During weaning, piglets are exposed to many stressors, including separation from the sow and the loss of sow milk. These abrupt changes in the piglets' diet often result in disturbances in digestive function and gastrointestinal disease(Reference Pluske, Hampson and Williams1). Post-weaning diarrhoea is a multifactorial condition that occurs after weaning, but is characterised by the proliferation of enterotoxigenic Escherichia coli (ETEC)(Reference Hopwood, Pluske, Hampson, Mosenthin, Zentek and Zebrowska2).
The antibiotics that are used as growth promoters appear to act by reducing pathogenic bacteria and modifying the microflora in the gut of the animal(Reference Radostits, Leslie, Fetrow and Radostits3). However, dietary antibiotics lead to the presence of drug residues in edible animal products. Goblet cells containing sulfated mucin are less susceptible to bacterial degradation and have a more predominant function in the absence of an appropriately developed immune system(Reference Davis, Brown, Baker, Bos, Dirain, Halbrook, Johnson, Maxwell and Rehberger4). Because antibiotic supplementation has been shown to reduce the number of these cells(Reference Davis, Brown, Baker, Bos, Dirain, Halbrook, Johnson, Maxwell and Rehberger4), antibiotic supplementation may result in reduced innate immune function. Thus, considering both the safety of the consumer and the profitability for the farmer, alternatives to antibiotics are needed.
Bovine lactoferricin (LFC), which is released by gastric pepsin cleavage of bovine lactoferrin (LF)(Reference Bellamy, Takase, Yamauchi, Wakabayashi, Kawase and Tomita5–Reference Kuwata, Yip, Yamauchi, Teraguchi, Hayasawa and Tomita7), is located on the 17–41 residues of the N-terminal part of LF, and shows more potent bactericidal and fungicidal activity than the native protein LF(Reference Kuwata, Yip, Yamauchi, Teraguchi, Hayasawa and Tomita7–Reference Tomita, Takase, Bellamy and Shimamura10). LF and LFC in the following text refer to the bovine forms. Several studies on LFC and related synthetic peptides have demonstrated that it shows broad-spectrum activity against both Gram-positive and Gram-negative bacteria(Reference Bellamy, Takase, Wakabayashi, Kawase and Tomita8, Reference Groenink, Walgreen-Weterings, van't Hof, Veerman and Nieuw11–Reference Yoo, Watanabe, Watanabe, Hata, Shimazaki and Azuma13). In addition, LF has been shown to have antifungal(Reference Bellamy, Wakabayashi, Takase, Shimamura and Tomita14, Reference Wakabayashi, Hiratani, Uchida and Yamaguchi15), antiviral(Reference Valenti, Antonini, Siciliano, Rega, Superti, Marchetti, Ammendolia, Seganti, Shimazaki, Tsuda, Tomita, Kuwata and Perraudin16, Reference Andersen, Osbakk, Vorland, Traavik and Gutteberg17) and anti-tumour activity(Reference Yoo, Watanabe, Watanabe, Hata, Shimazaki and Azuma13, Reference Iigo, Kuhara, Ushida, Sekine, Moore and Tsuda18), and to play a regulatory role in the adaptive immune response, as well as having anti-inflammatory properties(Reference Brock19, Reference Levay and Viljoen20). In addition to LFC, the N1-domain of LF contains a second antimicrobial peptide, designated lactoferrampin (LFA), with features of a hydrophobic domain containing tryptophan that are characteristic for antimicrobial peptides(Reference Marieke, van der, Jasper, Kamran, Enno, Jan and Arie21). LFC and LFA have different antimicrobial spectra(Reference van der Kraan, Nazmi, van't Hof, Amerongen, Veerman and Bolscher22). The fusion of LFC with LFA broadens their antimicrobial spectra in vitro (Reference Tang23). However, there are no reports on the effect of dietary supplementation with LFC on growth and health parameters in weaned piglets. Here we report such effects by dietary supplementation with a fusion protein of cipB and LFC–LFA (cipB–LFC–LFA, molecular weight 16 300 Da) obtained by gene engineering technology at the Institute of Subtropical Agriculture (Chinese Academy of Sciences, Beijing, China)(Reference Tang23). cipB protein, a Photorhabdus luminescens subsp. Akhurstii crystalline inclusion protein with a molecular weight of 11 300 Da, was used as a positive control for cipB–LFC–LFA in the present study. Colistin sulfate (CS), an antibiotic that is popularly used in pig feed, was selected as an antibiotic treatment.
