Background
Major depressive disorder (MDD) and bipolar disorders are major sources of disability worldwide. It is estimated that approximately 350 million people experience MDD or bipolar disorders globally.1 According to the World Health Organization, MDD is the leading cause of disability worldwide, with the annual attributable financial loss of $83 billion.2,Reference Greenberg, Kessler, Birnbaum, Leong, Lowe and Berglund3
A diagnosis of MDD or bipolar disorder is made by conducting a psychiatric interview and assessing the presence and impact of psychopathological symptoms, while ruling out any medical conditions that may be directly causing these symptoms. Although some biomarkers associated with a mood disorder diagnosis have been identified, to date, none of these biomarkers have been adopted in clinical practice.Reference Strawbridge, Young and Cleare4 In the absence of objective biomarkers to identify various disease states in psychiatry, treatment decisions are based largely on the patient's report of symptoms and a mental status examination.Reference Insel, Cuthbert, Garvey, Heinssen, Pine and Quinn5
Current pharmacological treatments for mood disorders
Pharmacological treatments for mood disorders were discovered serendipitously and they have remained fundamentally unchanged over the past several decades. Although antidepressant medications have evolved over the years, the therapeutic effect of all available antidepressants (with the possible exception of ketamine) is attributed to their action on at least one of the monoamine neurotransmitters.Reference Rosenblat, McIntyre, Alves, Fountoulakis and Carvalho6 Pharmacotherapy for acute bipolar depression comprises medications used primarily for the treatment of mania (i.e. lithium, atypical antipsychotics), MDD (i.e. antidepressants) or the maintenance phase of bipolar disorders (for example lamotrigine). Although these medications are effective for a significant proportion of patients, large studies suggest that up to 50% of patients do not achieve remission with standard treatments.Reference Rizvi, Grima, Tan, Rotzinger, Lin and McIntyre7–Reference Thomas, Kessler, Campbell, Morrison, Peters and Williams9 A possible explanation for this variability in treatment outcomes is that patients with mood disorders are a heterogeneous group. To achieve higher rates of remission, new studies could use the framework of ‘precision medicine’ and delineate subgroups of patients who are more likely to respond to specific treatments.
Immunopsychiatry
In recent years, several pre-clinical and clinical studies have investigated the association between the inflammatory response system and neuropsychiatric disorders, including MDD and bipolar disorders.Reference Husain, Strawbridge, Stokes and Young10–Reference Roman and Irwin17 The subspecialty of ‘immunopsychiatry’, which aims to study this association, is now an established area of research. In this selective review we will summarise the evidence of the putative pathophysiological association between the inflammatory response system and mood disorders. We will also provide an updated summary of findings from treatment trials of immunomodulating agents in patients with MDD and bipolar disorders.
Method
The literature was reviewed by searching Medline for clinical trials of immunomodulating agents as monotherapy or adjunctive treatments in MDD and bipolar disorders from inception until December 2019, as well as by reviewing relevant reviews and references in the field. Selected studies were randomised controlled trials (RCTs), cluster RCTs or cross-over trials of immunomodulating agents that had an active comparator or a placebo-arm. Participants had to meet criteria for MDD or bipolar disorders according to ICD-10, DSM-IV or DSM-5.18–20 We only reviewed studies in English.
Results
Are mood disorders associated with a proinflammatory state?
The immune system and its possible association with mental disorders has been studied since the late 1800s. In 1887, Julius Wagner-Jauregg induced malaria in patients to alleviate symptoms of ‘dementia paralytica’, later known to be the neuropsychiatric manifestations of syphilis.Reference Raju21 Almost a century later, Benjamin Hart coined the term ‘sickness behaviour’ to characterise the psychological and behavioural symptoms of an acute physical illness.Reference Hart22 In the early 1990s, the ‘macrophage theory’ of depression postulated that activated macrophages secrete proinflammatory cytokines that either precipitate or exacerbate a moodstate.Reference Smith23 Since then, numerous studies have linked the inflammatory response system to both MDD and bipolar disorders. Interest in this line of research has been fostered by the observation that nearly all chronic and autoimmune disorders are associated with a high comorbidity of depressive symptoms. For example, more than half of patients with rheumatoid arthritis or systemic lupus erythematosus experience depressive symptoms.Reference Capuron and Miller24 Similarly, patients with Crohn disease and comorbid depression experience exacerbations of co-occurring physical and depressive symptoms.Reference Mardini, Kip and Wilson25 In a recent meta-analysis of anti-cytokine agents in autoimmune disorders, these medications were reported to lead to a significant improvement of depressive symptoms.Reference Kappelmann, Lewis, Dantzer, Jones and Khandaker26 Although the effect size for these medications is small to moderate, it is comparable with the effect size observed with traditional antidepressant medications.Reference Cipriani, Furukawa, Salanti, Chaimani, Atkinson and Ogawa27,Reference Strawbridge, Carter, Marwood, Bandelow, Tsapekos and Nikolova28
During the past decade, many cross-sectional observational studies have investigated peripheral inflammatory biomarkers in MDD. A meta-analysis of 82 such studies comprising over 3000 patients reported that levels of interleukin (IL)-6, IL-10, Il-12, IL-13, IL-18, the soluble IL-2 receptor (sIL-2R), tumour necrosis factor – alpha (TNF-α), the soluble TNF receptor 2 (sTNFR2), and chemokine ligand 2 (CCL-2) are significantly elevated in individuals with MDD compared with healthy controls.Reference Köhler, Freitas, Maes, de Andrade, Liu and Fernandes29 In another recent meta-analysis of 37 studies comprising 13 541 patients with depression and 155 728 controls, half of those with depression showed low-grade inflammation as evidenced by a C-reactive protein (CRP) level >1 mg/L, and a quarter had a CRP level ≥3 mg/L.Reference Osimo, Baxter, Lewis, Jones and Khandaker30
