Social marginalisation in high-income countries is increasingly recognised as a risk factor for poor health, a high burden of multimorbidity and premature mortality.Reference Aldridge, Story, Hwang, Nordentoft, Luchenski and Hartwell1 Homeless people represent a population vulnerable to the ‘tri- morbidity’ of social marginalisation: mental illness, addiction and physical illness.Reference Luchenski, Maguire, Aldridge, Hayward, Story and Perri2 The health risks and consequences of living in precarious housing or absolute homelessness include high rates of psychotic disorders (up to 42%), substance use disorders (up to 54%), traumatic brain injury (up to 53%) and HIV infection (up to 21%).Reference Fazel, Geddes and Kushel3 These illnesses contribute to excess mortality, with risk of all-cause mortality estimated to be 4.83–11.60 times higher than expected.Reference Aldridge, Story, Hwang, Nordentoft, Luchenski and Hartwell1 Cross-sectional studies indicate multimorbid illness is associated with functional impairment in youth and older homeless or precariously housed people, however, the long-term trajectories of functioning are not well described.Reference Vila-Rodriguez, Panenka, Lang, Thornton, Vertinsky and Wong4,Reference Barbic, Jones, Woodward, Piercy, Mathias and Vila-Rodriguez5 Age-related functional impairments are disproportionately higher in homeless people over 50 years old, possibly creating vulnerability to early dementia and accelerated ageing.Reference Brown, Kiely, Bharel and Mitchell6,Reference Hurstak, Johnson, Tieu, Guzman, Ponath and Lee7 Cognitive functioning is of particular interest given its importance for daily living, yet it is an often overlooked health outcome. Cross-sectional studies report global cognitive impairment in approximately 25% of homeless people,Reference Depp, Vella, Orff and Twamley8 with selective deficits in attention, executive functioning and most prominently, verbal memory.Reference Brown, Kiely, Bharel and Mitchell6,Reference Hurstak, Johnson, Tieu, Guzman, Ponath and Lee7,Reference Ennis, Roy and Topolovec-Vranic9,Reference Gicas, Giesbrecht, Panenka, Lang, Smith and Vila-Rodriguez10 Cognitive dysfunction occurs across the age spectrum, including in homeless youth.Reference Fry, Langley and Shelton11,Reference Waclawik, Jones, Barbic, Gicas, O'Connor and Smith12 However, whether impairments are static, reversible or progressive is unclear.
Risk factors for cognitive dysfunction are not well delineated among marginalised people, but generally a multifactorial aetiology is presumed.Reference Depp, Vella, Orff and Twamley8,Reference Gicas, Giesbrecht, Panenka, Lang, Smith and Vila-Rodriguez10 Illnesses associated with premature or accelerated ageing, and found with greater prevalence in marginalised people, include schizophrenia, traumatic brain injury, alcohol dependence and HIV infection.Reference Guggenmos, Schmack, Sekutowicz, Garbusow, Sebold and Sommer13–Reference Pfefferbaum, Zahr, Sassoon, Kwon, Pohl and Sullivan16 Independent of age-related illnesses, cognitive dysfunction may be a critical marker of diminishing robustness in biological systems associated with premature mortality.Reference Schultz-Larsen, Rahmanfard, Kreiner, Avlund and Holst17,Reference Fried, Ferrucci, Darer, Williamson and Anderson18 Identifying vulnerabilities for poor long-term outcomes in homeless people is essential to establish priorities for early interventions.
Aims and hypotheses
To the best of our knowledge, cognition has never been examined longitudinally in a homeless or precariously housed sample. The current study involves a comprehensive 9-year prospective evaluation of over 350 people who are living in such a situation in a large urban center.Reference Vila-Rodriguez, Panenka, Lang, Thornton, Vertinsky and Wong4 The objectives were: (a) to describe trajectories of change in cognition, including verbal learning and memory, and inhibitory control; (b) to evaluate risk factors for cognitive change; and (c) to examine whether cognition is a predictor of mortality independent of chronic medical comorbidities. Older age and select risk factors (psychosis, alcohol use, traumatic brain injury, viral infection burden) are hypothesised to be associated with steeper rates of cognitive decline, and in turn, a greater risk of mortality. Our decision to focus on verbal learning, memory, and inhibitory control as our cognitive outcomes is reflected in the sensitivity of these measures to disruption from psychiatric and neurological illnesses, and the translational value given the tests we use are routinely administered in clinical neuropsychological assessments.
