The use of placebos in randomised control trials (RCTs) when effective treatments are available has been controversial. Reference Emanuel and Miller1–Reference Temple and Ellenberg3 There is now widespread agreement, however, that such studies can sometimes be ethical as long as certain conditions are met. Reference Emanuel and Miller1,Reference Temple and Ellenberg3,4 The Declaration of Helsinki now states that such RCTs are permissible when there is ‘compelling and scientifically sound methodological reasons’ to determine the efficacy or safety of an intervention without subjecting participants to additional risks of ‘serious or irreversible harm’. 4 In particular, placebo control trials of antidepressants are scientifically necessary due to their low assay sensitivity and do not appear to increase the risks of serious or irreversible harm. Reference Kim5,Reference Khan, Warner and Brown6 Even if such placebo control trials are permissible, they still raise issues regarding informed consent – an issue that has been relatively neglected. These trials ask individuals to bear special burdens for societal benefit. Thus, specific ethical attention to how that information is conveyed to potential individuals seems particularly important. Thus, for example, a recent draft guidance on informed consent by the US Food and Drug Administration states that disclosures ‘must include a description of the current medically recognised standard of care … [and when possible] quantified comparative estimates of risks and benefits (e.g., from the clinical literature)’ (Section 4, page 9–10). 7 For placebo control trials testing novel antidepressants, a decision analysis model has shown that a person with moderate depression can expect to have almost 25% greater chance of symptomatic benefit by choosing individualised psychiatric treatment over entering a placebo control trial (64.3% v. 39.5%). In this study, we conducted a randomised experimental survey to examine the potential impact of providing quantitative outcome information to potential participants in placebo control trials testing a novel antidepressant. We tested whether providing this quantitative trade-off information would affect prospective participants’ willingness to enrol in depression RCTs and examined the reasons for their preferences.
Method
Participants
The participants were recruited from the University of Michigan Depression Center. Upon check-in at the clinic, they received an introduction letter briefly explaining the study. Interested patients then picked up a survey in the waiting room. They either filled out the survey while waiting or returned it by mail in an unmarked envelope. Surveys were anonymous. The University of Michigan IRB approved the study with waiver of documentation of consent.
Measures
The main outcome measure was the choice between entering an 8-week RCT testing a new antidepressant for moderate depression, or pursuing treatment with a psychiatrist. This was measured on a 1–6 scale (1=‘I would definitely choose the research study’ and 6=‘I would definitely choose treatment with a psychiatrist’). Participants were then given the following prompt: ‘Please comment on why you chose that answer’. The survey text is in the Appendix.
To standardise the stimulus, all participants were asked to imagine having moderate depression using the description in the Montgomery–Asberg Depression Rating Scale. Reference Montgomery and Asberg9 They were then randomised to either the standard or enhanced disclosure regarding the use of placebos. The standard disclosure mentioned the probability of receiving the placebo and presented an alternative to participating in the study as ‘Instead of entering the research study, you can choose to receive regular treatment from a psychiatrist’. The enhanced disclosure included in addition a quantitative comparison of the participant's probability of experiencing improvement in depression symptoms, depending on whether the person entered the RCT or chose individual treatment. Based on a previous decision analysis model, the probabilities were presented as 40% for participating in the RCT v. 65% for individualised treatment; the details of the model can be found elsewhere. Reference Kim and Holloway8
Participants completed the 21-item Beck Depression Inventory (BDI). Reference Beck, Ward, Mendelson, Mock and Erbaugh10 They also rated their level of happiness on a scale of 1–10 (10 = ‘extremely happy [feeling ecstatic, joyous, fantastic]’ and 0 = ‘Extremely unhappy [utterly depressed, completely down]’). We collected demographic data including age, gender, race, ethnicity, highest education attainment and annual household income.
