Resistance to antipsychotic treatment is defined as failure to respond to two antipsychotics of adequate dose and duration 1 and occurs in 30% of individuals with schizophrenia. Reference Meltzer2 Clozapine is the only medication with evidence-based effectiveness in treatment-resistant schizophrenia, 1 being effective in 50–60% of cases, Reference Meltzer3 but is also associated with a broad range of adverse effects. A commonly accepted risk is clozapine-induced blood dyscrasia, typically neutropenia or agranulocytosis. Neutropenia is defined as a neutrophil count of less than 1.5×109/L, occurring at a rate of 2.7% of clozapine users at 1 year. Reference Alvir, Lieberman, Safferman, Schwimmer and Schaaf4 Patients who develop neutropenia may develop flu-like symptoms such as sore throat and pyrexia, with the peak risk occurring at 6–18 weeks. Less common is agranulocytosis, a life-threatening event defined as a neutrophil count of less than 0.5×109/L, occurring in approximately 0.8% of clozapine users at 1 year. Reference Alvir, Lieberman, Safferman, Schwimmer and Schaaf4
Dispensing of clozapine is dependent on a satisfactory full blood count (FBC) result and maintenance of normal white blood cell (WBC) and neutrophil counts during clozapine treatment. In established clozapine-induced neutropenia or agranulocytosis, clozapine is immediately discontinued, and given the increased risk of recurrence, Reference Dunk, Annan and Andrews5 re-challenge is contraindicated. However, re-challenge may be attempted under certain conditions, for example, due to an alternative cause of the reduced neutrophil count being identified, or after careful assessment of the clinical risks and benefits. This process involves collaboration with a haematologist and may involve the co-administration of lithium or granulocyte colony stimulating factor (GCS-F) in an attempt to promote granulocyte proliferation and increase the WBC and neutrophil counts. Despite carefully controlled re-challenge, a third of patients will develop a further blood dyscrasia, which is often more rapid in onset and more severe. Reference Dunk, Annan and Andrews5
In this report, we add to the limited existing evidence of successfully re-challenging clozapine for a third time with a patient with treatment-resistant schizoaffective disorder, following two episodes of agranulocytosis and one of episode of neutropenia.
Case report
J.B. is a 30-year-old Caucasian woman with a diagnosis of schizoaffective disorder. She was born and raised abroad before moving to the UK at the age of 21 years. J.B. first presented with psychotic symptoms at the age of 19 years. Over the next decade, she had multiple admissions to psychiatric hospitals, both informally and under Section 3 of the Mental Health Act. Her illness was characterised by mixed affective episodes associated with persecutory delusions, thought disorder and auditory hallucinations, often triggered by stress or periods of non-adherence to medication. During admission to our service, persecutory beliefs were about other patients, staff and visitors, and led to accusations of theft of her belongings, accusations of sexual assault and physical aggression. Periodically, she became suspicious about the side-effects of medications and declined to adhere to their administration. There was a significant labile component to her mood, fluctuating between laughing and verbal or physical aggression. Between episodes, J.B. was pleasant, engaged in recovery-oriented practices and appeared less preoccupied and distressed by positive psychotic symptoms.
During the first 7 years of her illness, J.B. was treated with multiple antipsychotics (i.e. quetiapine, aripiprazole, risperidone, olanzapine, amisulpride, haloperidol, sulpiride, zuclopenthixol decanoate and flupentixol decanoate), mood stabilisers (lithium carbonate and sodium valproate) and a course of electroconvulsive therapy (ECT) with minimal improvement documented in case notes. Given the treatment-resistant nature of her illness, clozapine was commenced.
J.B. had no significant past medical history and was not taking any medication for her physical health. Routine admission bloods including an autoimmune screen, hepatitis screen and liver function tests were all within normal ranges. J.B. smoked continually during all clozapine titrations, and the phenomenon of altered clozapine metabolism with smoking has been documented Reference Rostami-Hodjegan, Amin, Spencer, Lennard, Tucker and Flanagan6 ; we monitored clozapine serum levels and dosage adjustments were made.
Clozapine treatments
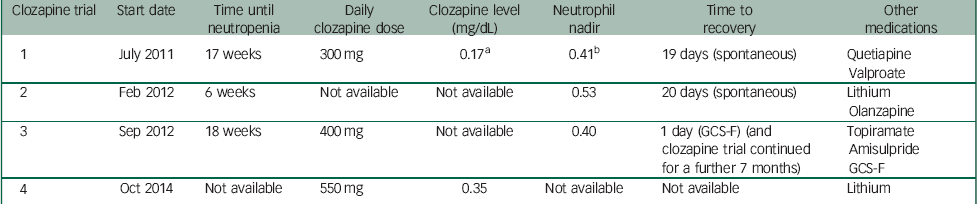
Table 1 provides a summary of clozapine trials and recorded measures.