Method
Study design and participants
Participants were recruited from single-room occupancy hotels (n = 310) and a downtown community courthouse (n = 65) located within a neighbourhood described as Canada's ‘poorest postal code’.Reference Honer, Cervantes-Larios, Jones, Vila-Rodriguez, Montaner and Tran19,Reference Krausz and Jang20 This single-room occupancy hotel accommodation meets a Canadian definition of ‘precarious housing’; falling below standards of adequacy, affordability or suitability and creating risk for homelessness.Reference Gaetz, Barr, Friesen, Harris, Hill and Kovacs-Burns21 People who were 18 years or older and English-speaking were eligible. Written informed consent was obtained from all participants. A full description of the nature and purpose of the study was provided to all participants with a minimum of 24 h to consider this information prior to enrolment. A small monetary honorarium was provided for each completed assessment.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human participants were approved by Clinical Research Ethics Board of the University of British Columbia and the Simon Fraser University Office of Research Ethics (H08-00521).
Cognitive measures
Cognitive tests were administered by research assistants, supervised by a neuropsychologist (A.E.T.), at baseline and annually thereafter. Verbal learning was assessed with the total immediate recall score and verbal memory with the total delayed recall score from the Hopkins Verbal Learning Test-Revised (HVLT-R).Reference Benedict, Schretlen, Groninger and Brandt22 Alternate forms were counterbalanced in the first 2 years (Forms 1 and 2) and then consecutively administered (Forms 3–6), re-starting with Form 1 at the seventh test administration. Inhibitory control, a component process of executive functioning, was assessed with the total correct score on the Stroop colour–word subtest. Mean demographically corrected T-scores (population mean 50, s.d. = 10) for each cognitive measure were derived from normative tables provided in the respective test manuals, providing a benchmark for expected level of cognitive performance in the absence of central nervous system dysfunction and facilitating comparison with other studies. Examiners rated the validity of each test score from 1, clearly invalid to 5, clearly valid. Tests scores rated as a 3 (questionably valid) or lower were considered invalid. This enables us to identify cognitive data that may have been invalidated by extraneous factors such as acute psychotic episodes, intoxication and extreme participant fatigue. Additional details are described elsewhere.Reference Gicas, Giesbrecht, Panenka, Lang, Smith and Vila-Rodriguez10
Clinical measures
Clinical data were collected at enrolment by psychiatrists and research assistants independent of cognitive testing. Using information obtained from an interview with a psychiatrist (including mental status and neurological examinations), a Mini-International Neuropsychiatric InterviewReference Sheehan, Lecrubier, Sheehan, Amorim, Janavs and Weiller23 and a review of medical records, all available data were used to make comprehensive psychiatric diagnoses according to DSM-IV-TR criteria.24 Psychotic disorders included schizophrenia, schizoaffective disorder, psychosis not otherwise specified, substance-induced psychosis, mood disorder with psychotic features, or delusional disorder.Reference Lieberman and First25 A diagnosis of clinical cognitive impairment was made by a psychiatrist (W.G.H.) according to DSM-IV-TR criteria for dementia, amnestic disorder, and cognitive disorder not otherwise specified (as per DSM-IV Appendix B research criteria for mild cognitive impairment) using information from participant histories, cognitive testing and neurological examination. For descriptive and statistical analyses, we used a T-score of less than or equal to 1.5 s.d. below the normative mean to define scores that fall within the clinically impaired range on a given test, in accord with existing guidelinesReference Petersen26 and with other studies in homeless people.Reference Hurstak, Johnson, Tieu, Guzman, Ponath and Lee7,Reference Waclawik, Jones, Barbic, Gicas, O'Connor and Smith12
A history of traumatic brain injury was ascertained using a structured medical history questionnaire and defined as the occurrence of at least one injury to the head associated with any duration of loss of consciousness, confusion and/or memory loss, or neuroradiological evidence of trauma on whole brain images collected on a 3T Phillips Achieva scanner, or evidence of persistent sequelae attributable to injury (for example seizures, need for anticonvulsants, organic personality disorder). We adopted an inclusive definition of traumatic brain injury by grouping individuals as either having a history based on the above criteria, or no history, a previously used approach.Reference Fann, Ribe, Pedersen, Fenger-Grøn, Christensen and Benros27 Blood samples were drawn and submitted for HIV, hepatitis B, hepatitis C (HCV), herpes simplex and cytomegalovirus serologies. Total virus exposure burden was quantified by summing seropositive results across the five viruses.Reference Noppert, Aiello, O'Rand and Cohen28 Quantitative polymerase chain reaction (qPCR) determined the presence of an active infection for participants who were HCV seropositive. The Charlson Comorbidity Index was used to quantify co-occurring medical conditions according to the Charlson weighting scheme and adding one point for each decade of life over 40 years.Reference Charlson, Pompei, Ales and MacKenzie29 Sociodemographic data was collected by research assistants using structured interviews. Duration of years living in homelessness or marginal housing within the neighbourhood was calculated as the most recent, relatively continuous period up to study entry. Mortality during the study was confirmed with Coroner's reports and hospital records.
