Huntington's disease is a progressive neurodegenerative disease for which there is no current effective treatment, except for symptomatic treatments to manage psychiatric and movement symptoms. In Huntington's disease, the risk of death by suicide has been estimated to be two to seven times greater than that observed in the general population.Reference Brogueira Rodrigues, Abreu, Damásio, Goncalves, Correia-Guedes and Coelho1–Reference Solberg, Filkuková, Frich and Feragen3 Suicide is the third most common cause of all deaths among patients with manifest Huntington's disease (after pneumonia and other infections), accounting for up to 6.6% of deaths,Reference Brogueira Rodrigues, Abreu, Damásio, Goncalves, Correia-Guedes and Coelho1 and estimates of lifetime prevalence of suicidal ideation among patients with Huntington's disease are as high as 20%.Reference Kachian, Cohen-Zimerman, Bega, Gordon and Grafman2,Reference Hubers, Reedeker, Giltay, Roos, van Duijn and van der Mast4,Reference van Duijn, Vrijmoeth, Giltay and Landwehrmeyer5 A previous study showed that death by suicide among people with neurological disorders occurs more often in patients with amyotrophic lateral sclerosis or Huntington's disease than in patients with multiple sclerosis, head injury, stroke or epilepsy.Reference Erlangsen, Stenager, Conwell, Andersen, Hawton and Benros6 This increased risk of suicidal behaviour frames the clinical management of Huntington's disease. Huntington's disease is a neurodegenerative disorder characterised by progressive motor dysfunction, neuropsychiatric symptoms and neurocognitive deterioration. Huntington's disease is an autosomal dominant hereditary disease caused by an expanded CAG trinucleotide repeat in the huntingtin gene on chromosome 4.Reference Walker7 In most Huntington's disease gene expansion carriers (HDGECs), clinical signs and symptoms become manifest in the fourth or fifth decade of their lives. with a survival time of around 15 years after clinical motor diagnosis onset.Reference Keum, Shin, Gillis, Mysore, Abu Elneel and Lucente8 Common neuropsychiatric symptoms are depressed mood, irritability, apathy and obsessive–compulsive behaviours that can present in all stages of the disease and may precede motor signs.Reference van Duijn, Craufurd and Hubers9 The increased occurrence of neuropsychiatric symptoms and neurocognitive deficits like depressed mood, impulsivity, impaired abstract thinking and impaired problem-solving capacity are risk factors for suicide.Reference Turecki and Brent10 Also, when facing the prospect of onset and steady progression of a disease for which there is currently no effective treatment, patients may feel entrapped and see suicide as an escape. The only two drugs approved for Huntington's disease (tetrabenazine and deutetrabenazine) are to treat chorea, and both are associated with an increased risk of depression and suicide,11 although there is disagreement as to whether tetrabenazine increases suicide risk.Reference Dorsey, Brocht, Nichols, Darwin, Anderson and Beck12–Reference Schultz, Killoran, Nopoulos, Chabal, Moser and Kamholz14 Although Huntington's disease is associated with an increased risk of suicide, the precise incidence in this population is not defined. Robust incidence estimates of suicide and suicide attempts in the HDGEC population are needed as a reliable reference for ongoing and future Huntington's disease therapeutic research and post-marketing surveillance, to help evaluate the suicidality risk of novel Huntington's disease therapeutics. Here, we aimed to estimate the incidence rate of suicide attempts and suicide in a global, prospective Huntington's disease cohort study, to provide robust references for Huntington's disease therapeutic development.
Method
Study population
Enroll-HD (https://www.enroll-hd.org/) is a prospective cohort study and global clinical research platform designed to facilitate clinical research in Huntington's disease.Reference Sathe, Ware, Levey, Neacy, Blumenstein and Muhlback15, Reference Landwehrmeyer, Fitzer-Attas, Giuliano, Gonçalves, Anderson and Cardoso16 Enroll-HD encompasses over 20 000 participants from approximately 160 active sites located in 19 countries across North America, Latin America, Europe and Australasia. Data are collected from participants annually, and are monitored for quality and accuracy using a risk-based monitoring approach. The data for this study were extracted from the Enroll-HD electronic data capture system database on 1 May 2019. Participants were classified as HDGECs or non-HDGECs based on CAG-repeat length, as determined in a central laboratory (Biorep Technologies, Inc). Individuals with a CAG-repeat length of ≥36 were classified as HDGECs (n = 15 924), and those with a CAG-repeat length of ≤35 were classified as non-HDGECs (n = 4988). The latter group comprised genotype-negative individuals and family members or individuals not related by blood to HDGECs. Individuals with unknown CAG-repeat length (n = 327) were excluded from analyses. This resulted in 20 912 participants who were available for analysis.