The primary objective of the present study was to determine the effect of dietary supplementation with the antimicrobial peptide bovine LFC–LFA replacing CS on growth performance, immune function, gut flora, intestinal mucosal morphology and antioxidant activity in piglets weaned at age 21 d and challenged with ETEC.
Materials and methods
Materials
cipB and cipB–LFC–LFA were provided by the Institute of Subtropical Agriculture (Chinese Academy of Sciences). They were obtained by the expression of the cipB and cipB–LFC–LFA genes in the expression host P. luminescens TZR001, as described previously(Reference Tang23), and their purity is 98 %. ETEC 0149, 0141 and 064 were purchased from the China Institute of Veterinary Drug Control (Beijing, China).
Animals, experimental design and diets
Sixty Landrace × Yorkshire castrated piglets were obtained from a local commercial swine herd on weaning at 21 d of age. The piglets were challenged with the ETEC mixture of three serotypes (0149, 0141 and 064) at 22 d of age. Each ETEC was cultured in tryptic soya broth (Shanghai Sangon Biological Engineering Technology & Service Co., Ltd, Shanghai, China) for 12 h, mixed and given to each pig as a single oral dose (109 cells) as described previously(Reference Davis, Brown, Baker, Bos, Dirain, Halbrook, Johnson, Maxwell and Rehberger4). Next day rectal swabs from each pig were plated on agar plates and scored as described previously: piglets scoring 0 (0 = have no β-haemolytic E. coli) were recorded as unaffected by diarrhoea; piglets scoring ≥ 1 ( ≥ 1 = have β-haemolytic E. coli) were recorded as affected by diarrhoea(Reference Montagne, Cavaney, Hampson, Lallès and Pluske24). Piglets scoring 3 (3 = mainly β-haemolytic E. coli) had been challenged with ETEC; eight piglets scored below 3, and so a further dose of E. coli was again given to these pigs (1010 cells) by oral medication. All pigs were weighed (5·42 (sem 0·59) kg) and assigned randomly into one of four groups (fifteen pigs per group). The experimental piglets were randomly allocated to different pens (one piglet per pen) in a temperature-controlled room, as described previously(Reference Tang, Yin and Nyachoti25). Feed and water were provided ad libitum.
The control diet formulated based on National Research Council requirements(26) contained 59·37 % maize, 25·00 % soyabean meal, 4·00 % fishmeal, 4·00 % dried whey powder, 5·00 % cream from bovine milk, 0·30 % limestone, 1·10 % monocalcium phosphate, 0·10 % anti-mould agent, 0·02 % antioxidant, 0·04 % vitamin premix (providing the following per kg of complete feed: 11 000 IU (3300 μg) vitamin A, 1100 IU (27.5 μg) vitamin D3, 22 IU (14.67 μg) vitamin E, 4 mg menadione as dimethylpyrimidinol bisulfate, 0·03 mg vitamin B12, 28 mg d-pantothenic acid, 33 mg niacin and 0·08 % choline chloride), 0·30 % trace mineral premix (providing the following per kg of complete feed: 165 mg Zn (ZnSO4), 165 mg Fe (FeSO4), 33 mg Mn (MnSO4), 16·5 mg Cu (CuSO4), 297 μg I (CaI2) and 297 μg Se (Na2SeO3)), 0·30 % salt, 0·06 % flavour, 0·23 % l-lysine-HCl (Tanke Industry Co. Ltd, Guangzhou, China), 0·05 % l-methionine (Tanke Industry Co. Ltd) and 0·05 % l-threonine (Tanke Industry Co. Ltd). The nutritional level of diets was as follows: 19·19 % crude protein, 0·583 % Ca, 0·464 % P, 1·198 % lysine, 0·397 % methionine, 0·850 % threonine and 14·3 MJ digestible energy/kg feed. CS, cipB and cipB–LFC–LFA were mixed with the vitamin premix, and then added to the diet, respectively. Each of the four groups of pigs was provided with one of the following diets: control (C), control supplemented with cipB at 100 mg/kg (C+B), control supplemented with cipB–LFC–LFA at 100 mg/kg (C+L) and control supplemented with CS at 100 mg/kg (C+CS).