Statistical analysis
Cognition as a longitudinal outcome
A series of linear mixed-effects models for longitudinal data were constructed for the three cognitive outcomes (verbal learning, verbal memory, inhibitory control). Random intercepts were included to account for between-subject variability and random slopes for time to account for repeated measures within subjects. Final models were determined by iteratively adding and removing fixed and random effects and comparing models using the Akaike Information Criterion and log likelihood ratio tests where appropriate. Age, gender and education were included as covariates. Time-invariant, primary risk factors (history of traumatic brain injury, alcohol dependence, psychotic disorder, total virus exposure) were added as fixed effects to the covariate model. Significant interaction terms (risk factor × time2) assessed by the log likelihood ratio test (P<0.05) were retained for inclusion in the final model. Mortality during the study was included as a fixed effect to mitigate a healthy survivor bias. Additional interactions between time and covariates were explored. The Pseudo-R 2 statistic was used to quantify the proportion of explained variance in the random effects, with effect sizes defined by Cohen's guidelines for squared multiple correlation change (i.e. 0.02, small; 0.13, medium; and 0.26, large).Reference Kwok, Underhill, Berry, Luo, Elliott and Yoon30 Checking of model assumptions, management of missing data, and sensitivity analyses are described in supplementary Appendix 1, available at https://doi.org/10.1192/bjo.2020.3.
Analyses of secondary risk factors were conducted for schizophrenia/schizoaffective disorder, substance-induced psychosis, HIV infection, HCV qPCR positive status, substance dependence diagnoses (stimulant, opioid, cannabis), and the Charlson Comorbidity Index. Duration (in years) living in the neighbourhood was used as a proxy for exposure to environmental impoverishment. Risk factors and their interactions were tested in separate models adjusted for demographic variables. Statistical analyses were performed in R (R Core Team, 2018) using the lme4 package.Reference Bates, Mächler, Bolker and Walker31
Cognition as a predictor of mortality
Survival analyses were conducted with left-truncated extended Cox regression models using age as the timescale to examine the association between cognition and risk of mortality. Participants who were lost to follow-up or who completed the study were right censored. Separate unadjusted regression models were conducted for the three cognitive variables, with cognitive scores entered as time-dependent variables. A test of the Schoenfeld residuals, and visual inspection of plots were used to assess proportionality. A time-varying coefficient was constructed by modelling the interaction between cognition and age. Significant interactions in the model suggest that the effect of cognition on risk of mortality varies by age, where the coefficient of the interaction term indicates the directional change in magnitude of the main effect for each additional year of life. Final adjusted models included the Charlson Comorbidity Index to control for the effect of co-occurring chronic physical illnesses on survival outcomes. Additional Cox regression analyses were conducted using clinical cognitive impairment status at study entry. Schoenfeld residuals were examined and age cut-points were considered where non-proportionality was evident. Analyses were conducted with R (R Core Team, 2018) using survival and survminer packages.Reference Therneau and Grambsch32
Results
Participant characteristics
Data were collected between 17 November 2008 and 22 August 2018. Of approximately 515 potential participants, 375 (72.8%) joined the study and 354 had completed a baseline cognitive assessment (see supplementary Fig. 1 for participant flow). Participant characteristics and risk factors for cognitive impairment are detailed in Table 1. The sociodemographic and clinical characteristics of our sample are comparable with other major Canadian studies of homeless people (see supplementary Table 1) and appear consistent with studies conducted in other high-income nations.Reference Fazel, Geddes and Kushel3 This provides support for the possible relevance of our sample to regional and international contexts.