Observation period
Enroll-HD baseline visit date (i.e. study entry) was considered as the beginning of the observation period for each participant. For participants who died or discontinued the study, date of death as reported on the Mortality form (or Reportable Event form in absence of the Mortality form) or date of Premature End form completion was used to define the end of the observation period. Participants who had not died nor formally discontinued, but had missed two consecutive annual visits (operationally defined as the elapsing of >2 years and 3 months since the due date of the previous in-person visit) were considered ‘lost to follow-up’; the date 2.25 years after the due date of their last Enroll-HD visit was used to define the end of the time-at-risk period. For all other (i.e. active) participants within the 2.25-year window after their last visit at the time of data cut, the date of the data cut (1 May 2019) was used to define the end of the time-at-risk period.
Pursuant to the above definition, the total observation period for HDGECs comprised 40 242 person-years, and for non-HDGECs comprised 13 148 person-years. Incidence rates were calculated per 100 000 person-years, according to the following formula: (observed events (within indicated time period) / person-years (within indicated time period)) × 100 000.
Variables
Data on ‘reportable events’ are recorded per Enroll-HD protocol, and comprise suicide attempt, completed suicide, mental health event requiring hospital admission, and death (other than suicide). Such events are recorded on a Reportable Events form, which can be completed at any time during or outside of a standard Enroll-HD visit. This form was the primary source for identifying suicide attempts and completed suicides. All Reportable Events forms are sent to the Data and Safety Monitoring Committee to assess site compliance to screening procedures and assessment protocols. Mortality forms, which are completed in the event of death, were used as a secondary source for identifying missed events. A quality-control process was used to screen and categorise identified events (Figs 1 and 2). Events dated before the Enroll-HD baseline visit date were excluded. Remaining events were then subject to clinical evaluation, as described below.

Fig. 1 Event flowchart: suicide attempts.
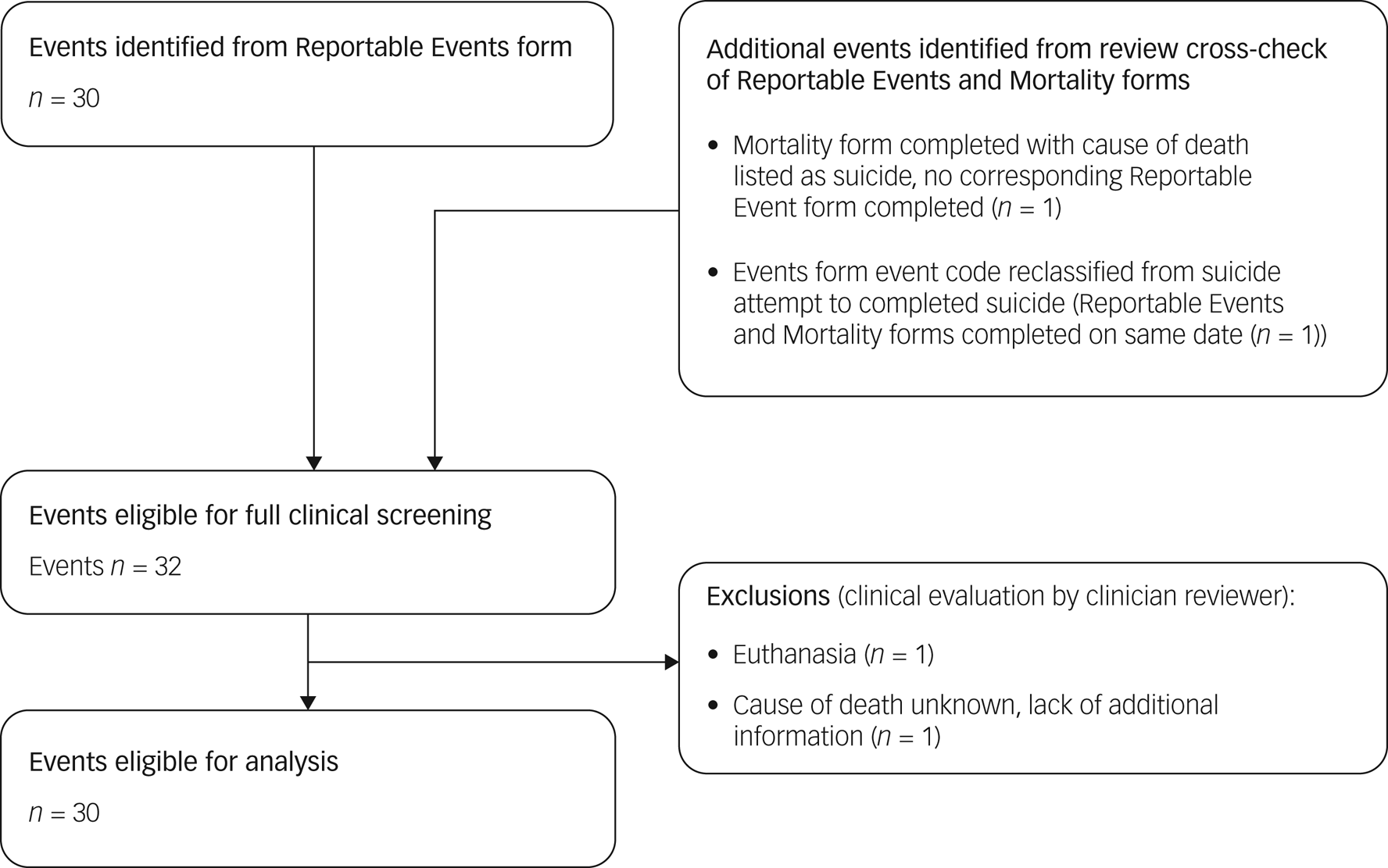
Fig. 2 Event flowchart: completed suicides.
Suicide attempts