Other meta-analyses have shown that traditional antidepressants (for example selective serotonin reuptake inhibitors (SSRIs) and serotonin–noradrenaline reuptake inhibitors) are associated with a reduction in IL-6, TNF-α and CCL-2Reference Köhler, Freitas, Stubbs, Maes, Solmi and Veronese31 and that persistently elevated TNF-α is associated with treatment resistance.Reference Liu, Wei, Strawbridge, Bao, Chang and Shi32,Reference Grosse, Carvalho, Birkenhager, Hoogendijk, Kushner and Drexhage33 Retrospective cohort studies indicate that inflammation may play a role in the onset of mood symptoms and that elevated peripheral inflammatory markers in early life are predictive of adult depressive symptomology.Reference Chu, Stochl, Lewis, Zammit, Jones and Khandaker34,Reference Khandaker, Stochl, Zammit, Goodyer, Lewis and Jones35
Within the literature, there is significant heterogeneity between studies and in particular, there are substantial differences in how comorbidities are controlled for. This is particularly important as protein-based inflammatory markers such as CRP fluctuate and are influenced by multiple factors including body mass index (BMI), medications, exercise, diet and substance use; all of which are difficult to account for and poorly reported in studies.Reference Horn, Long, Nelson, Allen, Fisher and Byrne36 Furthermore, there is evidence that IL-6 and CRP operate through multiple physiological pathways, which are not solely upregulated during the inflammatory response.Reference Giudice and Gangestad37 In addition, CRP has a second isoform that is produced in the absence of inflammation and is postulated to have a net anti-inflammatory effect.Reference Giudice and Gangestad37 Notwithstanding these limitations, current evidence supports an association between abnormal inflammatory processes and depressed mood, in at least a subset of individuals.
Much of the evidence in patients with bipolar disorders has been based on cross-sectional studies indicating that an activated inflammatory response may be associated with a mood state.Reference Sayana, Colpo, Simões, Giridharan, Teixeira and Quevedo38 As with MDD, several immune-related disorders, such as autoimmune disorders, obesity, type 2 diabetes and cardiovascular disease, have a higher rate of incidence in patients with bipolar disorders than in the general population.Reference Rosenblat and Mcintyre39 Patients with bipolar disorders are also at higher risk for inflammation-associated metabolic syndromes such as myocardial infarction, stroke, atherosclerosis and hypertension.Reference Rosenblat and McIntyre11,Reference Prieto, Schenck, Kruse, Klaas, Chamberlain and Bobo W40–Reference SayuriYamagata, Brietzke, Rosenblat, Kakar and McIntyre43
Examination of post-mortem brain tissue can provide valuable insights into a possible association between mood disorders and neuroinflammation. In a meta-analysis of post-mortem studies of brains of patients with MDD measuring cytokines, chemokines and cell-specific markers of microglia and astrocytes, two studies found increased markers of microglia in MDD and four studies found no differences between MDD and healthy controls.Reference Enache, Pariante and Mondelli44 Another similar meta-analysis had inconsistent results.Reference Giridharan, Sayana, Pinjari, Ahmad, da Rosa and Quevedo45 Several of the 51 studies included in the meta-analysis showed evidence of inflammation in post-mortem brain samples of patients with bipolar disorders. However, these 51 studies evaluated different biomarkers of neuroinflammation: presence of infiltrating peripheral immune cells in the central nervous system, cytokines levels or microglia activation and very few evaluated these different biomarkers in the same post-mortem brain sample. Eight of 15 studies in the meta-analysis found no effect of bipolar disorders on microglia cell markers; 9 of 17 studied did not find any effect of bipolar disorders on astrocyte cells, whereas eight found a decrease, and two reported both an increase and a decrease in different brain regions.Reference Giridharan, Sayana, Pinjari, Ahmad, da Rosa and Quevedo45 Given the heterogeneity of both the methods and results of these post-mortem studies, one cannot reach a conclusion from them whether there is a causal link between neuroinflammation and mood disorders. Future studies are needed to address this heterogeneity in the current post-mortem literature.
Given the limitations of post-mortem studies in mood disorders, the measurement of in-vivo indices related to neuroinflammation using brain imaging can provide additional evidence for a possible association between abnormal inflammatory processes and mood symptoms. In recent years, positron emission tomography (PET) has been used to evaluate glial cells in MDD. Translocator protein-18 kDa (TSPO) volume distribution (VT) is used as a measure of microglial activation because it is elevated in microglia that have morphological features of being activated. A PET study quantifying TSPO VT with an early TSPO radiotracer – [11C]PK11195 – showed increased microglial activation in patients with bipolar disorders (n = 14) compared with healthy controls (n = 11).Reference Haarman, Riemersma-Van der Lek, de Groot, Ruhé, Klein and Zandstra46
To our knowledge, no other published PET studies have investigated neuroinflammation in bipolar disorders. In a meta-analysis of PET studies in MDD, TSPO VT was elevated in patients with MDD compared with controls in the anterior cingulate cortex (standardised mean difference (SMD) = 0.78, 95% CI 0.41–1.16) and temporal cortex (SMD = 0.52, 95% CI 0.19–0.85). However, recent evidence shows that TSPO displays incomplete specificity for microglia, and hence may be an unreliable radiotracer of neuroinflammation.Reference Narayanaswami, Dahl, Bernard-Gauthier, Josephson, Cumming and Vasdev47 Future PET studies of central inflammation in mood disorders should use more sensitive radiotracers.
