQ group (VIQ ≥130) scored on average 1.83 points higher on manic features than the normal VIQ group (P=0.043).
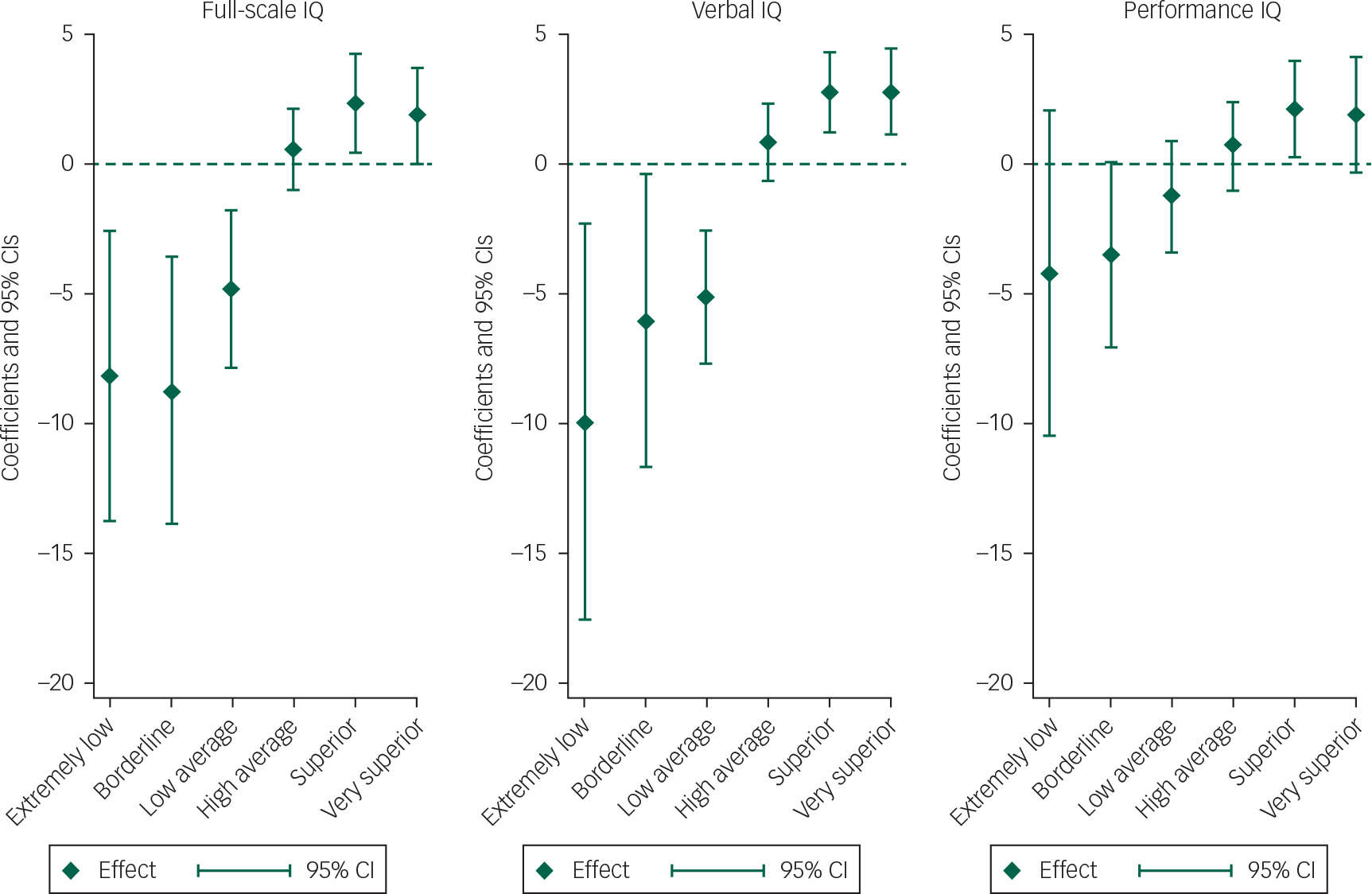
Fig. 2 Lifetime manic features score coefficients, by IQ type.a
a. Regression coefficients shown are from the multiple imputation model.