All events were subject to clinical evaluation by two independent reviewers (E.v.D. and J.J.W.). A decision tree, based on the Columbia-Suicide Severity Rating Scale (C-SSRS) definition of an ‘actual attempt’,Reference Posner, Brown, Stanley, Brent, Yershova and Oquendo17 was created (Fig. 3) to evaluate event narratives and include or exclude cases accordingly and consistently. Excluded events were categorised as follows: self-injurious behaviour (n = 4), interrupted attempt (n = 5), aborted attempt (n = 1) and suicidal ideation (n = 6). The National Toxicology Information Center (The Netherlands) was consulted to evaluate potential severity of drug overdoses. As in some cases the intent of the suicidal behaviour was not clear or clearly inferable, or inadequate information was provided to allow categorisation of an event, additional information was requested from Enroll-HD sites. Where additional information could not be provided, the event was included under the ‘liberal’ estimate, to avoid a possible underestimation of the incidence of suicide attempts. A ‘conservative’ estimate was generated based on conclusively reported cases only.

Fig. 3 Suicide attempt evaluation and classification decision tree.
Completed suicides
All reports of completed suicides were subject to clinical evaluation by one rater (E.v.D.). Narratives provided on the Reportable Events form were evaluated, alongside cause of death and narratives provided on associated Mortality forms. Additional information was requested for one case to assist in decision-making. Clinical evaluation resulted in the exclusion of two cases for reasons of euthanasia (n = 1) and cause of death listed as unknown with lack of information (n = 1).
Sociodemographic and clinical characteristics
Sociodemographic and clinical variables were used to characterise participants (Table 1). Clinical variables included psychiatric measures (e.g. prior suicidal ideation per C-SSRS; depression, anxiety, apathy and irritability subscale scores per the short version of the Problem Behavior Assessment) and clinical assessments encompassing other Huntington's disease-specific disease domains: motor (e.g. Unified Huntington's Disease Rating Scale (UHDRS) total motor score (TMS)), function (e.g. UHDRS total functional capacity (TFC)) and cognition (e.g. Symbol Digit Modality Test (SDMT)). To characterise the full sample, variable values ascertained at the baseline visit were used. To characterise participants who experienced an event, variable values were ascertained at the Enroll-HD visit before the event. Only age and CAG-age product (CAP) score were determined based on age on event date. CAP score, calculated per the following formula, is indicative of cumulative exposure to mutant huntingtin (akin to ‘pack-years’ for assessing tobacco exposure in smokers):Reference Zhang, Long and Mills18
where L = 30 and K = 6.49.
Table 1 Participant characterisation

HDGECs, Huntington's disease gene expansion carriers; IQR, interquartile range; CAP, CAG-age product; TFC, total functional capacity; TMS, total motor score; SDMT, Symbol Digit Modality Test; C-SSRS, Columbia-Suicide Severity Rating Scale; PBA-s, Problem Behaviors Assessment - Short.