Analysis
A Mann–Whitney rank sum test was used to compare the willingness to enrol scores in the standard disclosure and the enhanced disclosure arms. Because the decision between RCT and individual treatment is dichotomous, our primary analysis also dichotomised the responses, with responses 1–3 being assigned as preferring the RCT and responses 4–6 being assigned as preferring individual psychiatric treatment. We then used χ 2 tests to compare the disclosure arms. All tests performed were two-sided. For a 1:1 randomised experimental design with n = 140 in each arm, with α of 0.05, the power to detect an effect of 15% difference in the lower range (25% v. 40% willing to participate) is 0.77.
We performed a content analysis of comments that participants provided when asked to explain their answer to the willingness to enrol in RCT question. The coding process was iterative. Two research assistants reviewed the comments independently, developed the codes with the senior author, then coded them and resolved any discrepancies in discussion.
For each disclosure arm, we performed an exploratory logistic regression to examine the effects of age, gender, race, annual income, education and average BDI score (1–10 happiness scale was not included owing to high correlation with BDI score).
Results
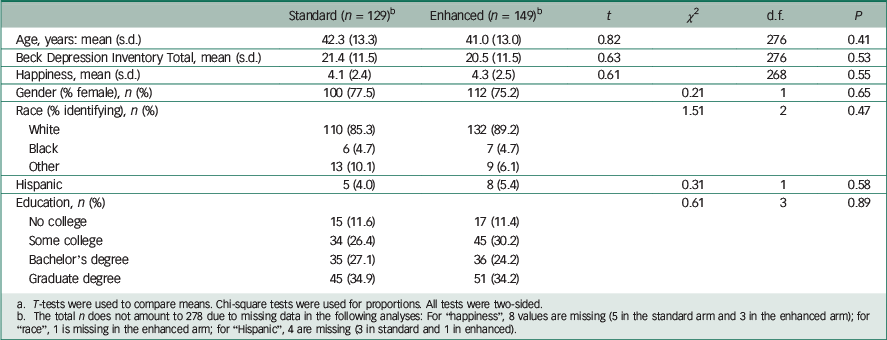
A total of 278 patients participated: 129 received the standard disclosure and 149 received the enhanced version. Demographic and clinical characteristics between the two arms were similar, indicating successful randomisation (Table 1). On average, the participants showed moderate levels of depression on the BDI. Reference Beck, Ward, Mendelson, Mock and Erbaugh10
Table 1 Comparison of participant characteristics in standard v. enhanced disclosure arms a
| Standard (n = 129) b | Enhanced (n = 149) b | t | χ 2 | d.f. | P | |
|---|---|---|---|---|---|---|
| Age, years: mean (s.d.) | 42.3 (13.3) | 41.0 (13.0) | 0.82 | 276 | 0.41 | |
| Beck Depression Inventory Total, mean (s.d.) | 21.4 (11.5) | 20.5 (11.5) | 0.63 | 276 | 0.53 | |
| Happiness, mean (s.d.) | 4.1 (2.4) | 4.3 (2.5) | 0.61 | 268 | 0.55 | |
| Gender (% female), n (%) | 100 (77.5) | 112 (75.2) | 0.21 | 1 | 0.65 | |
| Race (% identifying), n (%) | 1.51 | 2 | 0.47 | |||
| White | 110 (85.3) | 132 (89.2) | ||||
| Black | 6 (4.7) | 7 (4.7) | ||||
| Other | 13 (10.1) | 9 (6.1) | ||||
| Hispanic | 5 (4.0) | 8 (5.4) | 0.31 | 1 | 0.58 | |
| Education, n (%) | 0.61 | 3 | 0.89 | |||
| No college | 15 (11.6) | 17 (11.4) | ||||
| Some college | 34 (26.4) | 45 (30.2) | ||||
| Bachelor's degree | 35 (27.1) | 36 (24.2) | ||||
| Graduate degree | 45 (34.9) | 51 (34.2) | ||||
a T-tests were used to compare means. Chi-square tests were used for proportions. All tests were two-sided.
b The total n does not amount to 278 due to missing data in the following analyses: For “happiness”, 8 values are missing (5 in the standard arm and 3 in the enhanced arm); for “race”, 1 is missing in the enhanced arm; for “Hispanic”, 4 are missing (3 in standard and 1 in enhanced).