The pigs were individually weighed on an empty stomach at the end of the experiment. Feed intake and diarrhoea (score ≥ 1 as described above) were recorded daily during the 3-week period. At the end of the experiment, 10 ml blood were drawn from the orbital sinus of five pigs per treatment with the closest body weight to obtain a serum sample and these animals were euthanised to evaluate intestinal microbiota and gut morphology. The animal protocol was approved by the Animal Care Committee of the Institute of Subtropical Agriculture.
Assay of serum immune and biochemical index, and liver biochemical index concentrations
Serum was obtained after blood centrifugation at 3000 rpm for 20 min and stored at − 20°C. Total IgA, IgM and IgG were determined in serum using radial immuno-diffusion kits (Triple J Farms, Bellingham, WA, USA). Serum Fe and total Fe-binding capacity were determined colorimetrically using reagent kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). GPx, NO synthase (NOS), peroxidase (POD), superoxide dismutase and total antioxidant content (T-AOC) were determined in serum and liver by colorimetric methods described with reagent kits supplied as above.
Analysis of gut microbiota
Intestinal digesta of the distal ileum, caecum and mid-colon were collected aseptically. Intestinal bacteria were evaluated using conventional culture methods as described previously(Reference Namkung, Gong, Yu and de Lange27). For conventional culture, intestinal digesta were diluted with sterile phosphate buffer solution. For lactic acid bacteria, De Man–Rogosa–Sharp (MRS) agar plates were incubated anaerobically at 37°C for 48 h (Oxoid, Basingstoke, Hants, UK). For bifidobacteria, lipovitellin–salt–mannitol–cysteine (LSM-C) agar plates were incubated anaerobically at 37°C for 48 h (Oxoid). For coliforms, MacConkey agar plates were incubated aerobically at 37°C for 24 h (Oxoid).
Analysis of gut morphology
Gut samples for the evaluation of histology were collected from the jejunum, 1 m posterior to the pyloric sphincter, and fixed in 10 % buffered formalin solution. Serial sections (5 μm) were cut and stained with periodic acid–Schiff(Reference McManus28) to evaluate villus morphology. Villus height was considered to be the distance from the crypt opening to the tip of the villus, while crypt depth was measured from the base of the crypt to the level of the opening(Reference Kik, Huisman and van der Poel29).
Data treatment and analysis
Diarrhoea percentage (%) = daily total number of piglets with diarrhoea of each treatment/(daily total number of piglets of each treatment) × 100.
Intestinal bacterial data were log-transformed (log 10 colony-forming units/g digesta).
Statistical analysis
All data are presented as means and with their standard errors. All data except for diarrhoea percentage from the experiment were subjected to one-way ANOVA using the general linear model (GLM) procedure of SAS statistical software (SAS Institute, Inc., Cary, NC, USA) according to a completely randomised one-factorial design. The diarrhoea percentages from the experiment were subjected to two-way (treatment and time) ANOVA using the GLM procedures of SAS statistical software (SAS Institute). Duncan's multiple-range test was performed to identify differences among groups. Significance was set at P < 0·05.
Results
Feed intake, growth performance and diarrhoea percentage
Piglets fed the C+L or C+CS diet had higher daily weight gain and daily feed intake (P < 0·05) than pigs fed the C or C+B diet (Table 1). Piglets fed the C+L or C+CS diet had lower faecal scores (P < 0·05) than piglets fed the C or C+B diet (Table 1). However, there were no differences in growth performance and faecal score between pigs fed the C+L and C+CS diets (P>0·05). There were also no differences in growth performance and faecal score between pigs fed the C and C+B diets. Feed conversion did not differ among the four groups (P>0·05) (Table 1). Compared with the C and C+B diets, dietary supplementation with cipB–LFC–LFA or CS increased recovery from diarrhoea (P < 0·05) (Fig. 1). The effect of dietary supplementation with cipB–LFC–LFA on the incidence of diarrhoea in piglets weaned at age 21 d was the same as that with CS (P>0·05) (Fig. 1).
Table 1 Effects of supplementary fusion protein cipB–lactoferricin–lactoferrampin (C+L) compared with an unsupplemented basal diet (C), a control of the basal diet supplemented with cipB alone (C+B) or the basal diet supplemented with the antibiotic colistin sulfate (C+CS) on growth performance in piglets weaned at age 21 d
(Mean values and pooled standard errors for fifteen pigs per treatment)
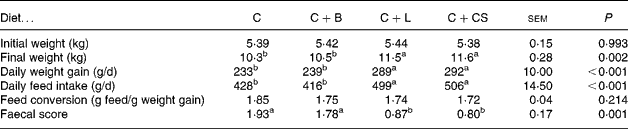
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).