As 18 participants had more than one suicide attempt during the observation period, the number of unique events and the number of unique participants differs. For characterisation of suicide attempts, descriptive statistics were generated based on the number of unique events.
Deaths (all cause)
To determine proportionate mortality attributable to suicide in Enroll-HD, data on deaths (all causes) were required. The Mortality form (completed in the event of participant death) and the Reportable Events form were the reference sources for this variable.
The research participants who contribute their data and samples to Enroll-HD give voluntary written informed consent in accordance with General Data Protection Regulation (European Union). The informed consent was purposefully developed to ensure research use and protect the confidentiality and privacy of the contributors. Institutional review board/independent ethics committee approval, consistent with local regulations, has been obtained by each Enroll-HD site. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Statistical methods
Analyses were limited to generation of descriptive statistics. Incidence/event rate statistics were generated for suicide attempts and completed suicides, using all available data. Incidence statistics are also presented by year of study and region. Proportionate mortality statistics and respective 95% confidence interval for suicide were also generated. The 95% confidence interval estimation for the binomial proportions was based on the bootstrap percentile-t method as described by Mantalos and Zografos.Reference Mantalos and Zografos19 Strengthening the Reporting of Observational studies in Epidemiology guidelines were followed in reporting. All descriptive statistics are presented by gene status.
Missing or incomplete event dates were imputed according to the rules outlined in Supplementary Appendix 1 available at https://doi.org/10.1192/bjo.2021.969. Additional information regarding event narratives was requested from study sites where insufficient information was provided to include or exclude or categorise events. Missing data for other variables were not imputed. Missing data points by variable are indicated in Table 1. Statistical analyses were performed with R version 3.4.2 for Windows (R Core Team; Vienna, Austria; https://www.R-project.org/).
Results
Frequency statistics
Of the 20 912 Enroll-HD participants considered, representing a total of 53 390 participant-years, a total of 30 completed suicides were observed; 29 were observed in HDGECs and one was observed in a non-HDGEC (Table 1 and Fig. 2). For suicide attempts, a total of 126 events with clear or clearly inferable intent were observed in 111 unique participants (conservative estimate); 123 of these events were observed in HDGECs and three in non-HDGECs (Figs 1 and 4). The total number of suicide attempts increased to 156 events in 133 unique participants when inclusion of events was expanded to incorporate events in which intent was unclear (liberal estimate); 151 of these events were observed in HDGECs and five in non-HDGECs (Table 1 and Figs 1 and 5).