Table 1 Baseline characteristics and risk factors for change in cognition over time
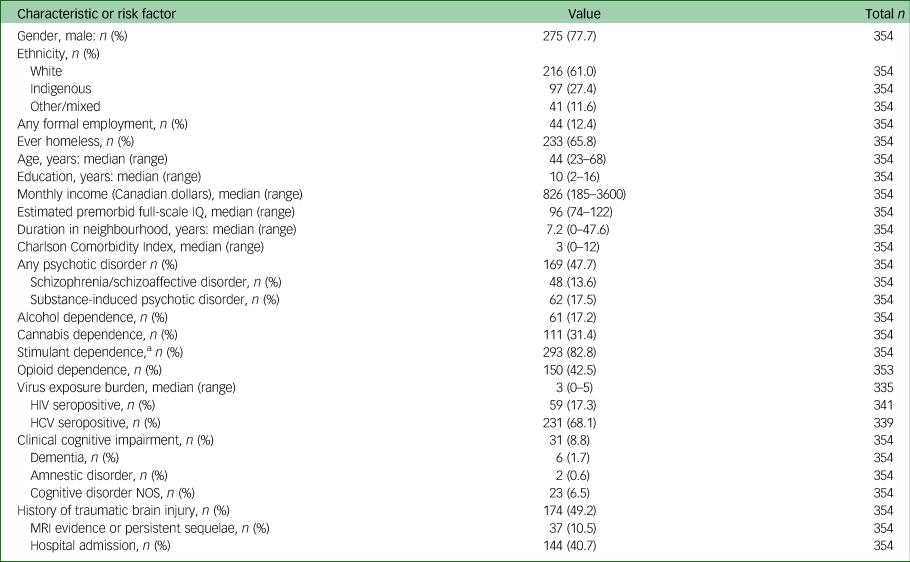
HCV, hepatitis C; NOS, not otherwise specified.
a. Amphetamine, methamphetamine or cocaine.
At baseline evaluation, the mean age-adjusted T-score for verbal learning was 30.5 (s.d. = 10.2), falling in the clinically impaired range, with 68.1% of the participants scoring at or below the 1.5 s.d. cut-off used to define impairment on a given test. The mean end-point verbal learning T-score remained within the clinically impaired range (mean 33.6, s.d. = 12.7), with 57.6% of the participants scoring at or below the cut-off. For verbal memory, the mean baseline T-score was also in the clinically impaired range (mean 31.5. s.d. = 11.0), with 62.9% of participants scoring at or below the cut-off. The mean end-point verbal memory T-score remained in the impaired range (mean 33.9, s.d. = 12.8), with 59.2% of participants scoring in this range. Mean inhibitory control T-scores were within normal clinical limits at baseline (mean 48.8, s.d. = 9.8) and end-point (mean 51.0, s.d. = 11.0), with 10.0% and 8.7% scoring within the clinically impaired range, respectively.
Trajectories of cognitive change over time, and effects of primary risk factors
Participants included in the models had on average 5.0 (s.d. = 2.6) cognitive assessments with a mean of 1.3 years (s.d. = 0.5) between assessments, and a mean of 6.0 years (s.d. = 2.3) total follow-up time.
Verbal learning
Verbal learning (Table 2) was characterised by an initial improvement in performance followed by a declining pattern of performance over time (quadratic time function), but this did not meet the threshold for statistical significance. Higher verbal learning scores at baseline were associated with younger age, being female, more years of education, lower total virus exposure and absence of a psychotic illness. None of the primary risk factors altered the trajectory of cognitive change over time. Addition of risk factors to the model accounted for 1.6% of the variance in verbal learning trajectories (consistent with a small to trivial effect size).
Table 2 Mixed-effects models of cognitive change over time and associations with risk factorsa
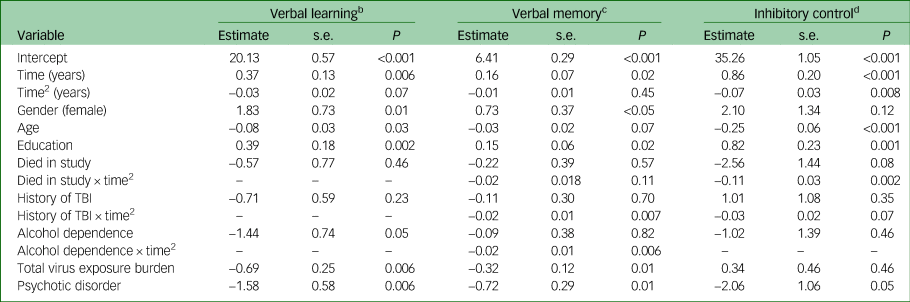
TBI, traumatic brain injury; ‘–’ indicates that the interaction was not included in the final adjusted model.
a. Models fit using restricted maximum likelihood estimation. Time2 is a quadratic function (time × time).
b. n = 295. Observations = 1670.
c. n = 294. Observations = 1634.
d. n = 289. Observations = 1606.