Putative pathophysiological models linking inflammation to MDD and bipolar disorder
Mechanistic studies are yet to confirm how inflammation may induce a mood episode in a subset of individuals. However, current theories on the ‘inflammatory model’ of depression postulate that in a subset of individuals with depression, psychosocial stress leads to the activation of the sympathetic nervous system and subsequent release of catecholamines (for example, norepinephrine), which stimulates bone marrow production and the release of myeloid cells (for example, monocytes) into the periphery. Monocytes are the main producers of inflammatory cytokines; once they enter the periphery, it is hypothesised that they can encounter stress-induced damage-associated molecular patterns (DAMPs) and microbial-associated molecular patterns (MAMPs) leaked from the gut, for example bacteria and bacterial products.Reference Miller and Raison48 These DAMPs and MAMPs then lead to the activation of inflammatory signalling pathways and the release of proinflammatory cytokines including TNF and IL-6, which can cross the blood–brain barrier through cellular, humoral and neural routesReference Miller and Raison48 (see Fig. 1).
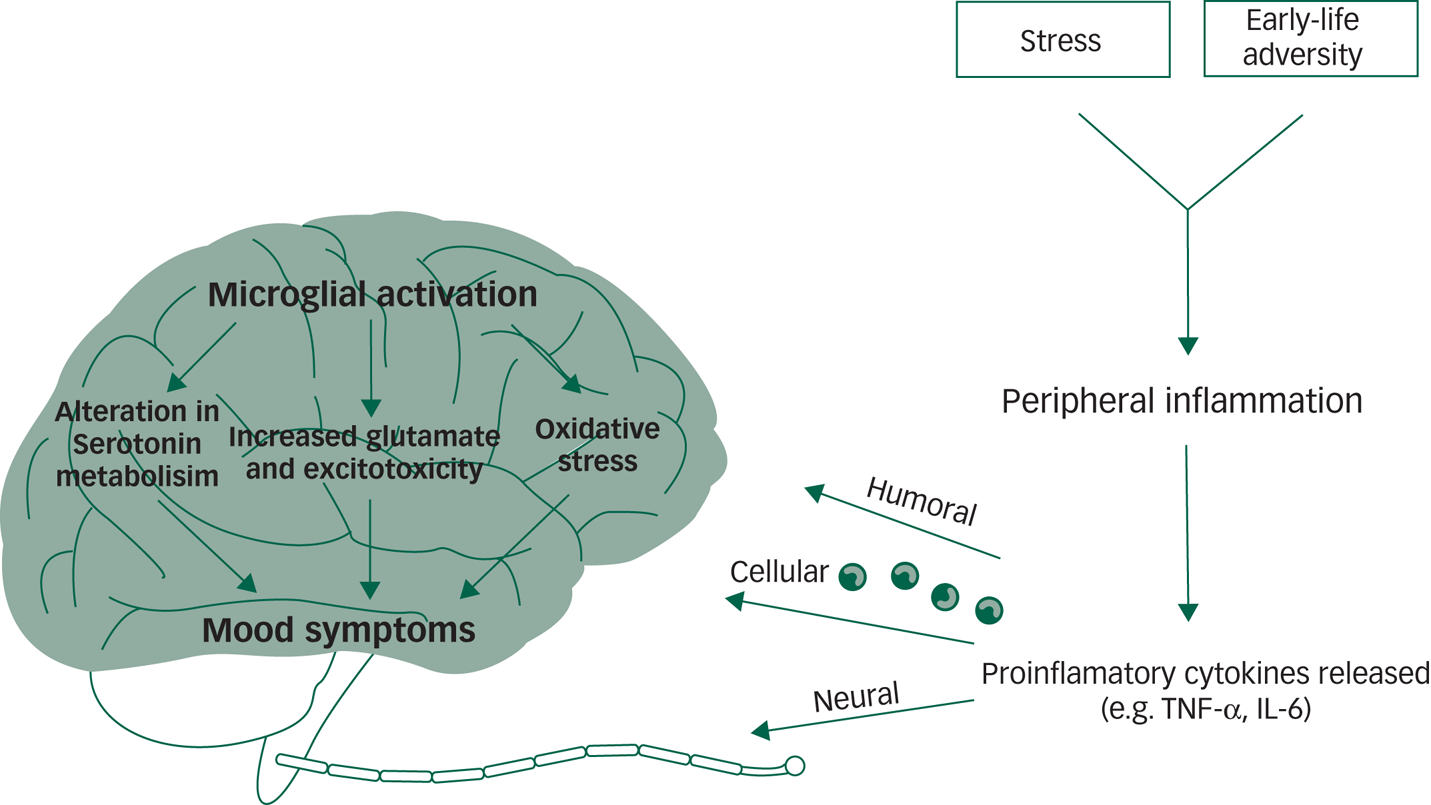
Fig. 1 Inflammation and mood disorders.