Fig. 4 Incidence rates of suicide attempts in Enroll-HD (conservative estimate). left panel: HDGECs; right panel: non-HDGECs; incidence rates are displayed by year, and overall (red bar). *Period of observation began 25 July 2012 and ended 1 May 2019. HDGECs, Huntington's disease gene expansion carriers.
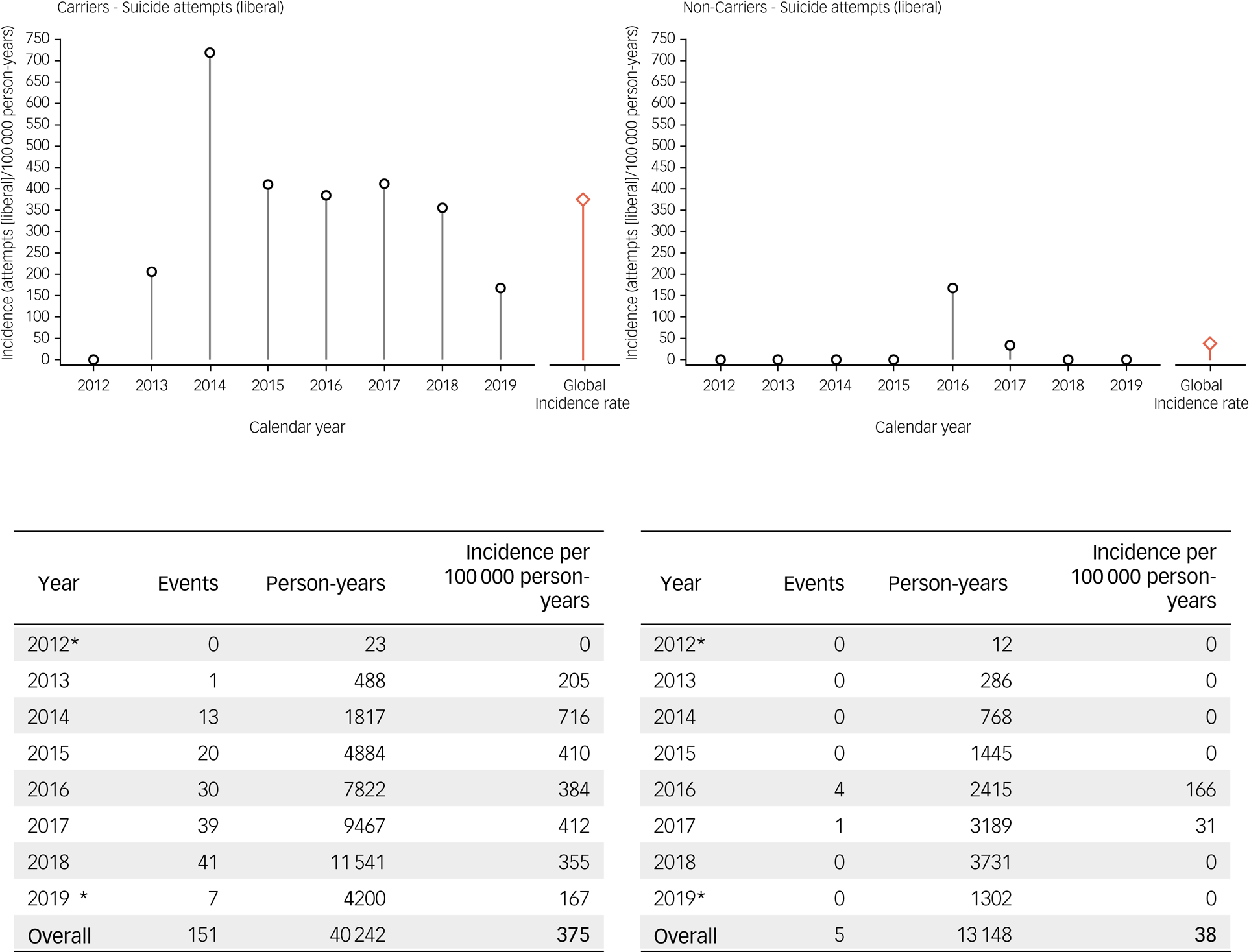
Fig. 5 Incidence rates of suicide attempts in Enroll-HD (liberal estimate). left : HDGECs; right: non-HDGECs; incidence rates are displayed by year, and overall (red bar). *Period of observation began 25 July 2012 and ended 1 May 2019. HDGECs, Huntington's disease gene expansion carriers.
Multiple suicide attempts were observed in 18 participants. Fourteen participants reported two attempts, three reported three attempts and one reported four attempts. Of the 30 participants who died by suicide, two had reported a prior suicide attempt. The median length of time between event date and date of Reportable Event form completion for completed suicides and suicide attempts was 28 days (interquartile range 3–155). A total of 659 deaths (all causes, including suicide) were observed in our analysis sample over the time period examined; 637 in HDGECs and 22 in non-HDGECs.
Suicide attempts
Incidence rates of suicide attempts in Enroll-HD, using the conservative and liberal estimate, respectively, are presented by gene status and year (2012–2019) in Figs 4 and 5, and by gene status and region (North America, Europe, Latin America, Australasia) in Tables 2 and 3. For suicide attempts where intent was clear or clearly inferable (i.e. conservative estimate), the global overall incidence rate observed in HDGECs was 306 per 100 000 person-years (Fig. 4). The analogous figure in non-HDGECs was 23 per 100 000 person-years (Fig. 4). When all suicide attempts were considered, including events where intent was not clear or clearly inferable (i.e. liberal estimate), the global overall incidence rate in HDGECs was 375 per 100 000 person-years, and 38 per 100 000 person-years in non-HDGECs (Fig. 5).
Table 2 Comparative incidence rates for suicide attempts (liberal) in Enroll-HD, by geographical region

; HDGECs, Huntington's disease gene expansion carriers.
Table 3 Comparative incidence rates for suicide attempts (conservative) in Enroll-HD, by geographical region

HDGECs, Huntington's disease gene expansion carriers.
Completed suicides
Suicide rates in Enroll-HD are presented by gene status and year in Fig. 6, and by gene status and region in Table 4. Considering all available data, the overall incidence rate of suicide was 72 per 100 000 person-years in HDGECs, and 8 per 100 000 person-years in non-HDGECs. . The proportionate mortality attributable to suicide in Enroll-HD is 4.6% (29 out of 637) in HDGECs (95% CI 2.84–5.87%), and 4.5% (1 out of 22) in non-HDGECs (95% CI 0–13.51%).