Verbal memory
Like verbal learning, an initial improvement in performance was observed. Higher verbal memory scores at baseline were associated with being female, more years of education, lower total virus exposure and the absence of a psychotic illness. A history of traumatic brain injury and alcohol dependence at baseline were associated with significantly greater decline in verbal memory over time (Table 2, Fig. 1). Addition of risk factors and their interactions to the model accounted for 5.8% of the variation in verbal memory change over time (small effect size).
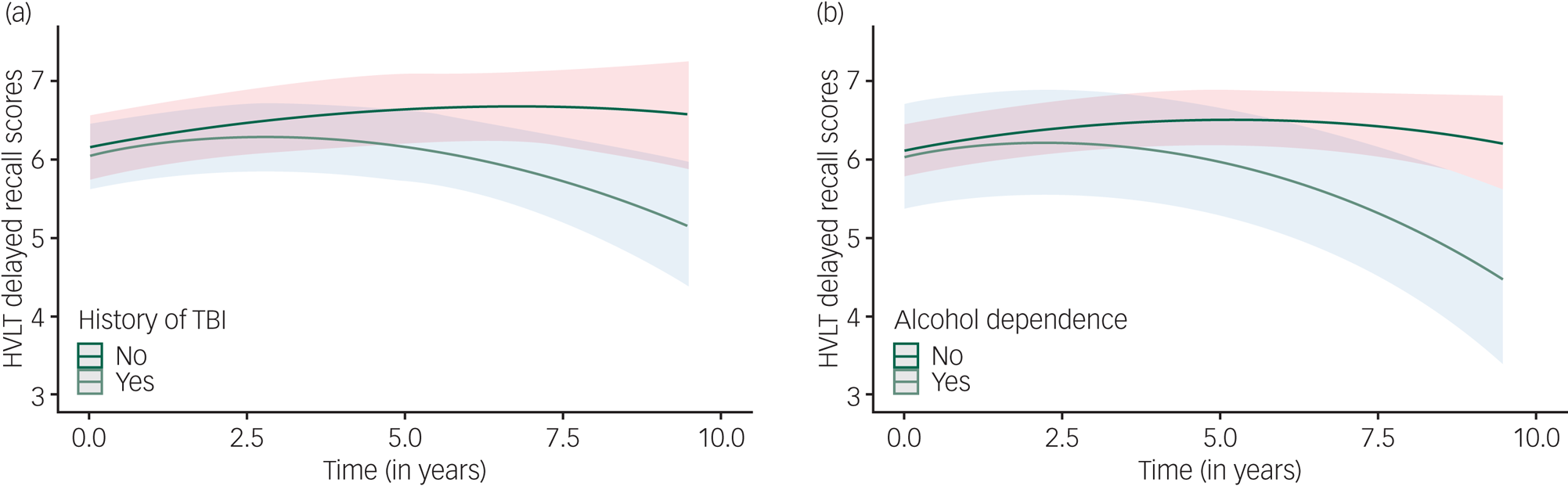
Fig. 1 Change in verbal memory over time as a function of (a) traumatic brain injury or (b) alcohol dependence.
Inhibitory control
The trajectory of change in inhibitory control was characterised by an initial improvement in scores followed by a pattern of significant decline over time. Of note, relatively greater decline in inhibitory control was observed for people who died during follow-up (Table 2). Higher inhibitory control scores at baseline were associated with younger age and more years of education. The risk factors and their interactions accounted for 23.0% of the pattern of change over time (medium to large effect size).
Effects of secondary risk factors
Longer duration living in an impoverished neighbourhood was associated with lower scores in verbal learning and memory at baseline, and significantly greater decline in inhibitory control over time (supplementary Table 2). Other secondary risk factors contributing to level of cognitive performance at baseline included: schizophrenia/schizoaffective disorder, HIV seropositive status, stimulant and opioid (but not cannabis) dependence, and Charlson score. Aside from duration of living in the neighbourhood, no other secondary risk factor was associated with change over time in cognitive function.