In the brain, the activated central inflammatory response influences neurotransmitter systems via metabolic or molecular pathways and by increasing the expression and functioning of presynaptic reuptake pumps.Reference Miller and Raison48,Reference Bin, Lindler, Owens, Daws, Blakely and Hewlett49 Several cytokines activate indoleamine 2,3-dioxygenase, which breaks down tryptophan, the primary precursor of serotonin, into kynurenine.Reference Raison, Dantzer, Kelley, Lawson, Woolwine and Vogt50,Reference Maes, Leonard, Myint, Kubera and Verkerk51 This shunting of the production of serotonin towards kynurenine, combined with increased reuptake from the presynaptic serotonin pump, can lead to serotonin depletion. Furthermore, the kynurenine pathway is hypothesised to disrupt glutamate metabolism, leading to reduce astrocytic glutamate reuptake and stimulation of astrocyte glutamate release, leading to excess glutamateReference Tavares, Tasca, Santos, Alves, Porciúncula and Emanuelli52,Reference Tilleux and Hermans53 and reduced brain-derived neurotrophic factor (BDNF).Reference Hardingham, Fukunaga and Bading54 Inflammatory effects on growth factors such as BDNF in the dentate gyrus of the hippocampus may affect learning and memory in patients with mood disorders.Reference Miller and Raison48
Proinflammatory cytokine effects on dopamine can also inhibit several aspects of reward motivation in corticostriatal circuits involving the basal ganglia, ventromedial prefrontal cortex and subgenual and dorsal anterior cingulate cortex, while also activating circuits in the amygdala and hippocampus.Reference Miller and Raison48 These physiological changes may present clinically as decreased motivation (anhedonia), avoidance, arousal, fear and alarm (anxiety) – core symptoms of depression and significant perpetuating factors for a depressive mood state.Reference Miller and Raison48
Although there are fewer studies examining putative associations between abnormal inflammatory processes and bipolar disorders, similar pathways may be involved. These include alterations in dopamine and glutamate metabolism and changes in proinflammatory cytokines, potentially leading to mitochondrial dysfunction and consequent increase in apoptosis, cell membrane damage and protein aggregation.Reference Berk, Kapczinski, Andreazza, Dean, Giorlando and Maes55 In the context of bipolar disorders, increased dopamine release is postulated to lead to the formation of free radicals, causing oxidative damage.Reference Rees, Florang, Anderson and Doom56 Mitochondrial electron transport chain dysfunction may in turn produce more free radicals.Reference Green, Brand and Murphy57 Activation of a proinflammatory cytokine receptor, such as TNF-α and IL-6 can lead to apoptosis through caspase activation and nitric oxide production.Reference Berk, Kapczinski, Andreazza, Dean, Giorlando and Maes55
Glutamate may also cause excitotoxic damage in bipolar disorders through activation of the N-Methyl-d-aspartate receptor, which increases calcium influx and consequent nitric oxide production, leading to nitrosative damage to DNA, proteins and lipids.Reference Berk, Kapczinski, Andreazza, Dean, Giorlando and Maes55,Reference Zuo, Wu, Yao, Cao, Wu and Tanaka58 Oxidative and nitrosative damage can induce cell membrane damage, protein aggregation and apoptosis. This cascade has been hypothesised to lead to changes that result in the manifestation of depressive, manic and mixed episodes in bipolar disorders.Reference Berk, Kapczinski, Andreazza, Dean, Giorlando and Maes55
Use of immunomodulatory agents for the treatment of MDD and bipolar disorder
Given the prevalence of treatment-resistant depression in patients with MDD or bipolar disorders, it is likely that a subset of these patients do not respond to current standard antidepressant treatments because their treatment does not engage the appropriate neurobiological target. Conversely, if an activated inflammatory response is associated with MDD and bipolar disorders, therapeutic interventions targeting this pathophysiological process may confer benefit, at least in a subset of individuals.
In recent years, several studies have assessed the effect of immunomodulatory agents in neuropsychiatric disorders including MDD and bipolar disorders. These studies have most commonly evaluated non-steroidal anti-inflammatory drugs (NSAIDS), cytokine inhibitors and pleiotropic agents such as minocycline or N-acetylcysteine (NAC). Herein, we review evidence from selected clinical trials of these agents in MDD and bipolar disorders. Tables 1 and 2 outline the studies included.
Table 1 Identified clinical trials of anti-inflammatory agents in major depressive disorder (MDD)

NSAIDs, non-steroidal anti-inflammatory drugs; NARI, selective noradrenaline reuptake inhibitor; o.d., once daily; HRSD, Hamilton Rating Scale for Depression; SSRI, selective serotonin reuptake inhibitor; b.i.d., twice daily; TAU, treatment as usual; MADRS, Montgomery–Åsberg Depression Rating Scale; CRP, C-reactive protein; NR, not reported.
Table 2 Identified clinical trials of anti-inflammatory agents in bipolar disorders

MADRS, Montgomery–Åsberg Depression Rating Scale; LSM, least squares mean; NOS, not otherwise specified; MDE, major depressive episode; TAU, treatment as usual; b.i.d., twice daily; ASA, aspirin; CRP, C-reactive protein; NR, not reported.