Fig. 6 Incidence rates of completed suicide in Enroll-HD. left: HDGECs; right: non-HDGECs; incidence rates are displayed by year, and overall (red bar). *Period of observation began 25 July 2012 and ended 1 May 2019. HDGECs, Huntington's disease gene expansion carriers.
Table 4 Comparative incidence rates for completed suicides in Enroll-HD, by geographical region

HDGECs, Huntington's disease gene expansion carriers.
Sociodemographic and clinical characteristics
Sociodemographic and clinical characteristics of participants who attempted or completed suicide are provided in Table 1.
Suicide attempts
Of the 151 suicide attempts observed in HDGECs (liberal estimate), 58% (n = 87) were observed in females, and median age at event was 48 years. With regards to disease stage and severity, 81% (n = 122) of events were observed in manifest individuals, with a median CAP score at event of 105. Median TFC score at event was 8, median TMS at event was 30 and median SDMT score was 21. Prior suicidal ideation, as assessed by the C-SSRS, had been reported by 60% (n = 59).
Completed suicides
Of those HDGECs who died by suicide, 35% (n = 10) were female, and median age of event was 55 years. With regards to disease stage and severity, 93% (n = 27) were manifest, with a median CAP score of 112. Median TFC score was 9, median TMS was 38 and median SDMT score was 20. Prior suicidal ideation, as assessed by the C-SSRS, had been reported by only 29% (n = 6).
Discussion
For the first time, robust estimates of the incidence of suicide attempts and completed suicides are provided in a large and globally representative Huntington's disease cohort, encompassing a broad disease-stage spectrum. Our results confirm the elevated occurrence of completed suicides and suicide attempts in HDGECs that were previously reported in smaller and mostly retrospective studies. Besides their relevance in clinical practice, the incidence estimates calculated here will be a useful reference to evaluate therapeutic safety, since suicidality is an event of interest in the development of any drug targeting the central nervous system, as per Food and Drug Administration guidance.20 Completed suicides and even suicide attempts in the context of clinical trials are relatively scarce and therefore difficult to evaluate, so knowing the estimated suicide/suicide attempt incidence in a representative cohort is important.
We provide both conservative and liberal estimates of suicide attempt incidence rate in Enroll-HD because the intent of the suicidal act was not always clear from event narratives. When only the conservative approach was used, this may have underestimated the incidence of suicide attempts. Including events in which the intent is unclear or questionable in our calculations is in line with clinical practice. The estimates from both approaches resulted in a range for the annual incidence of 306–375 per 100 000 person-years in HDGECs, and 23–38 per 100 000 person-years in non-HDGECs, where the lower values represent estimates from conservative approaches. Regional differences were also noted, although the limited number of events in certain regions restricts meaningful comparison. Comparable incidence estimates of suicide attempts in the general population are not known, but the World Health Organization has estimated that the 12-month prevalence of suicide attempts is approximately 0.3%.Reference Borges, Nock and Haro Abad21
Although many prior studies have reported on suicidality in Huntington's disease with regards to suicidal ideation and risk factors for suicidal ideation,Reference Hubers, Reedeker, Giltay, Roos, van Duijn and van der Mast4,Reference Anderson, Eberly, Groves, Kayson, Marder and Young22,Reference Honrath, Dogan, Wudarczyk, Görlich, Votinov and Werner23 there is limited data on suicide attempts and completed suicides in Huntington's disease. In the neurobiological predictors of Huntington's disease study (PREDICT-HD),Reference Fiedorowicz, Mills, Ruggle, Langbehn and Paulsen24 an observational study of premanifest HDGECs, 13 suicide attempts over 2718 person-years were reported, which is equal to a suicide attempt incidence of 478 per 100 000 person-years (Table 5), which is higher than our HDGEC estimate. In a randomized, double-blind, placebo-controlled trial of coenzyme Q10 in Huntington's disease (2CARE),Reference McGarry, McDermott, Kieburtz, Fung, McCusker and Peng25 a clinical trial conducted in patients with early-manifest Huntington's disease, 22 suicide attempts were reported over <3045 person-years, which is equivalent to at least 722 per 100 000 person-years. Data from a phase 2, dose-finding, randomized, parallel-group, double-blind, placebo-controlled study, evaluating the safety and efficacy of Pridopidine 45 mg, 67.5 mg, 90 mg, and 112.5 mg twice-daily versus placebo for symptomatic treatment in patients with Huntington's disease (PRIDE-HD)Reference Reilmann, McGarry and Grachev26, a clinical trial that enrolled manifest patients, reported four suicide attempts over <204 person-years regardless of treatment group considered, which is equivalent to at least 1961 per 100 000 person-years, although the limited sample size and limited prospective follow-up period calls into question the accuracy of this estimate.