All model assumptions were deemed met. We re-ran the primary analyses excluding 27 participants who met criteria for clinical cognitive impairment (as well as eligibility for inclusion in the mixed-effects models), and the results remained unchanged. A diagnosis of clinical cognitive impairment was not associated with differential trajectories of cognitive change. Overall, our sensitivity analyses did not suggest any significant sources of bias in our data (see supplementary Table 3 and supplementary Appendix 1 for details).
Cognitive function and early mortality
As of 22 August 2018, participants were followed for a median of 6.4 years (interquartile range 3.9–8.6 years). During 2260 person-years of observation, 70 of 375 (18.7%) participants died. Causes of death were: physical illness 30 (42.9%), accidental overdose 24 (34.3%), trauma 4 (5.7%), suicide 1 (1.4%), and unknown 11 (15.7%).
Unadjusted models
In unadjusted Cox regressions, significant Schoenfeld tests indicated that verbal learning and inhibitory control interacted with age, but there was no statistically significant effect of verbal learning over time on risk of mortality (Table 3). For inhibitory control, each additional correct response on the inhibitory control task (within a 45 s interval) at study entry was associated with a 6.6% lower risk of mortality, and this effect increased by 0.3% for every additional year of life. For each 1-point increase in the Charlson Comorbidity Index score at study entry, the risk of mortality was 9.9% higher, and was consistent across age. Verbal memory scores were not associated with risk of mortality.
Table 3 Cox regression models of association between cognition and mortalitya
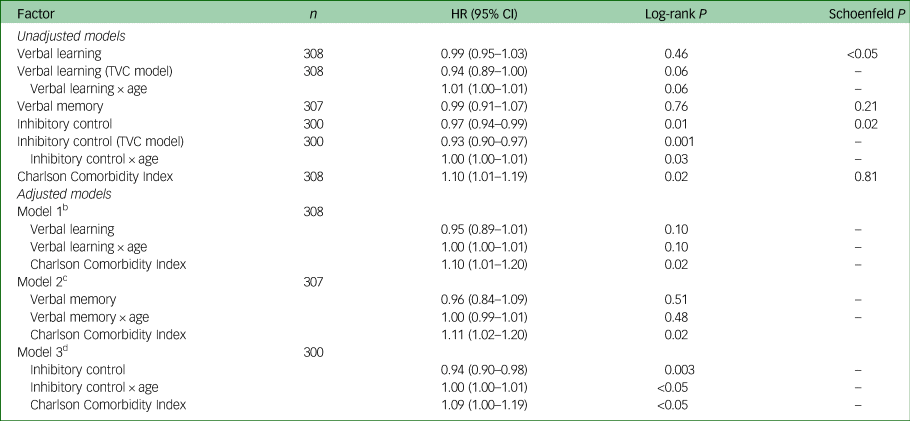
HR, Hazard ratio; TVC, time-varying coefficient.
a. Age (over time) was centred to mean age at study entry (43 years).
b. Observations = 1474; events = 56.
c. Observations = 1450; events = 56.
d. Observations = 1417; events = 53.
Adjusted models
In the final model adjusted for comorbidities, inhibitory control remained a significant predictor of mortality (Table 3). Higher scores at study entry were protective, and this effect increased with age. These effects were not seen with verbal learning or memory, and were not apparent diagnostically at baseline. A diagnosis of clinical cognitive impairment at study entry was not associated with risk of mortality in those under 55 years (hazard ratio (HR) = 1.75, CI = 0.58–4.29, P = 0.32) or 55 years and older (HR = 0.24, CI = 0.03–1.79, P = 0.16). This age cut-point was suggested through inspection of the Schoenfeld residual plot (Schoenfeld P = 0.02). The results were similar when demographically corrected cognitive T-scores were used to classify impairment (≤1.5 s.d. below the mean).