Fig. 1 Effects of supplementary fusion protein cipB–lactoferricin–lactoferrampin (–Δ–) compared with an unsupplemented basal diet (–○–), a control of the basal diet supplemented with cipB alone (–□–) or the basal diet supplemented with the antibiotic colistin sulfate (– × –) on the incidence of diarrhoea in piglets weaned at age 21 d. Values are means with their standard errors (2·14) represented by vertical bars. a,b Lines with unlike letters were significantly different (P < 0·05).
Gut flora
Dietary supplementation with cipB–LFC–LFA or CS decreased the concentration of E. coli in the ileum, caecum and colon (P < 0·05) and increased the concentration of lactobacilli and bifidobacteria in the ileum, caecum and colon (P < 0·05) compared with the C and C+B groups (Table 2). The concentration of bifidobacteria in the ileum of the C+L group was lower than that in the C+CS group (P < 0·05) (Table 2). However, there were no differences in the concentration of E. coli and lactobacilli in the ileum, caecum and colon, or in the concentration of bifidobacteria in the caecum and colon between the C+L and C+CS groups (P>0·05) (Table 2). There were also no differences in the concentration of E. coli, lactobacilli and bifidobacteria in the ileum, caecum and colon between the C and C+B groups (Table 2).
Table 2 Effects of supplementary fusion protein cipB–lactoferricin–lactoferrampin (C+L) compared with an unsupplemented basal diet (C), a control of the basal diet supplemented with cipB alone (C+B) or the basal diet supplemented with the antibiotic colistin sulfate (C+CS) on the gut flora of piglets weaned at age 21 d (log 10 colony-forming units/g digesta)
(Mean values and pooled standard errors for five pigs per treatment)
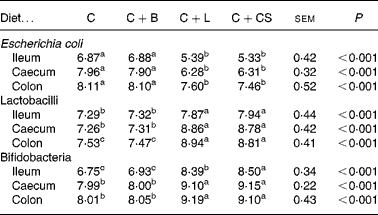
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Intestinal mucosal morphology
The villus height of the jejunum and ileum in the C+L group was greater than that in the C and C+B groups (P < 0·05), and the villus height:crypt depth ratio of the jejunum in the C+L group was greater than that in the C group (P < 0·05) (Table 3). However, there were no differences in crypt depth between the C+L group and the other groups (P>0·05) (Table 3). There were also no differences in crypt depth or villus height:crypt depth ratio in the jejunum between the C+L group and the C+CS group (P>0·05) (Table 3).
Table 3 Effects of supplementary fusion protein cipB–lactoferricin–lactoferrampin (C+L) compared with an unsupplemented basal diet (C), a control of the basal diet supplemented with cipB alone (C+B) or the basal diet supplemented with the antibiotic colistin sulfate (C+CS) on the intestinal mucosal morphology in piglets weaned at age 21 d
(Mean values and pooled standard errors for five pigs per treatment)
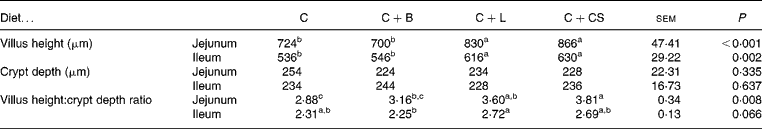
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Indices of antioxidant levels in serum and liver
As shown in Table 4, compared with the C and C+B groups, pigs fed C+L or C+CS had higher levels of GPx, POD and T-AOC in both serum and liver (P < 0·05). Serum POD and T-AOC in the C+L group were lower than those in the C+CS group (P < 0·05) (Table 4).