Table 5 Comparative incidence rates for suicide attempts in HDGECs

HDGECs, Huntington's disease gene expansion carriers.
The incidence rate of completed suicides among HDGECs in Enroll-HD is 72 per 100 000 person-years, which is about four to six times the incidence rate of 11.9 and 21.1 suicides per 100 000 person-years for the general population in the UK and USA, respectively.27 This is notably lower than the incidence rate of suicide of 203.6 per 100 000 person-years observed in a Danish cohort of symptomatic patients with Huntington's disease, although this estimate was based on a much smaller cohort of patients (n = 1228),Reference Erlangsen, Stenager, Conwell, Andersen, Hawton and Benros6 and the suicide rate in the general population in Denmark has historically been high.Reference Nordentoft and Erlangsen28 We found a slightly higher incidence of completed suicide among HDGECs in Enroll-HD in Europe (70 per 100 000 person-years) compared with North America (58 per 100 000 person-years); however, no conclusions can be drawn because of the small numbers after regional stratification (Table 4). The incidence rate of completed suicide in non-HDGECs in Enroll-HD is 8 per 100 000 person-years, which is close to the global age-standardised completed suicide rate of 10.5 per 100 000.27
In the PREDICT-HD study,Reference Fiedorowicz, Mills, Ruggle, Langbehn and Paulsen24 a completed suicide rate of 37 per 100 000 person-years was reported (one event over 2718 person-years), and in the 2CARE study, 164 per 100 000 person-years (five events over <3045 person-years) was reported,Reference McGarry, McDermott, Kieburtz, Fung, McCusker and Peng25 whereas no completed suicides were reported in the PRIDE-HD study (Table 6).Reference Reilmann, McGarry and Grachev26 In the PREDICT-HD study, participants were strictly defined as premanifest individuals at the time of inclusion, and participants are therefore in a less advanced disease stage compared with the Enroll-HD population, whereas in Enroll-HD, the majority of HDGECs were manifest at baseline (68%; see Table 1). Clinical trial populations (2CARE, PRIDE-HD) are distinct from natural cohorts because participants with severe mental health problems or with an a priori increased suicide risk are often excluded, which likely decreases the risk of suicidality events in trial populations. On the other hand, the strict monitoring of participants in trials and the higher frequency of visits may inflate the reporting.
Table 6 Comparative incidence rates for completed suicides in HDGECs

HDGECs, Huntington's disease gene expansion carriers.
We found that 4.6% (95% CI 2.84–5.87%) of all reported HDGEC deaths in Enroll-HD were from suicide, which is consistent with an analysis of death certificates of patients with manifest Huntington's disease in Denmark (5.6%)Reference Sørensen and Fenger29 and the 6.6% of patients with manifest Huntington's disease that was previously assessed in REGISTRY, the European observational study that has been incorporated into Enroll-HD.Reference Brogueira Rodrigues, Abreu, Damásio, Goncalves, Correia-Guedes and Coelho1 However, the Norwegian Cause of Death Registry reported a lower proportionate mortality attributable to suicide of 2.3% in patients with Huntington's disease;Reference Solberg, Filkuková, Frich and Feragen3 this is still double the approximately 1.2% suicide mortality in the general population.30
Little is known about risk factors for suicide attempts and completed suicide in Huntington's disease. Earlier studies showed that HDGECs with suicidal ideation are more often depressed, anxious or aggressive/irritable, and use more psychotropic medication.Reference van Duijn, Vrijmoeth, Giltay and Landwehrmeyer5,Reference Fiedorowicz, Mills, Ruggle, Langbehn and Paulsen24,Reference Hubers, van Duijn, Roos, Craufurd, Rickards and Landwehrmeyer31 It is known from the general population that suicidal behaviour is associated with feelings of entrapment that result in an attempt to escape from an unbearable situation;Reference O'Connor and Kirtley32 having an incurable, progressive neurodegenerative disease may cause such a feeling of entrapment. The prospect of a loss of independence or having mental or neurocognitive problems may accelerate the transition from suicidal ideation to suicidal acts.