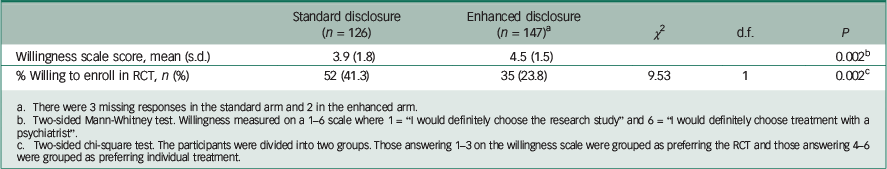
The mean willingness to enrol score (on a scale of 1–6) was 3.9 for those receiving standard disclosure forms and 4.5 for those receiving the enhanced version (P=0.002, Mann–Whitney rank sum test; Table 2).
Table 2 Effect of standard v. enhanced disclosure on depressed patients’ willingness to enter a placebo-controlled antidepressant clinical trial v. individual psychiatric treatment
| Standard disclosure (n = 126) |
Enhanced disclosure (n = 147) a |
χ 2 | d.f. | P | |
|---|---|---|---|---|---|
| Willingness scale score, mean (s.d.) | 3.9 (1.8) | 4.5 (1.5) | 0.002 b | ||
| % Willing to enroll in RCT, n (%) | 52 (41.3) | 35 (23.8) | 9.53 | 1 | 0.002 c |
a There were 3 missing responses in the standard arm and 2 in the enhanced arm.
b Two-sided Mann-Whitney test. Willingness measured on a 1–6 scale where 1 = “I would definitely choose the research study” and 6 = “I would definitely choose treatment with a psychiatrist”.
c Two-sided chi-square test. The participants were divided into two groups. Those answering 1–3 on the willingness scale were grouped as preferring the RCT and those answering 4–6 were grouped as preferring individual treatment.
A greater proportion of respondents in the standard arm was willing to enrol in the RCT than those in the enhanced arm (41.3% v. 23.8%, P = 0.002, χ 2 test).
Some persons made more than one codable comment so that there were 158 total comments coded for the standard disclosure arm and 175 total comments coded for the enhanced disclosure arm. The comments per person ratio was 1.22 for the standard arm and 1.17 for the enhanced arm. Overall proportion of comments about direct personal benefit (50.0%, 79/158 for the standard disclosure arm; 48.0%, 84/175 for the enhanced arm) and altruism (8.2%, 13/158 for standard arm; 9.1%, 16/175 for enhanced arm) were similar in the two arms.
Because the list of codes was not identical for those preferring RCT compared with those preferring individual psychiatric treatment, the qualitative results are presented separately in Tables 3 and 4.
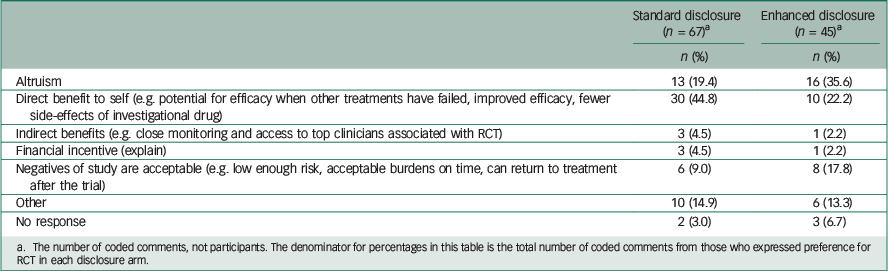
Table 3 Comparison of reasons given for willingness to enroll in RCT, by disclosure arm
| Standard disclosure (n = 67) a |
Enhanced disclosure (n = 45) a |
|
|---|---|---|
| n (%) | n (%) | |
| Altruism | 13 (19.4) | 16 (35.6) |
| Direct benefit to self (e.g. potential for efficacy when other treatments have failed, improved efficacy, fewer side-effects of investigational drug) | 30 (44.8) | 10 (22.2) |
| Indirect benefits (e.g. close monitoring and access to top clinicians associated with RCT) | 3 (4.5) | 1 (2.2) |
| Financial incentive (explain) | 3 (4.5) | 1 (2.2) |
| Negatives of study are acceptable (e.g. low enough risk, acceptable burdens on time, can return to treatment after the trial) | 6 (9.0) | 8 (17.8) |
| Other | 10 (14.9) | 6 (13.3) |
| No response | 2 (3.0) | 3 (6.7) |
a The number of coded comments, not participants. The denominator for percentages in this table is the total number of coded comments from those who expressed preference for RCT in each disclosure arm.