Discussion
Main findings
Our 9-year longitudinal study of people living in a socially marginalised neighbourhood found progressive impairment in select cognitive domains and an association with mortality. Cognitive trajectories showed a pattern of initial improvement in performance, likely attributable to practice effects. These effects are thought to be most prominent following initial repeat testing sessions, because of familiarity with test materials and/or procedures, but diminish with additional repeat testing.Reference Wilson, Capuano, Yu, Yang, Kim and Leurgans33 Subsequent decline in verbal memory was most notable for individuals with a history of traumatic brain injury or alcohol dependence at baseline, controlling for the effects of sociodemographic factors, psychotic disorders and virus burden on baseline level of performance. Significant decline in inhibitory control was observed, with greater decline for those who died during follow-up and for those who spent more years living in an impoverished environment. In survival analyses, better inhibitory control predicted lower risk of mortality; this protective effect was stronger with increasing age. Risk of mortality did not differ by clinical cognitive impairment status at study entry, suggesting findings are not merely a reflection of pre-existing global cognitive deficit or dementia.
Age and cognitive decline
Patterns of cognitive decline in this comparatively young community sample appear to emerge somewhat earlier than might be expected. A longitudinal evaluation of healthy community-dwelling participants reported decline in memory and reasoning abilities beginning in the 60s.Reference Salthouse34 In a large US national sample of healthy adults, decline in immediate word list recall (verbal learning) began in the 40s, followed by decline in delayed word list recall (verbal memory) and executive functioning (reasoning, verbal fluency, working memory) beginning in the 50s and later, though these effects were reported to be very small to trivial.Reference Hughes, Agrigoroaei, Jeon and Bruzzese35 In comparison, we observed decline in verbal memory and inhibitory control (a select component of executive functioning) beginning in the latter half of the 40s. However, these comparison cohorts differ from our sample in sociodemographic composition and therefore do not provide optimal benchmarks.Reference Salthouse34,Reference Hughes, Agrigoroaei, Jeon and Bruzzese35 Although it is tempting to speculate a pattern of premature ageing, this hypothesis requires further investigation with a demographically matched control group that more closely approximates the current sample.
Individual-level risk factors
Select risk factors previously implicated in accelerated ageing had an impact on cognitive decline in this sample. Alcohol dependence was associated with greater decline in verbal memory, whereas dependence on other non-prescribed substances was not. Heavy alcohol consumption with increasing age is linked with greater impairment in learning, memory and motor functions, but not attention and executive functioning, and is considered to reflect a dual premature-accelerated model of cognitive ageing.Reference Woods, Porges, Bryant, Seider, Gongvatana and Kahler36
Traumatic brain injury in our sample is associated with abnormalities of neural connectivity and cross-sectional cognitive impairment,Reference Schmitt, Thornton, Rawtaer, Barr, Gicas and Lang37 and is now observed to be associated with steeper memory decline. A study of nearly 2.8 million people from Denmark found an increased risk of all-cause dementia in people with a history of traumatic brain injury.Reference Fann, Ribe, Pedersen, Fenger-Grøn, Christensen and Benros27 Existing evidence appears strongest for a link between severe traumatic brain injury and accelerated ageing.Reference Wood38 We also found that people who died during follow-up showed steeper decline in inhibitory control. This is consistent with evidence of accelerated cognitive decline several years prior to death in older adults with and without dementia.Reference Karr, Graham, Hofer and Muniz-Terrera39
Environmental-level risk factors
Social and environmental deprivation may also be a major determinant of accelerated cognitive ageing.Reference Clarke, Weuve, Barnes, Evans and Mendes de Leon40 In the current study, longer time living within an impoverished neighbourhood was associated with greater decline in inhibitory control, possibly reflecting the cumulative effects of socioeconomic disadvantage, unsafe living conditions and social stressors. Lack of community resources for cognitive enrichment in day-to-day life may also contribute. These findings highlight the importance of considering individual-level and environment-level risk factors for cognitive decline.
Factors associated with mortality
Trajectories of cognitive functioning appear to have significant bearing on premature mortality. In a cross-sectional study of over 1100 community-dwelling older adults using a short form of the Mini-Mental Status Examination, the association between global cognition and mortality was reported to be distinct from the effects of chronic disease.Reference Schultz-Larsen, Rahmanfard, Kreiner, Avlund and Holst17 In the present longitudinal study using more detailed neuropsychological testing, better inhibitory control was associated with a 6.6% decreased risk of mortality, and this protective effect of cognition became larger by 0.3% for every additional year of life, controlling for co-occurring chronic medical illnesses. Cognition itself may be an independent marker of health and reflective of diminished integrity in neural systems, associated with effects on brain structure and connectivity.Reference Gicas, Giesbrecht, Panenka, Lang, Smith and Vila-Rodriguez10,Reference Gicas, Jones, Panenka, Giesbrecht, Lang and Vila-Rodriguez41 This model is consistent with the concept of frailty, associated with decreased physiological reserve as a consequence of lifetime aggregation of effects from biological ageing or subthreshold disease-related insults.Reference Fried, Ferrucci, Darer, Williamson and Anderson18 Cognitive dysfunction, therefore, may be an emergent clinical expression of frailty and may help to explain the stronger relationship between cognition and risk of mortality over time.