The key strength of our study is the substantial observation period (53 390 person-years) on which the analyses here are based, affording confidence in the robustness of the estimates generated. Other strengths are the broad representation of the Huntington's disease population in our sample, including extensive age and disease-stage coverage; the high-quality monitored data and the rigorous review and screening process that was applied to evaluate and categorise identified events; and the suicidal attempts estimates that encompass a conservative or more liberal range.
Our study also has some limitations. First, although Enroll-HD aims to recruit a large and population-representative sample, it is likely that there is a degree of selection bias; for instance, patients with Huntington's disease with severe impairments or severe psychiatric disorders may be less likely to participate. It is also possible that HDGECs who do not participate in Enroll-HD have other characteristics that are associated with risk of suicide attempt or suicide, such as people who are ashamed of their gene status or have difficulties in coping with the disease. Further, those participating in Enroll-HD may have unusually positive expectations of research and the development of effective therapeutics relative to those outside of the study. Second, per study protocol, individuals under 18 years of age are only able to participate if they have clinically diagnosed features of Huntington's disease and a confirmatory genetic test, restricting our sample of HDGECs in this age bracket. There is also limited ethnic diversity in Enroll-HD; Huntington's disease is principally a disease of Caucasian ancestry, and typically less prevalent in other ancestral groups, although there are exceptions. Another limitation is that most clinical characteristics were determined at the Enroll-HD visit before any events, as opposed to at the time of these events. Furthermore, lifetime prevalence of suicidal ideation may be underestimated as only one item of the C-SSRS was used, and reports of suicidal attempts may have been affected by recall bias or denial. It is also known that participant disclosure of information about suicidal ideation or behaviour is influenced by the associated stigma and therefore underreported.Reference Klonsky, May and Saffer33 Given our reliance on reporting of events by participant or companion/caregiver, it is possible our incidence calculations are underestimated. Finally, it is likely that our incidence values specifically for 2019 may be underestimated, given the observed delay in event reporting.
The incidence of completed suicide and suicide attempts are greatly elevated in the Huntington's disease population, and this will be a critical feature of the disease until we have effective treatments. Our incidence estimates, based on a very large, prospectively assessed cohort, may serve as a reference for the Huntington's disease therapeutics community to assist in drug safety profile evaluation and, as therapeutic interventions become available, these metrics may also be valuable in assessing progress in clinical care. Future research may wish to leverage the comprehensive Enroll-HD dataset to investigate factors associated with suicide and suicidal attempt.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjo.2021.969
Data availability
Data available on request due to privacy/ethical restrictions. The data that support the findings of this study are available on request (specified dataset request; SPS) pending review by the Enroll-HD Scientific Review Committee; https://enroll-hd.org/forresearchers/access-data/.
Author contributions
E.v.D. conceptualised and designed the study, and reviewed and revised the manuscript. A.R.F. and D.A. prepared the data-set, analysed the data, and reviewed and revised the manuscript. J.J.W. conceptualised and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. E.N. and C.S. conceptualised the study and reviewed and revised the manuscript.
Funding
This work was funded by the CHDI Foundation, a non-profit biomedical research organisation exclusively dedicated to collaboratively developing therapeutics that substantially improve the lives of individuals with Huntington's disease. Analyses were conducted at the Associação para a Investigação e Desenvolvimento da Faculdade de Medicina [Association for Research and Development of the Faculty of Medicine] under a CHDI Foundation-sponsored research agreement (A-15627). Data used in this work were generously provided by the participants in the Enroll-HD study and made available by CHDI Foundation, Inc. Enroll-HD is a clinical research platform and longitudinal observational study for Huntington’s disease families intended to accelerate progress towards therapeutics; it is sponsored by CHDI Foundation. Enroll-HD would not be possible without the vital contribution of the research participants and their families. The individuals who contributed to the collection of the Enroll-HD data are also gratefully acknowledged; see https://www.enroll-hd.org/acknowledgments/.
Declaration of interest
E.v.D. received consultancy honorary fees from Lundbeck. A.R.F., D.A., J.J.W. and E.N. have nothing to disclose. C.S. received consultancy honorary fees from Pfizer, vTv Therapeutics, Kyowa, Neuraly, Pinteon Therapeutics and Green Valley Pharmaceuticals.















eLetters
No eLetters have been published for this article.