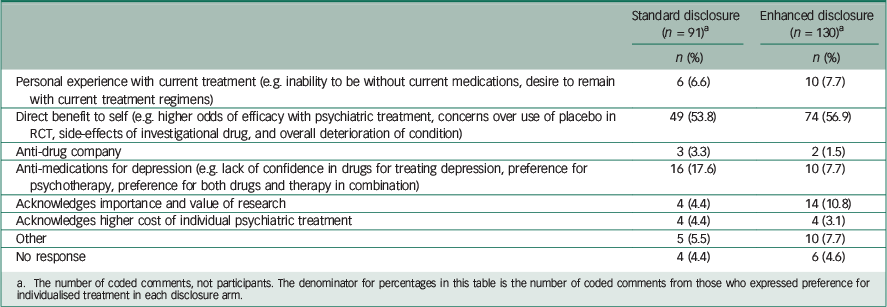
Table 4 Comparison of reasons given for unwillingness to enroll in RCT, by disclosure arm
| Standard disclosure (n = 91) a |
Enhanced disclosure (n = 130) a |
|
|---|---|---|
| n (%) | n (%) | |
| Personal experience with current treatment (e.g. inability to be without current medications, desire to remain with current treatment regimens) | 6 (6.6) | 10 (7.7) |
| Direct benefit to self (e.g. higher odds of efficacy with psychiatric treatment, concerns over use of placebo in RCT, side-effects of investigational drug, and overall deterioration of condition) | 49 (53.8) | 74 (56.9) |
| Anti-drug company | 3 (3.3) | 2 (1.5) |
| Anti-medications for depression (e.g. lack of confidence in drugs for treating depression, preference for psychotherapy, preference for both drugs and therapy in combination) | 16 (17.6) | 10 (7.7) |
| Acknowledges importance and value of research | 4 (4.4) | 14 (10.8) |
| Acknowledges higher cost of individual psychiatric treatment | 4 (4.4) | 4 (3.1) |
| Other | 5 (5.5) | 10 (7.7) |
| No response | 4 (4.4) | 6 (4.6) |
a The number of coded comments, not participants. The denominator for percentages in this table is the number of coded comments from those who expressed preference for individualised treatment in each disclosure arm.
Among those who preferred the RCT (Table 3), 19.4% of comments in the standard arm mentioned altruism, whereas 35.6% of comments in the enhanced disclosure arm mentioned it. A total of 44.8% of comments from those in the standard disclosure arm who preferred RCT mentioned direct benefit, whereas only 22.2% of comments from those willing to participate in the RCT in the enhanced arm mentioned it. Among those who preferred individualised treatment over the RCT (Table 4), the comments were fairly similar between the two arms, except perhaps the standard arm had a higher proportion indicating scepticism about medication treatment for depression (17.6% v. 7.7%).
In exploratory logistic regression models for each arm, in which willingness to enrol (dichotomised) was the dependent variable, none of the patient characteristics was a significant predictor of willingness to participate in the RCT (age, gender, race, ethnicity, annual income, education and average BDI score).
Discussion
The use of placebos in RCTs when effective treatments exist is accepted as ethical under certain conditions. Such trials continue to be the standard for testing the safety and efficacy of antidepressants. Reference Endicott, Lam, Hsu, Fayyad, Boucher and Guico-Pabia11–Reference Sambunaris, Bose, Gommoll, Chen, Greenberg and Sheehan16 However, even if they are necessary to advance medical knowledge, how should researchers communicate to the potential participants the burden they are asked to bear? Specifically, what is the potential impact of providing a quantitative estimate of this trade-off to potential participants?