Use of immunomodulatory agents for the treatment of MDD
In the first published RCT of the selective cyclooxygenase (COX)-2 inhibitor celecoxib for the treatment of MDD, celecoxib (in addition to reboxetine) was more efficacious than placebo in reducing depressive symptoms.Reference Müller, Schwarz, Dehning, Douhe, Cerovecki and Goldstein-Müller59 The reduction in scores on the Hamilton Rating Scale for Depression (HRSD) from baseline to end of trial was 55% in the celecoxib/reboxetine group compared with 33% in the placebo group; 75% of the participants in the celecoxib/reboxetine group were responders compared with 45% in the placebo group.Reference Berk, Kapczinski, Andreazza, Dean, Giorlando and Maes55 Following this initial encouraging pilot study, there have been favourable results in trials of celecoxib in MDD as an augmenting agent to sertraline and fluoxetine.Reference Akhondzadeh, Jafari, Raisi, Nasehi, Ghoreishi and Salehi60–Reference Abbasi, Hosseini, Modabbernia, Ashrafi and Akhondzadeh62
Two studies assessed celecoxib as an augmenting agent to sertraline; both reported significantly greater changes in HRSD (mean difference 3.35 (95% CI 1.08–5.61), t(38) = 2.99, P = 0.005 and (13.7 (s.d. = 3.8) v. −8.8 (s.d. = 4.5)) in the celecoxib group compared with placebo group.Reference Majd, Hashemian, Hosseinib, Shariatpanahi and Sharifid61,Reference Abbasi, Hosseini, Modabbernia, Ashrafi and Akhondzadeh62 The third compared celecoxib with fluoxetine and reported a significant difference between the two treatments at the end-point (week 6) (t = 3.35, d.f. = 38, P = 0.001), also reporting a significant group × time interaction.Reference Akhondzadeh, Jafari, Raisi, Nasehi, Ghoreishi and Salehi60
Further candidate inflammatory targets for treatment of MDD would include inflammatory cytokines, adhesion molecules and cellular components of the inflammatory response.Reference Miller, Haroon and Felger63 Cytokine inhibitors (for example infliximab) target inflammatory cytokines such as TNF-α, IL-1, IL-6R, and IL-12/23 and the cell adhesion molecule α4-integrin. They are approved by the US Food and Drug Administration and recommended by the UK National Institute for Health and Care Excellence for the treatment of autoimmune disorders including rheumatoid arthritis, ulcerative colitis, Crohn disease, psoriasis, multiple sclerosis and ankylosing spondylitis. A recent systematic review of these cytokine inhibitors in patients without a diagnosis of MDD showed that these drugs lead to an overall improvement in depressive symptoms compared with placebo, independent of their effect on physical health.Reference Kappelmann, Lewis, Dantzer, Jones and Khandaker26 In RCT in patients with treatment-resistant MDD, there was no overall difference between infliximab and placebo, even when controlling for baseline level of inflammation.Reference Raison, Rutherford, Woolwine, Shuo, Schettler and Drake64 A phase II trial of an anti-IL-6 mAb (sirukumab) in patients with MDD and elevated plasma CRP (ClinicalTrials.gov Identifier: NCT02473289) is awaiting publication.
Another candidate immunomodulatory agent for the treatment of MDD is the anti-inflammatory tetracycline antibiotic minocycline. This pleiotropic agent is postulated to have antidepressant action by inhibiting neurotoxic factors (i.e. proinflammatory cytokines, reactive oxygen species) released by activated microglia, and by inducing neuroprotective factors (i.e. anti-inflammatory cytokines, antioxidants, neurotrophic factors) released by astrocytes.Reference Soczynska, Mansur, Brietzke, Swardfager, Kennedy and Woldeyohannes65 Minocycline has been studied as a monotherapy or an augmenting agent in patients with MDD. As a monotherapy, it was shown to have clinically significant improvements in depressive symptoms compared with placebo in patients with HIV and comorbid MDD.Reference Emadi-Kouchak, Mohammadinejad, Asadollahi-Amin, Rasoulinejad, Zeinoddini and Yalda66
Three clinical trials have evaluated minocycline as an augmenting agent in MDD: two (one open-label study, one RCT) reported significant reductions in depressive symptoms with minocycline;Reference Miyaoka, Wake, Furuya, Liaury, Ieda and Kawakami67,Reference Husain, Chaudhry, Husain, Khoso, Rahman and Hamirani68 the third one, an RCT in 41 patients with treatment-resistant MDD, reported a mean change in HRSD scores of over 18 points in the minocycline group, compared with −0.2 in the placebo group (effect size −1.21, P < 0.001).Reference Husain, Chaudhry, Husain, Khoso, Rahman and Hamirani68 Given the large effect size and small sample size, this study is now being replicated in a larger sample (ClinicalTrials.gov Identifier: NCT03947827).
In a separate RCT of minocycline in 71 patients with MDD (not necessarily treatment resistant), the investigators reported no significant difference in the reduction on the Montgomery–Åsberg Depression Rating Scale (MADRS) across the 12 weeks of treatment between the minocycline and placebo groups, effect size 0.46 (95% CI −7.1 to 3.2), P = 0.02. However, there was a significant improvement in psychosocial function in the minocycline group.Reference Dean, Kanchanatawan, Ashton, Mohebbi, Ng and Maes69 In a recent meta-analysis, the antidepressant effect of minocycline had an overall moderate and statistically significant effect size (−0.78, 95% CI −0.4 to −1.33, P = 0.005).Reference Rosenblat and McIntyre70
NAC is an over-the-counter supplement that has peripheral and central immunomodulatory effects. NAC has been studied across multiple psychiatric diagnoses,Reference Zheng, Zhang, Cai, Yang, Qiu and Ungvari71 appearing safe and effective for schizophrenia, but lacking consistent evidence of efficacy in MDD and bipolar disorders. For MDD, one published RCT in a reasonably large sample (n = 252) showed that adjunctive NAC was not efficacious in reducing depressive symptoms compared with placebo.Reference Berk, Dean, Cotton, Jeavons, Tanious and Kohlmann72 More recently, a smaller study (n = 57) compared NAC versus placebo in patients with MDD or bipolar disorders, with evidence of inflammation at baseline (CRP>3 mg/L) or low baseline inflammation (CRP < 3 mg/L).Reference Porcu, Urbano, Verri, Sabbatini Barbosa, Baracat and Vargas73 Those with high baseline inflammation had a significantly greater reduction in depressive symptoms with NAC than with placebo but the analysis did not separate those with MDD or bipolar disorders (P = 0.04).Reference Porcu, Urbano, Verri, Sabbatini Barbosa, Baracat and Vargas73 These findings suggest that NAC may be effective in patients who exhibit an inflammatory biotype.