Table 1 Summary of clozapine trials and recorded measures
| Clozapine trial | Start date | Time until neutropenia | Daily clozapine dose | Clozapine level (mg/dL) | Neutrophil nadir | Time to recovery | Other medications |
|---|---|---|---|---|---|---|---|
| 1 | July 2011 | 17 weeks | 300 mg | 0.17 a | 0.41 b | 19 days (spontaneous) | Quetiapine Valproate |
| 2 | Feb 2012 | 6 weeks | Not available | Not available | 0.53 | 20 days (spontaneous) | Lithium Olanzapine |
| 3 | Sep 2012 | 18 weeks | 400 mg | Not available | 0.40 | 1 day (GCS-F) (and clozapine trial continued for a further 7 months) | Topiramate Amisulpride GCS-F |
| 4 | Oct 2014 | Not available | 550 mg | 0.35 | Not available | Not available | Lithium |
GCS-F, granulocyte colony stimulating factor.
a Clozapine level: 0.17 mg/dL is equivalent to 170 ng/mL.
b Neutrophil nadir: 0.41×109/L is equivalent to 410/mm3 (cells).
Trial 1
J.B. was first commenced on clozapine in July 2011. At this time, she was also receiving quetiapine and sodium valproate. Pre-treatment FBC showed a WBC count of 5.8×109/L and a neutrophil count of 2.9×109/L. WBC and neutrophil counts remained within normal limits until week 17, when J.B. developed agranulocytosis with a nadir WBC count of 2.0×109/L and a neutrophil count of 0.41×109/L. The daily dose of clozapine was 300 mg, with a plasma clozapine concentration of 0.17 mg/dL. Clozapine was immediately stopped and olanzapine was commenced as an alternative. The blood dyscrasia persisted for 19 days in total before cell counts recovered. Case records from the previous hospital indicated a significant improvement in her clinical state with clozapine, and discontinuation was associated with a rapid increase in her psychotic symptoms.
Trial 2
A second clozapine challenge was initiated in February 2012. Sodium valproate was discontinued prior to the second clozapine trial. At this time, J.B. was concurrently treated with olanzapine 10 mg and lithium carbonate 300 mg daily. An FBC prior to re-challenge with clozapine showed a WBC count of 8.0×109/L and a neutrophil count of 4.4×109/L. Six weeks after re-commencement of clozapine, J.B. developed a neutropenia, with a nadir neutrophil count of 0.53×109/L and a WBC of 1.9×109/L. Clozapine was immediately discontinued following consultation with a haematologist. The neutrophil count normalised over the next 20 days, during which time she was treated with an increased dose of olanzapine 15 mg and sulpiride 200 mg daily.
Trial 3
In September 2012, after a careful risk–benefit assessment and in collaboration with a haematologist, a third clozapine trial was commenced. On this occasion, J.B. was being treated with topiramate 400 mg and amisulpride 400 mg daily. She was commenced on weekly subcutaneous injections of GCS-F, with 480 mcg of filgrastim. After 18 weeks of treatment, she developed agranulocytosis with neutrophils dropping to 0.40×109/L and a WBC count of 2.6×109/L. By this time, clozapine had been titrated to 400 mg daily. Following consultation with a haematologist, J.B. continued clozapine treatment with an additional dose of filgrastim 480 mcg given that day. This resulted in a good response, with the neutrophil count increasing to 19.2×109/L and the WBC to 23.10×109/L the following day. A plan was formulated to increase the frequency of filgrastim injections to twice weekly in the event of further neutropenia; however, this was not required. J.B. was maintained on clozapine, with weekly filgrastim for a further 7 months, and was discharged to a rehabilitation service. However, she subsequently became non-adherent with clozapine.