Limitations
Limitations should be considered. First, the extent of cognitive decline in the present sample was not directly compared with a healthy cohort of individuals with the same age and education, necessary for more definitive understanding of the role of premature and/or accelerated cognitive ageing. Second, the deleterious effects of alcohol and traumatic brain injury may have been underestimated because of the challenges of retrospectively assessing patterns of alcohol use, as well as severity and number of traumatic brain injuries. Additionally, we did not examine all possible cognitive domains and therefore our understanding of trajectories of change in this sample remains incomplete. We also did not examine other clinical conditions, such as mood disorders, anxiety and post-traumatic stress disorder, which are more likely to fluctuate over time and may co-vary with the level of cognitive performance at any given time point. Future studies will need to address these factors in order to provide a more complete clinical picture.
Contrary to our initial hypothesis, age, psychosis and virus exposure burden did not predict cognitive change, possibly requiring longer follow-up periods. An examination of symptoms of psychosis, rather than diagnosis, may also reveal unique covariation with cognition over time. Alternatively, for some risk factors, the association with decline may be masked by practice effects inherent in longitudinal designs.Reference Salthouse34
Lastly, the numbers of available participants limited the ability to assess possible synergistic effects of comorbidities. Nonetheless, this study has strengths in the breadth of risk factors evaluated and frequency of follow-up over 9-years. Similar risk factors are reported in homeless and marginally housed populations worldwide,Reference Aldridge, Story, Hwang, Nordentoft, Luchenski and Hartwell1,Reference Fazel, Geddes and Kushel3 and the findings may be more broadly relevant. We used well-validated neuropsychological measures that could be practically implemented in clinical services for this population, yielding unique information about cognitive status beyond what global screening tools can provide (for example Mini-Mental Status Examination).
Implications
Our findings of domain-specific cognitive decline and an association with mortality raise the question as to whether a dual premature-accelerated pathway of cognitive ageing is operant in homeless and precariously housed people. Alcohol use and traumatic brain injury may be modifiable risk factors, amenable to early intervention to mitigate risk for cognitive decline. Inhibitory control appears to be a protective factor; building cognitive reserve early on could buffer against decline and death. Replicating the current findings in other homeless and precariously housed samples, and across more cognitive domains, has a degree of urgency proportionate to globally increasing public health relevance.
Funding
This study was funded by grants from the Canadian Institutes of Health Research (W.G.H., CBG-101827, MOP-137103), BC Mental Health and Addictions Services (W.G.H.), and the William and Ada Isabelle Steel Fund (A.E.T.). W.G.H. was supported by the Jack Bell Chair in Schizophrenia.
Acknowledgements
We would like to thank the Hotel Study staff and volunteers for their efforts in data collection and management. We also wish to thank the study participants for their time.
Data availability
All authors have full ongoing access to the database used for carrying out this study. Data cannot be made publicly available due to possible privacy breaches and other ethical and legal obligations to the study participants. These restrictions are outlined by the University of British Columbia's Clinical Research Ethics Board and Simon Fraser University's Research Ethics Board. Inquiries regarding data can be made to the Clinical Research Ethics Board of the University of British Columbia (ethics.research.ubc.ca) and the study principal investigator (W.G.H.) at william.honer@ubc.ca.
Author contributions
K.M.G., A.A.J., A.E.T. and W.G.H. were involved in formulating research questions, study design, data analysis and interpretation and writing the article. A.E.T., W.J.P., A.M.B., D.J.L., F.V.-R., R.M.P., G.W.M. and W.G.H. were involved in study conceptualisation and data acquisition and/or interpretation. A.P., E.L., K.W., O.L. and T.B. were involved in data acquisition. All authors critically revised the manuscript for intellectual content and approved the final version.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjo.2020.3.






eLetters
No eLetters have been published for this article.