A recent meta-analysis evaluated the efficacy and safety of anti-inflammatory agents as monotherapy or adjunctive treatment for MDD.Reference Bai, Guo, Feng, Deng, Li and Nie74 The drugs included in this review were celecoxib, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), lovastatin, atorvastatin, simvastatin, minocycline, pioglitazone, modafinil and NAC. Overall, in this analysis of 26 RCTs, anti-inflammatory agents reduced depressive symptoms (SMD = −0.55, 95% CI −0.75 to −0.35, I 2 = 71%) and were associated with higher response (relative risk (RR) = 1.52, 95% CI 1.30 to 1.79, I 2 = 29%) and remission rates (RR = 1.79, 95% CI 1.29 to 2.49, I 2 = 41%) compared with placebo.Reference Bai, Guo, Feng, Deng, Li and Nie74 Anti-inflammatory agents were shown to be safe, with the only significant difference between them and placebo being the incidence of gastrointestinal adverse events.Reference Bai, Guo, Feng, Deng, Li and Nie74 The authors asserted that, based on the evidence of their analysis, anti-inflammatory agents are effective and relatively safe treatment options for MDD. However, some key limitations detract from this conclusion. The authors included medications that do not have direct anti-inflammatory effects: omega-3, pioglitazone, statins, and modafinil (which has therapeutic mechanisms similar to traditional monoaminergic antidepressants). They also did not include a number of studies that appeared to have met their inclusion criteria and that have been included in previous meta-analyses.Reference Husain, Strawbridge, Stokes and Young10,Reference Köhler-Forsberg, N. Lydholm, Hjorthøj, Nordentoft, Mors and Benros75 Moreover, although the safety of these medications was reported as favourable, adverse events were poorly reported in the original studies.Reference Köhler-Forsberg, N. Lydholm, Hjorthøj, Nordentoft, Mors and Benros75
The latter comment regarding safety is salient as the literature would suggest safety is yet to be reliably established, particularly with the short duration of most trials.Reference Köhler-Forsberg, N. Lydholm, Hjorthøj, Nordentoft, Mors and Benros75 NSAIDs are well documented to have more concerning side-effects than traditional antidepressants, specifically gastric bleeding and impaired renal function. Further, celecoxib is known to increase the risks of cardiovascular events.Reference Husain, Strawbridge, Stokes and Young10 This risk is not completely understood in the context of MDD and bipolar disorders, which are both conditions associated with an independent risk for cardiovascular disease.Reference Goldstein, Carnethon, Matthews, McIntyre, Miller and Raghuveer41,Reference Goldstein, Schaffer, Wang and Blanco76,Reference Penninx77 With respect to the cytokine inhibitors, these have been associated with opportunistic infections such as tuberculosis because of immunosuppression.Reference Cao, Qasem, Sharp, Abdelli and Naser78 Given the high risks of immunosuppression and adverse side-effects with these agents, it is important to proceed cautiously when designing clinical trials in patients with mood disorders especially given the high degree of physical comorbidity in this population.Reference Kopylov, Vutcovici, Kezouh, Seidman, Bitton and Afif79,Reference Singh, Cameron, Noorbaloochi, Cullis, Tucker and Christensen80
Use of immunomodulatory agents for the treatment of bipolar disorder
Open-label studies of minocycline have shown significant improvements in depressive symptoms in patients with bipolar disorder depression.Reference Soczynska, Kennedy, Alsuwaidan, Mansur, Li and McAndrews81,Reference Murrough, Huryk, Mao, Iacoviello, Collins and Nierenberg82 A recent meta-analysis of eight published RCTs of immunomodulatory agents in bipolar disorder depression showed an overall effect size of −0.40 (95% CI −0.14 to −0.65, P = 0.002), suggesting a moderate antidepressant effect with good overall tolerability.Reference Rosenblat, Kakar, Berk, Kessing, Vinberg and Baune12 Included drugs were DHA/EPA, celecoxib, aspirin, pioglitazone and NAC. Subgroup analysis of each class of drug revealed non-significant effect sizes, with the exception of NAC. This analysis was limited by the dearth of studies in bipolar disorders limiting the power.