Our primary finding is that enhanced disclosure consisting of evidence-based, quantitative outcome data has a significant impact on willingness to participate in RCT. Participants in the enhanced disclosure arm were significantly less likely (0.58 times as likely) than those in the standard disclosure arm to prefer the RCT over individual treatment. Further, our qualitative analysis of the respondents’ rationales for their choices shows that this difference is likely because of the fact that participants in the enhanced arm made logical use of the information and their choices are more aligned with their preferences than those in the standard disclosure arm. This conclusion is supported by the following.
The overall proportions of comments reflecting altruism and direct benefits to self are similar in both arms; that is, there was no overall difference between the two arms in terms of proportion of altruistic v. direct benefit-related comments. However, when the respondents’ comments reflecting their reasons for choosing the RCT option are examined, the distribution of those reasons diverges between the two disclosure arms. Those in the standard arm expressed direct personal benefit reasons much more than altruism reasons (44.8% v. 19.4%); this pattern is reversed in the enhanced disclosure arm (22.2% direct benefit v. 35.6% altruism comments). Since the enhanced disclosure makes clear that enrolling in an RCT is disadvantageous for participants, we conclude that the enhanced disclosure arm shows greater congruence between choice of RCT and the reasons for that choice. This is further supported by the fact that in the enhanced arm, 22.2% of the comments among those choosing the RCT mentioned direct benefit, whereas 56.9% of comments among those choosing individualised psychiatric treatment mentioned direct benefit. In contrast, in the standard disclosure arm, comments among those choosing the RCT and those choosing individualised treatment mentioned direct benefit at a closer level of frequency (44.8% and 53.8%), suggesting that motivation for direct benefit does not distinguish those preferring the RCT from those preferring individual treatment among those in the standard arm. Thus, although enhanced disclosures may result in fewer persons volunteering in RCTs with placebo controls when effective treatments exist, such enhanced disclosures allow potential participants to make decisions more in line with their values regarding altruism and benefits to self.
Before discussing the potential implications of these results, we note several limitations. First, although the decision analysis model on which the trade-off information was based was conducted using standard methods and an extensive evidence base, all models are limited by their assumptions. We refer readers to the model for details. Reference Kim and Holloway8 Second, the hypothetical nature of the study may limit extrapolation to actual decision-making by potential participants. Third, the impact of the enhanced disclosure may be smaller in reality because by highlighting the trade-off information, we drew attention to it. On the other hand, research ethics boards might reasonably require researchers to highlight this trade-off because at the heart of risk–benefit analyses for these trials is the issue of forgoing treatment. In that case, our study may accurately reflect the potential impact of implementing an enhanced disclosure. Another limitation is that our content analysis, like all qualitative analyses, depends on judgments of interpretation; other teams of researchers might have developed different coding schemes. Also, we did not subject the categorisations of comments to formal statistical testing given the low numbers for some codes and their post-hoc nature. Furthermore, our participants were already connected to a clinic for their depression treatment; we cannot infer from the results the potential behaviours of persons who are not connected to a mental health professional with little access to care outside of the RCT at the time they are offered to participate in one.
Despite these limitations, our study has the strength of a randomised experimental design ensuring high internal validity, evidence-based and rigorously derived quantitative outcome estimates for the enhanced disclosure information, and respondents from a typical population from which participants are drawn for depression RCTs, as reflected in their having moderate depression symptoms with demographic profiles within the range of those in published antidepressant RCTs. Reference Endicott, Lam, Hsu, Fayyad, Boucher and Guico-Pabia11,Reference Kennedy, Avedisova, Gimenez-Montesinos, Belaidi and de Bodinat13,Reference Boulenger, Loft and Olsen15
Do our results support the practice of providing ‘quantitative comparative estimates’ 7 of benefits to research participants when they are invited to take part in a placebo control RCT when effective treatments exist? What are the potential objections or obstacles to such a policy? First, it may not always be possible to provide a quantitative estimate of comparative outcomes. However, when a review of the clinical literature would yield such data, it should not be difficult to arrive at a reasonable range of estimates that would be useful for the potential participants. If that is the case, our evidence supports disclosure of that information. However, even if a quantitative estimate is not available, at least the lack of clinical equipoise (between entering RCT and receiving individual treatment) should be disclosed to the subject. They should be told that they are more likely to experience relief in symptoms if they seek individualised treatment, rather than simply be given a neutral description that other alternatives and treatments are available.