Compared with children with IQ scores in the average range (90–109), as IQ increased, from ‘high average IQ’, ‘superior IQ’ and ‘very superior IQ’, so the mean score for lifetime manic features increased. Similarly, as IQ decreased, from ‘low average IQ’, ‘borderline IQ’ to ‘extremely low IQ’, the mean score for lifetime manic features decreased. As detailed within Table 4, this was true for both unadjusted analyses and analyses which adjusted for gender, ethnicity, maternal social class, maternal age >40 years, maternal education, maternal history of depression, child left-handedness and version of questionnaire. Although there was no clear evidence that the observed trends for the categorised VIQ and PIQ variables were different from each other (P=0.11), the AIC statistic for the regression model with VIQ was 15160.9, compared with 15194.3 for the regression model with PIQ, suggesting that VIQ was a better predictor of lifetime manic features than PIQ. There was evidence that the relationship between VIQ and lifetime manic features was non-linear (likelihood ratio test for a quadratic relationship P=0.002) but no evidence of a non-linear effect for PIQ (P=0.340; Fig. 2). Table 5 demonstrates that these findings were also consistent for imputed data analyses. Furthermore, on tests of interaction, there was no evidence that effects were different for males and females.
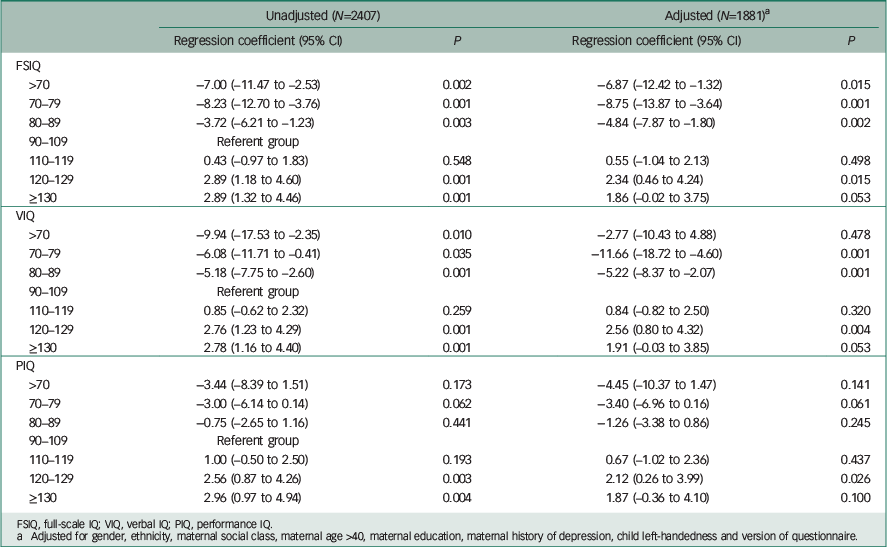
Table 4 FSIQ, VIQ and PIQ at age 8 years and lifetime manic features score in young adulthood
| Unadjusted (N=2407) | Adjusted (N=1881) a | |||
|---|---|---|---|---|
| Regression coefficient (95% CI) | P | Regression coefficient (95% CI) | P | |
| FSIQ | ||||
| >70 | −7.00 (–11.47 to −2.53) | 0.002 | −6.87 (–12.42 to −1.32) | 0.015 |
| 70–79 | −8.23 (–12.70 to −3.76) | 0.001 | −8.75 (–13.87 to −3.64) | 0.001 |
| 80–89 | −3.72 (–6.21 to −1.23) | 0.003 | −4.84 (–7.87 to −1.80) | 0.002 |
| 90–109 | Referent group | |||
| 110–119 | 0.43 (–0.97 to 1.83) | 0.548 | 0.55 (–1.04 to 2.13) | 0.498 |
| 120–129 | 2.89 (1.18 to 4.60) | 0.001 | 2.34 (0.46 to 4.24) | 0.015 |
| ≥130 | 2.89 (1.32 to 4.46) | 0.001 | 1.86 (–0.02 to 3.75) | 0.053 |
| VIQ | ||||
| >70 | −9.94 (–17.53 to −2.35) | 0.010 | −2.77 (–10.43 to 4.88) | 0.478 |
| 70–79 | −6.08 (–11.71 to −0.41) | 0.035 | −11.66 (–18.72 to −4.60) | 0.001 |
| 80–89 | −5.18 (–7.75 to −2.60) | 0.001 | −5.22 (–8.37 to −2.07) | 0.001 |
| 90–109 | Referent group | |||
| 110–119 | 0.85 (–0.62 to 2.32) | 0.259 | 0.84 (–0.82 to 2.50) | 0.320 |
| 120–129 | 2.76 (1.23 to 4.29) | 0.001 | 2.56 (0.80 to 4.32) | 0.004 |