Since the publication of this meta-analysis, a relatively large (n = 99) 2 × 2 factorial design trial of minocycline and aspirin showed no main effect for either treatment, although participants with elevated IL-6 appeared to have a more favourable response to adjunctive minocycline compared with placebo.Reference Savitz, Teague, Misaki, Macaluso, Wurfel and Meyer83 Future studies are needed to assess the efficacy and safety of minocycline in treating bipolar depression; we are aware of at least one large study currently underway to assess the efficacy of both minocycline and celecoxib for bipolar depression.Reference Husain, Chaudhry, Hamirani, Minhas, Kazmi and Hodsoll84
An early study of NAC in bipolar depression was promising with NAC treatment leading to a significant improvement on the MADRS (least squares mean difference –8.05 (95% CI –13.16 to 2.95), P = 0.002) compared with placebo.Reference Berk, Copolov, Dean, Lu, Jeavons and Schapkaitz85 However, a meta-analysis that included two RCTs in bipolar disorders found there was no significant group differences in antidepressant effects (n = 124, SMD = −0.59, 95% CI −1.48 to 0.3, I2 = 83% P = 0.19).Reference Zheng, Zhang, Cai, Yang, Qiu and Ungvari71
Subsequently there have been several studies assessing its efficacy in treating bipolar disorder depression. The largest of these studies was in 148 patients with bipolar disorder depression who were treated adjunctively with either NAC, NAC plus a nutraceutical agent that may increase mitochondrial biogenesis or placebo.Reference Berk, Turner, Malhi, Ng, Cotton and Dodd86 The analysis of the primary outcome was negative, with no difference in change in depressive symptoms among the three groups. However, there was a significant improved delayed response (20 weeks post-discontinuation) in the NAC plus nutraceutical group.Reference Berk, Turner, Malhi, Ng, Cotton and Dodd86 In another study of the efficacy of adjunctive NAC in 80 patients with bipolar disorder acute depression, NAC was not superior to placebo.Reference Ellegaard, Licht, Nielsen, Dean, Berk and Poulsen87
However, in a recent study comparing NAC, aspirin, NAC plus aspirin and placebo, at 16 weeks,
NAC plus aspirin was associated with a higher rate of response (67%), than NAC alone (57%), placebo (55%) or aspirin (33%) alone.Reference Bauer, Green, Colpo, Teixeira, Selvaraj and Durkin88
To our knowledge only one RCT has prospectively stratified patients based on evidence of inflammation. A recent 12-week RCT evaluated the effects of adjunctive intravenous infliximab for the treatment of bipolar depression in patients with biochemical (i.e. elevated CRP ≥ 5 mg/L) or phenotypical (i.e. obesity, diabetes type 1 or 2, inflammatory bowel disease, rheumatological disorder, daily cigarette smoking, or migraine headaches) evidence of inflammation. Despite this stratification, infliximab showed no reduction in overall depressive symptoms compared with placebo.Reference McIntyre, Subramaniapillai, Lee, Pan, Carmona and Shekotikhina89 The authors did, however, report an association between early childhood adversity and response to infliximab, suggesting a possible subgroup of patients who could benefit from this form of treatment.
There is some evidence from a few small studies suggesting that immunomodulatory medications are beneficial in mania. In a recent meta-analysis of three RCTs of celecoxib, aspirin and NAC, respectively, the overall effect size for treatment of manic symptoms was −0.72 (95% CI −1.31 to −0.13, P = 0.02).Reference Husain, Strawbridge, Stokes and Young10
In a secondary analysis of a large study of 482 patients with bipolar disorders receiving either lithium or quetiapine, the trajectory of either depressive or manic symptoms did not significantly differ in patients taking concomitant NSAIDs acetaminophen (n = 177) and, those who did not.Reference Köhler-Forsberg, Sylvia, Thase, Calabrese, Deckersbach and Tohen90 However, anti-inflammatory drugs in this study were not the study medication and were only self-reported by patients. Given the small number of reliable studies and their small samples, one cannot draw firm conclusions from their findings, although they suggest that further, well-designed trials investigating the antimanic effects of immunomodulatory agents are warranted.
Overall, although there are some small promising studies in bipolar disorders, results from recent large replication studies have been negative. Some post hoc analyses suggest that immunomodulatory agents may be effective for a subset of patients (for example those with elevated IL-6 or those with a history of childhood adversity). However, further work is required to confirm these findings and establish whether some clinical or biological phenotypes may be more responsive to these drugs.
Discussion
Main findings
This critical review highlights converging evidence for a bidirectional relationship between an activated inflammatory response and the onset and persistence of mood symptoms in a subset of patients. However, few prospective studies have been conducted thus far and using this approach could provide vital insight into a putative causal link between inflammation and mood disorders. Current evidence suggests that an activated inflammatory response is associated with treatment resistance and may predict poor response to traditional antidepressant medications.Reference Chamberlain, Cavanagh, De Boer, Mondelli, Jones and Drevets91,Reference Arteaga-Henríquez, Simon, Burger, Weidinger, Wijkhuijs and Arolt92 However, results from clinical trials of immunomodulatory agents in mood disorders have been conflicting and the efficacy of these drugs is yet to be established.