Trial 4
Following admission to our service in October 2013, a range of pharmacotherapeutic interventions were attempted, including zuclopenthixol decanoate, quetiapine, olanzapine, amisulpride and lithium, all with minimal beneficial effect. In October 2014, following specialist consultations, the multidisciplinary team agreed collaboratively with the patient and family to re-challenge with clozapine for a third time under close supervision. She was deemed to have capacity to be part of each of these decisions. Following consultation with a haematologist and pharmacist, lithium carbonate was commenced prior to clozapine initiation, and weekly FBCs were measured over the first year of clozapine treatment. It was planned that in the event of the neutrophil count dropping below 2×109/L, a single dose of 105 mcg of lenograstim was to be administered subcutaneously, and clozapine was to be continued with daily blood monitoring. In the event of the neutrophil count dropping below 1.5×109/L, a single dose of 105 mcg lenograstim was to be administered, and clozapine treatment immediately terminated. Lenograstim was to be used in preference to filgrastim because of increased experience with its use in our clinical setting.
Pre-treatment FBC demonstrated a WBC count of 8.43×109/L and a neutrophil count of 5.7×109/L. Standard clozapine titration was carried out as per Maudsley Guidelines, Reference Taylor, Paton and Kapur7 and clozapine was increased to a dose of 550 mg daily administered in liquid form. Lithium carbonate at 1200 mg daily was concurrently used, with serum lithium levels of 0.55–0.95 mmol/L attained. Weekly FBCs across the first year demonstrated stable WBC and neutrophil counts, with no episodes of neutropenia or the need for rescue lenograstim. Consistently, therapeutic plasma clozapine concentrations of around 0.35–0.40 mg/L were attained, without further haematological complications despite continuation of clozapine, and a dramatic improvement in mental state was observed.
Discussion
Owing to the severity and distressing nature of this young woman's psychotic and mood symptoms and the lack of response to a number of antipsychotic medications, clozapine was re-challenged following specialist consultations and close monitoring practices. The results were promising given the failure of three previous attempts and thus support the need for measured re-challenging and augmentation practices. In a recent systematic review, clozapine re-challenge was successful in 70% (78/112 patients) after an episode of neutropenia, with 20% (3/15 patients) successfully re-challenged after agranulocytosis. Reference Manu, Sarpal, Muir, Kane and Correll8 The co-administration of lithium (33/35 patients) or GCS-F (7/11 patients), in a targeted strategy, decreased the likelihood of developing a further blood dyscrasia. Reference Manu, Sarpal, Muir, Kane and Correll8 We reported previously that a clozapine re-challenge was successful in 79% of patients (n=19) admitted to our service. Reference Meyer, Gee, Whiskey, Taylor, Mijovic and Gaughran9
The effective treatment of schizoaffective disorder often requires the use of several psychopharmacological agents, Reference Correll and Gallego10 and polypharmacy may have played a part in this case. The first trial of clozapine was concurrent with the use of sodium valproate. Sodium valproate can cause a dose-dependent inhibition of granulopoiesis, Reference Mintzer, Billet and Chmielewski11 which when combined with clozapine may have led to the agranulocytosis. In the second trial, clozapine re-challenge occurred in combination with lithium carbonate. Lithium carbonate has demonstrated the ability to increase circulating neutrophils and granulocyte colony formation. Reference Murphy, Goodwin and Bunney12–Reference Blier, Slater, Measham, Koch and Wiviott14 Although a neutropenia occurred during this trial, it is interesting to note that the neutrophil count did not drop as low as seen in the first trial. During the third trial, J.B. was treated with concurrent G-CSF, and although agranulocytosis still occurred, neutrophil recovery was 1 day, which was significantly shorter than the recovery seen in the first and second trials. We are unable to explain this transient episode of agranulocytosis and the lack of any recurrent neutropenia over the following 7 months of clozapine treatment with concurrent G-CSF (with no G-CSF dose change). We cannot exclude that a transient increase in plasma clozapine concentrations was related to the onset of agranulocytosis (we have no data relating to plasma clozapine concentrations at that time, nor do we know if the patient had ceased smoking at or around this time). Alternatively, the presence of a viral infection may have contributed to the onset of agranulocytosis, though this was not clinically documented.
During the fourth trial, lithium carbonate was used with therapeutic serum levels attained. Previous work has suggested that serum lithium levels greater than 0.4 mmol/L are required for granulopoiesis. Reference Blier, Slater, Measham, Koch and Wiviott14 Given the low dose of lithium carbonate used during the second trial, it may be that there were insufficient lithium levels to promote granulocyte colony formation at that time and that the optimal serum lithium levels in the fourth trial supported the maintenance of normal neutrophil counts.