| ≥130 | 2.78 (1.16 to 4.40) | 0.001 | 1.91 (–0.03 to 3.85) | 0.053 |
| PIQ | ||||
| >70 | −3.44 (–8.39 to 1.51) | 0.173 | −4.45 (–10.37 to 1.47) | 0.141 |
| 70–79 | −3.00 (–6.14 to 0.14) | 0.062 | −3.40 (–6.96 to 0.16) | 0.061 |
| 80–89 | −0.75 (–2.65 to 1.16) | 0.441 | −1.26 (–3.38 to 0.86) | 0.245 |
| 90–109 | Referent group | |||
| 110–119 | 1.00 (–0.50 to 2.50) | 0.193 | 0.67 (–1.02 to 2.36) | 0.437 |
| 120–129 | 2.56 (0.87 to 4.26) | 0.003 | 2.12 (0.26 to 3.99) | 0.026 |
| ≥130 | 2.96 (0.97 to 4.94) | 0.004 | 1.87 (–0.36 to 4.10) | 0.100 |
FSIQ, full-scale IQ; VIQ, verbal IQ; PIQ, performance IQ.
a Adjusted for gender, ethnicity, maternal social class, maternal age >40, maternal education, maternal history of depression, child left-handedness and version of questionnaire.
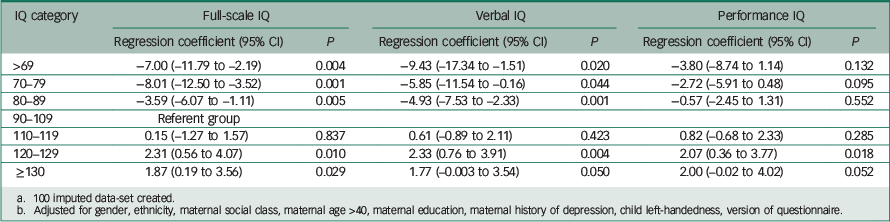
| IQ category | Full-scale IQ | Verbal IQ | Performance IQ | |||
|---|---|---|---|---|---|---|
| Regression coefficient (95% CI) | P | Regression coefficient (95% CI) | P | Regression coefficient (95% CI) | P | |
| >69 | −7.00 (–11.79 to −2.19) | 0.004 | −9.43 (–17.34 to −1.51) | 0.020 | −3.80 (–8.74 to 1.14) | 0.132 |
| 70–79 | −8.01 (–12.50 to −3.52) | 0.001 | −5.85 (–11.54 to −0.16) | 0.044 | −2.72 (–5.91 to 0.48) | 0.095 |
| 80–89 | −3.59 (–6.07 to −1.11) | 0.005 | −4.93 (–7.53 to −2.33) | 0.001 | −0.57 (–2.45 to 1.31) | 0.552 |
| 90–109 | Referent group | |||||
| 110–119 | 0.15 (–1.27 to 1.57) | 0.837 | 0.61 (–0.89 to 2.11) | 0.423 | 0.82 (–0.68 to 2.33) | 0.285 |
| 120–129 | 2.31 (0.56 to 4.07) | 0.010 | 2.33 (0.76 to 3.91) | 0.004 | 2.07 (0.36 to 3.77) | 0.018 |
| ≥130 | 1.87 (0.19 to 3.56) | 0.029 | 1.77 (–0.003 to 3.54) | 0.050 | 2.00 (–0.02 to 4.02) | 0.052 |
a 100 imputed data-set created.
b Adjusted for gender, ethnicity, maternal social class, maternal age >40, maternal education, maternal history of depression, child left-handedness, version of questionnaire.
Discussion
In this large birth cohort we found that better performance on IQ tests at age 8 years, across the IQ range, was predictive of higher scores on a dimensional measure of lifetime manic features assessed in young adulthood. This association persisted after adjusting for a range of child and maternal confounding factors and there was some evidence that the association was strongest for childhood VIQ score. It is therefore possible that childhood IQ, particularly VIQ, may represent a marker of risk for prodromal or early stage bipolar disorder, although formal diagnostic assessments and longer periods of follow-up will be required to clarify this.