Interpretation of our findings
One potential explanation for the inconsistent findings from RCTs of immunomodulatory agents is that these treatments have not been targeted towards an ‘inflamed’ subset of patients. Very few studies included in this review stratified treatment based on a priori markers of an activated immune response. Only about 30% of individuals with MDD show peripheral evidence of inflammation and immunomodulatory agents are only likely to benefit this specific subset of individuals.Reference Osimo, Baxter, Lewis, Jones and Khandaker30 Nonetheless, results from the recent study of infliximab for bipolar depression has shown that stratifying patients based on peripheral markers of inflammation such as CRP or phenotypic evidence of inflammation does not necessarily confer benefit from immunomodulatory agents.Reference McIntyre, Subramaniapillai, Lee, Pan, Carmona and Shekotikhina89
Choice of candidate peripheral biomarkers
A major gap in the current evidence relates to the choice of candidate biomarkers that could be utilised to stratify patients to an ‘inflamed’ subtype. Historically, when utilised, studies have used individual peripheral inflammatory markers such as CRP, IL-6 or TNF-α. This approach is limited as acute phase proteins and inflammatory cytokines are highly influenced by multiple factors including diet, BMI and smoking status. Moreover, each individual marker may have varying degrees of inflammatory versus anti-inflammatory properties.Reference Horn, Long, Nelson, Allen, Fisher and Byrne36,Reference Giudice and Gangestad37 Recent studies suggest that using composite measures may be a more suitable approach. One such study developed a multisystem data-driven composite inflammatory biomarker, using POLG, ADARB1, OGG, 8-oxoGuo, leucocytes and age, to distinguish patients with bipolar disorders from healthy controls with a sensitivity of 73% and specificity of 71%.Reference Munkholm, Vinberg, Pedersen, Poulsen, Ekstrøm and Kessing93
Utilising computer generated binary clustering, another study was able to stratify patients with MDD to ‘inflamed’ and ‘uninflamed’ subtypes using peripheral immune cell counts. The inflamed subgroup had increased monocyte, CD4+, neutrophil counts, CRP and IL-6. This subgroup also had more severe depressive symptoms.Reference Lynall, Turner, Bhatti, Cavanagh, de Boer and Mondelli94 Furthermore, there is evidence that symptom clusters of depression may contribute to the ‘inflamed’ subtype; for example anhedonia has been associated with increased CRP and atypical depressive symptoms (increased appetite, weight gain) have been associated with increased CRP, IL-1RA and IL-6.Reference Felger, Haroon, Patel, Goldsmith, Wommack and Woolwine95,Reference Simmons, Burrows, Avery, Kerr, Taylor and Bodurka96
Combining peripheral and central markers of inflammation
To date, most studies have focused solely on peripheral markers of inflammation and a more informative approach may be to assess these alongside sophisticated neuroimaging techniques such as PET imaging or more invasive measures of central inflammation, for example in cerebrospinal fluid. This would allow the assessment of the correlation between the central markers of inflammation and the more pragmatic peripheral markers. For instance, a recent study, utilising a composite approach, measured the blood serum concentration of several products synthesised by activated microglia and to some extent astroglia – for example prostaglandin E2 (PGE2), prostaglandin F2 alpha (PGF2α), and TNF-α – and controlled by CRP. This study showed that ln(PGE2/CRP) and ln(TNF-α/CRP) consistently correlated with TSPO VT (and hence microglial activation and neuroinflammation) in patients with MDD.Reference Attwells, Setiawan, Wilson, Rusjan, Miler and Xu97 If this approach leads to the identification of peripheral markers that are surrogate markers of neuroinflammation, they can then be used in future RCTs of immunomodulatory therapeutics. Taken together, utilising peripheral and central markers of inflammation and targeting specific symptom clusters in patients with mood disorders may provide more consistent results in clinical trials of repurposed or novel immunomodulatory drugs.
Addressing the heterogeneity of mood disorders
Finally, it is also important to consider the heterogeneity of mood disorders as an ongoing challenge in designing RCTs of any novel intervention. Using the Research Domain Criteria approach to examine specific symptom subsets (for example anhedonia, motivation, suicidal ideation) rather than solely relying on traditional assessment scales, may be a more sensitive way to assess the efficacy of immunomodulatory agents. Future trials should use the existing evidence on inflammatory biomarkers to recruit and stratify participants who may be more likely to respond to repurposed or novel immunomodulatory medications. Although further trials of these agents remain warranted, they must incorporate enriched patient samples and mechanistic evaluations into their design. Otherwise, the promise of translating the use of these agents to the clinic is likely to remain unfulfilled.
Acknowledgements
The authors would like to thank Rachel Copeland for creating Fig. 1.
Author contributions
B.D.M.J. conducted the literature search, literature review and drafted the manuscript. M.I.H. conceived the idea for the study, helped with the literature review and drafted the manuscript. Z.J.D., A.F.C., R.S., A.H.Y. and B.H.M. contributed to the literature review and helped with drafting the manuscript. All authors reviewed and approved the final manuscript.
Funding
There was no direct funding that led to the completion of this review. This work was supported in part by an Academic Scholars Award to M.I.H. from the Department of Psychiatry, University of Toronto. This report represents independent research part-funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Declaration of interest
A.H.Y. has been commissioned to provide lectures and advice to all major pharmaceutical companies with drugs used in affective and related disorders. A.H.Y. has undertaken investigator-initiated studies funded by Astra Zeneca, Eli Lilly, Lundbeck and Wyeth. R.S. has received honorarium for speaking from Lundbeck. B.H.M. currently receives research support from Brain Canada, the Canadian Institutes of Health Research, the CAMH Foundation, the Patient-Centered Outcomes Research Institute (PCORI), the US National Institute of Health (NIH), Capital Solution Design LLC (software used in a study funded by CAMH Foundation) and HAPPYneuron (software used in a study founded by Brain Canada). Within the past 5 years he has also received research support (medications for NIH-funded clinical trials) from Bristol-Myers, Eli Lilly and Pfizer. He directly owns stocks of General Electric (less than $5000). M.I.H. is a principal investigator for a trial sponsored by COMPASS Pathways Limited. None of the companies have a financial interest in this research.
ICMJE forms are in the supplementary material, available online at https://doi.org/10.1192/bjo.2020.43.






eLetters
No eLetters have been published for this article.