Despite neutropenia and agranulocytosis being well-documented complications of clozapine treatment, the mechanism of action remains unclear. Reference Whiskey and Taylor15 There are several hypotheses, but due to its idiosyncratic nature it has been difficult to perform mechanistic studies to investigate these in a systematic fashion. Clozapine-induced agranulocytosis (CIA) has a large genetic component; genetic data suggest dysfunction in the human leucocyte antigen (HLA) system, which comprises genes that modulate immune system function. Recent work has suggested that there is an association with several genetic variants in the HLA-DQB1 locus, a single amino acid at HLA-DQB1 (126Q) and an amino acid change in the extracellular binding pocket of HLA-B (158T). Reference Goldstein, Jarskog, Hilliard, Alfirevic, Duncan and Fourches16
Both toxic and immune-mediated mechanisms have also been suggested as being important in the aetiology of CIA. The prevailing thinking is that many idiosyncratic drug reactions like CIA are secondary to the formation of reactive metabolites. Clozapine has been shown in vitro to be oxidised by the myeloperoxidase system to reactive electrophilic nitrenium ions. This oxidation to bioactive nitrenium occurs in the liver and peripheral neutrophils. These bioactive metabolites are proposed to directly affect bone marrow and circulating cells. The reactive nitrenium ions may bind to neutrophils to cause cell death. They may trigger an immune response in susceptible individuals leading to neutrophilic precursors in the bone marrow being targeted (equivalent to what occurs in a drug hypersensitivity reaction). It is possible that the target in agranulocytosis could be stromal cells and neutrophil precursors in the bone marrow, Reference Pereira and Dean17 whereas the main target in neutropenia may only be the peripheral blood neutrophils. Reference Duggal and Singh18 Clozapine further appears to accelerate the process of apoptosis through oxidative mitochondrial stress, rendering the neutrophils highly susceptible to oxidant-induced apoptosis. Apoptosis is induced in weeks 4–12 after starting clozapine, corresponding to the period when agranulocytosis is most likely to occur. Reference Fehsel, Loeffler, Krieger, Henning, Agelink and Kolb-Bachofen19 The recurrence of CIA does not usually occur very rapidly on re-challenge (i.e. within days), although this should not be taken to be indicative of a non-immune-mediated mechanism. In general, a subsequent neutropenic event on clozapine re-challenge tends to occur earlier and is more severe, consistent with an immune-mediated event. Reference Dunk, Annan and Andrews5 Further, a majority of patients treated with clozapine exhibit evidence of a systemic inflammatory response with raised cytokines, fever or paradoxical increases in WBC seen in the earlier stages of treatment. Reference R⊘ge, M⊘ller, Andersen, Correll and Nielsen20 Other potential causes hypothesised as primary or contributory factors to neutropenia associated with clozapine include benign ethnic neutropenia (BEN), which may be present in up to a quarter of individuals of African or Middle Eastern descent, Reference Haddy, Rana and Castro21 and the concurrent use of other drugs with potential to affect bone marrow function. Reference Flanagan and Dunk22
It is justifiable that there is widespread reluctance to instigate a clozapine re-challenge following an episode of neutropenia or agranulocytosis. We conclude that, in an appropriate environment and under the care of a highly specialised expert multidisciplinary team, it is appropriate to instigate a re-challenge with clozapine in selected patients with treatment-resistant schizophrenia or schizoaffective disorder following a careful risk–benefit analysis and collaborative discussion with the patient and families. The present case offers some data to help understand the mechanisms of CIA; although it is possible that there is not a common mechanism in all such cases, on the basis of this successful re-challenge, the suggestion that CIA is due to direct toxicity or an immune-mediated mechanism appears to be less likely. At the patient level, this fourth clozapine challenge represents an increase in quality of life for J.B. whose medical therapeutic options were otherwise exhausted. For future research directions, we propose that potential biomarkers of clozapine immune response be prospectively assessed in new clozapine starters. This would allow differentiation between those who develop CIA and those with immune tolerance; the prediction of those at risk of CIA; investigation of the relationship between CIA and neutropenia and plasma clozapine concentrations; identification of enzymes responsible for the bioactivation of clozapine; and phenotyping of peripheral leucocytes to identify cells which precipitate neutropenia or which allow for the development of immune tolerance.
Funding
S.S. is supported by a European Research Council Consolidator Award (grant number 311686), and some of this work was supported by a Medical Research Council New Investigator award to S.S. (grant number G0901868) and developed by the National Institute for Health Research (NIHR) Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. The funders of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report.





eLetters
No eLetters have been published for this article.