Findings in the context of previous work
These findings are consistent with the recent twin study by Higier and colleagues Reference Higier, Jimenez, Hultman, Borg, Roman and Kizling12 which found that supra-normal levels of verbal proficiency might represent a trait marker for bipolar disorder and they are in line with the work by Gale and colleagues Reference Gale, Batty, McIntosh, Porteous, Deary and Rasmussen16 who suggested that high intelligence may be a risk factor for ‘pure’ or ‘non-comorbid’ bipolar disorder in men. Although MacCabe and colleagues Reference MacCabe, Lambe, Cnattingius, Sham, David and Reichenberg14 looked at the association between educational attainment (rather than IQ) and later risk of bipolar disorder, they found a positive association for men (but not for women). It is notable that, as with Higier and colleagues’ twin study, we found that the association between IQ and later features of bipolar disorder applied to both men and women. We did not find that lower childhood IQ predicted manic features in adulthood.
More broadly, our finding that higher IQ scores (particularly VIQ) measured in childhood were associated with higher scores on a dimensional measure of lifetime manic features assessed in early adulthood is also consistent with research suggesting that the genetic propensity to bipolar disorder may be associated with a range of creative abilities, especially in areas where verbal proficiency may prove advantageous to the individual, such as in literature or in leadership roles. Reference Richards, Kinney, Lunde, Benet and Merzel2,Reference Murray and Johnson5,Reference Santosa, Strong, Nowakowska, Wang, Rennicke and Ketter29 To some extent, this is supported by studies which have identified that the clinical states of mania and hypomania are associated with both greater combinatory thought processes Reference Solovay, Shenton and Holzman30 and greater associational fluency. Reference Henry, Weingartner and Murphy31–Reference Levine, Schild, Kimhi and Schreiber33
Most neurocognitive research on bipolar disorder has found that individuals, as well as to a lesser extent their non-bipolar first-degree relatives, have subtle deficits in verbal memory, sustained attention and executive function Reference Bourne, Aydemir, Balanzá-Martínez, Bora, Brissos and Cavanagh34–Reference Bora, Yucel and Pantelis36 and that these impairments are associated with functional impairment. Reference Malhi, Ivanovski, Hadzi-Pavlovic, Mitchell, Vieta and Sachdev37,Reference Torres, DeFreitas, DeFreitas, Bond, Kunz and Honer38 These deficits may also get worse over time with repeated episodes of illness. Reference Gildengers, Mulsant, Begley, Mazumdar, Hyams and Reynolds Iii39,Reference Moorhead, McKirdy, Sussmann, Hall, Lawrie and Johnstone40 In this regard, it could be considered surprising that we did not find an association between lower childhood IQ and manic features in young adulthood. However, the neurodevelopmental trajectory of bipolar disorder (in contrast to schizophrenia) is not characterised by significant motor delays and cognitive difficulties in childhood, with many individuals who later go on to develop bipolar disorder performing at least as well as the general population Reference Demjaha, MacCabe and Murray41 (although bipolar disorder with an onset in childhood may have a neurodevelopmental profile which is closer to that of schizophrenia). Reference Arango, Fraguas and Parellada42 It is therefore possible that our findings refer to a group of young adults in the early or prodromal stages of bipolar disorder, a proportion of whom may not ultimately go on to develop the full-blown disorder.
Strengths and limitations
The study analysed data from a large birth cohort that is representative of the UK general population in terms of ethnicity, social class and educational attainment. The assessment of childhood IQ at age 8 years, approximately 14 years before the assessment of manic features, as well as the depth of baseline data that were available for assessing the impact of confounding factors, all represent major methodological strengths.