Second, our results show that if enhanced disclosures were required, recruitment would be more difficult but still feasible. This would raise the cost and effort of conducting placebo control clinical trials when effective treatments exist. The ethical gain (increased transparency with better decision-making by participants) would thus have a cost but one cannot expect ethics to be always without costs. In addition to the ethical gains, the more realistic expectations regarding potential benefits among participants could have important scientific benefits. Specifically, attrition bias is a perennial concern because average dropout rates in pivotal trials of new antidepressants is 37%. Reference Leon, Mallinckrodt, Chuang-Stein, Archibald, Archer and Chartier17 It is plausible that better informed participants with more realistic expectations might be more compliant, with fewer dropouts, thus improving the quality of such studies.
In conclusion, the debate over the ethical permissibility of withholding effective treatments in placebo control trials must also address the important ethical issue of how to inform participants in such trials of the special burden they will bear. Although there are important challenges to disclosing quantified comparative outcomes information to potential participants, our results provide evidence that such information, when available, will likely make a significant and ethically important difference in potential participants’ decision-making.
Acknowledgements
The views expressed are the authors’ and do not represent the views or policies of the NIH, DHHS, or the US government.
Funding
Supported by the senior author's (S.K.) research fund, the University of Michigan.
Appendix
Survey text
Please read the following description and circle a number on the scale below that best fits which option you would choose, then briefly describe why you would make that choice. There are no right or wrong answers.
Imagine that you have depression. You feel sad or downhearted a lot of the time. You often feel edgy, uncomfortable, and tense or panicked, but can usually overcome these feelings with great effort.
You have difficulty dropping off to sleep, and often have light or restless sleep. You don't have much of an appetite, and food just doesn't taste as good as it once did. You often feel tired or run down. You're not very interested in sex.
You frequently have a hard time collecting your thoughts, which sometimes makes it hard to read or have a conversation. You have a hard time starting activities, and are not able to enjoy your usual interests as much as you used to.
You often feel like a failure and feel pessimistic about your future. You feel weary of life and occasionally feel that life is not worth living, although you don't have any real plans or intentions of ending your life.
Suppose you have the kind of depression symptoms described [above]
You see an advertisement in the clinic recruiting depressed patients into an 8-week long research study testing a promising new drug for the treatment of depression. If you join the study, you have a 1 in 2 chance of getting a placebo (a sugar pill). If you don't get a placebo pill, you will get the new drug.
The benefits of entering the research study are that you would get close clinical monitoring, and any drugs you receive would be at no cost to you. Also, if you got the new drug and it worked, then your depression might get better. Finally, you would be helping researchers and the drug company develop a new treatment for depression, which could benefit future patients with depression.
If you enter the research study, you would have a 50% (1 in 2) chance of getting the placebo and 50% chance of getting the new drug. The new drug can cause upset stomach in some people. Instead of entering the research study, you can choose to receive regular treatment from a psychiatrist.
[For those randomised to the Enhanced Disclosure arm, the following two bullet points were added.]
-
If you enter the research study , you have about a 40% (or 40 out of 100) chance of feeling better by the end of 8 weeks.
-
If you receive regular treatment with a psychiatrist, you have about a 65% (or 65 out of 100) chance of feeling better by the end of 8 weeks.
Would you participate in the research study or would you choose to receive regular treatment with a psychiatrist?

Please comment on why you chose that answer.
_________________________________________________________
_________________________________________________________
_________________________________________________________
_________________________________________________________








eLetters
No eLetters have been published for this article.