Our primary outcome of interest was a dimensional score for lifetime manic features, derived from 28 items selected from the HCL-32 questionnaire. Reference Court, Forty, Jones, Gordon-Smith, Jones and Craddock18 Clearly, as noted above, higher scores on this instrument are not diagnostic of bipolar disorder but we would argue that higher transformed scores on this measure are a good quantitative assessment of propensity to bipolar disorder, particularly in a population sample of young adults who are likely to be still below the age threshold for experiencing a first episode of mania. Reference Leboyer, Henry, Paillere-Martinot and Bellivier43,Reference Hamshere, Gordon-Smith, Forty, Jones, Caesar and Fraser44 The use of dimensional measures of psychopathology in this way is consistent with a view of mental disorders as quantitative traits. Reference Plomin, Haworth and Davis45 Furthermore, the dimensional nature of the modified questionnaire fits with prior research supporting a dimensional nature of vulnerability to mania. Reference Meyer and Keller46 This is also in line with recent recommendations that dimensional, rather than strict categorical approaches, such as those suggested within the Research Domain Criteria, Reference Cuthbert and Insel47,Reference Insel, Cuthbert, Garvey, Heinssen, Pine and Quinn48 may be more useful for understanding the aetiology of psychiatric conditions. Reference Insel49 There is now good evidence that lifetime features of mania occur along a spectrum of mood and psychotic disorders that includes (but is likely not limited to) the categorical diagnoses of recurrent major depressive disorder, bipolar disorder and schizophrenia. Reference Cardno and Owen50–Reference Craddock and Owen54
Possible limitations of the HCL-32 instrument include the fact that, as a self-report measure, it may be subject to reporting biases, for example, in areas such as sexual activity, risk taking and alcohol use, where young adults might be more likely to respond affirmatively to these statements. However, this instrument has been used very widely in young adult populations and is well validated with respect to screening for bipolar disorder in clinical and young adult population settings. Reference Meyer, Schrader, Ridley and Lex22 The opening statement specifically asks respondents to remember ‘a period when you were in a high state, not related to recreational drug use.’ It is still possible that scores on this questionnaire may have been related to current drug or alcohol use but unfortunately we did not have a contemporaneous measure which would allow us to take this into account within our analyses. Further, it is unlikely that drug or alcohol use would have confounded the IQ measures because they were taken at age 8 years.
Another important limitation relates to the extent to which our study sample is representative of the original ALSPAC cohort and, more broadly, the general population. It is likely that there was an attrition bias in this study. Compared with the rest of the ALSPAC cohort, our study sample had higher IQ, higher maternal social class, higher maternal education level and lower rates of maternal depression. There was also a greater proportion of females and participants of White ethnicity. This attrition represents a limitation of this study but to minimise this possibility we used multiple imputation (Table 5). Within these imputed analyses our findings were similar to the complete case analyses.
Implications
In evolutionary terms, our findings suggest that there may be some selective advantage associated with propensity to serious recurrent disorders of mood such as bipolar disorder. Higher IQ in childhood is likely to advantageous for the long-term reproductive fitness of our species but it is possible that the trade-off for this is greater liability to recurrent depression and/or bipolar disorder. Our findings open the way for more detailed genetic investigations of the relationship between intelligence and bipolar disorder (including, for example, polygenic risk score approaches), as well as assessments of the extent to which childhood IQ might represent an endophenotypic marker for later bipolar disorder. Future work should also assess the contribution of copy number variations (CNVs) to this relationship between IQ and bipolar disorder, given that large CNVs are more strongly associated with schizophrenia, autism and intellectual disability than with bipolar disorder. Reference Green, Rees, Walters, Smith, Forty and Grozeva55 Finally, our findings support quite recent evidence that VIQ or verbal proficiency may represent biomarkers or endophenotypes for bipolar disorder Reference Higier, Jimenez, Hultman, Borg, Roman and Kizling12 and in this regard should stimulate future work on the genetic relationship between VIQ and recurrent disorders of mood such as bipolar disorder.
In summary, this prospective study has identified that performance on childhood IQ tests at age 8 years was positively correlated across the IQ range with lifetime features of bipolar disorder experienced in early adulthood, even after adjusting for a range of confounding factors. Clinically, these findings may have implications for the development of approaches which seek to identify subgroups of adolescents and young adults who may be at higher risk of developing bipolar disorder, for example, those with exceptional verbal proficiency in the context of other known risk factors for bipolar disorder, such as high genetic loading, Reference Craddock and Sklar56 exposure in utero to influenza Reference Canetta, Bao, Co, Ennis, Cruz and Terajima57 or childhood trauma. Reference Etain, Aas, Andreassen, Lorentzen, Dieset and Gard58
Funding
This research was funded by a Strategic Start-up Grant to DJS from the University of Glasgow.
Acknowledgements
We thank all families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. The UK Medical Research Council and the Wellcome Trust (Grant ref: 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors and DJS will serve as guarantor for the contents of this paper.











eLetters
No eLetters have been published for this article.