Table 4 Effects of supplementary fusion protein cipB–lactoferricin–lactoferrampin (C+L) compared with an unsupplemented basal diet (C), a control of the basal diet supplemented with cipB alone (C+B) or the basal diet supplemented with the antibiotic colistin sulfate (C+CS) on serum and liver concentration of glutathione peroxidase (GPx), nitrogen oxide synthase (NOS), peroxidase (POD), superoxide dismutase (SOD) and total antioxidant content (T-AOC) in piglets weaned at age 21 d
(Mean values and pooled standard errors for five pigs per treatment)
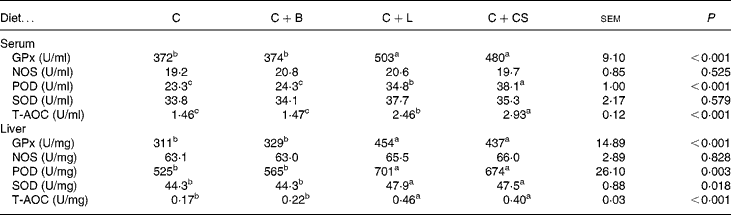
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Serum iron, total iron-binding capacity and immunoglobulins
Dietary supplementation with cipB–LFC–LFA or CS increased serum Fe, total Fe-binding capacity and IgA, IgG, and IgM relative to the animals in the C and C+B groups (P < 0·05) (Table 5). Serum IgM in the C+L group was lower than that in the C+CS group (P < 0·05) (Table 5).
Table 5 Effects of supplementary fusion protein cipB–lactoferricin–lactoferrampin (C+L) compared with an unsupplemented basal diet (C), a control of the basal diet supplemented with cipB alone (C+B) or the basal diet supplemented with the antibiotic colistin sulfate (C+CS) on serum Fe2+, total iron-binding capacity and immunoglobulins in piglets weaned at age 21 d
(Mean values and pooled standard errors for five pigs per treatment)
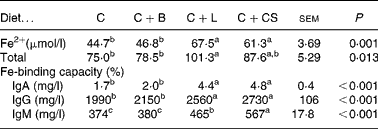
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Discussion
cipB–LFC–LFA is a fusion protein of the cipB protein and the LFC–LFA peptide which is released by pepsin in the animal stomach. Because the present results showed that dietary supplementation with cipB had no effects on growth performance, immune function, gut flora and intestinal mucosal morphology in piglets weaned at age 21 d and challenged with ETEC, we conclude that LFC–LFA is responsible for the observed effects on growth performance, immune function, gut flora and intestinal mucosal morphology.
The results also showed that dietary supplementation with LFC–LFA decreased the concentration of E. coli while it increased both lactobacilli and bifidobacteria in the gut. The observed faecal score and diarrhoea results were a reflection of this. The effects of the treatment on bacterial concentration are related to the occurrence of diarrhoea(Reference Callesen, Halas, Thorup, Bach, Kim, Mullan, Hampson, Wilson and Pluske30). Dietary supplementation with LFC–LFA reduced faecal score and the percentage of diarrhoea in the present experiment, so the increase in lactobacilli and bifidobacteria concentrations could result, at least partly, from increased DM concentration due to reduced diarrhoea.
Some previous studies have also reported that LF and LFC had positive effects on pathogenic and beneficial bacteria. Arnold et al. (Reference Arnold, Cole and McGhee31) found that LFC under 50 μm could directly kill E. coli (Reference Arnold, Cole and McGhee31). Ellison et al. reported that the concentrations of lactobacilli and bifidobacteria in infants fed breast milk were significantly greater than those in infants fed milk powder; this difference is believed to be related to the presence of LFC in breast milk(Reference Ellison, Giehl and Laforce32). LFC, a cationic peptide with broad antibacterial activity, shows membrane-disruptive properties(Reference Ulvatne, Haukland, Olsvik and Vorland33), and contains a high proportion of basic amino acid residues. It has been demonstrated that the highly cationic property of LF is responsible for the ability of LF to bind glycosaminoglycan(Reference Mann, Romm and Migliorini34), heparin and lipopolysaccharide(Reference Elass-Rochard, Roseanu, Legrand, Trif, Salmon, Motas, Montreuil and Spik35). It has been suggested that LF exerts its effect at the surface of the bacterial membrane(Reference Bellamy, Wakabayashi, Takase, Shimamura and Tomita14) and the positive charges within the peptide are thought to promote interaction with membrane components. As the number of positive charges increases, the number of interactions with negatively charged membrane components also increases(Reference Hwang, Zhou, Shan, Arrowsmith and Vogel9, Reference Nikaido and Nakae36). LFA has a hydrophobic domain containing tryptophan, which is involved in the insertion of hydrophobic peptides into cell membranes(Reference Marieke, van der, Jasper, Kamran, Enno, Jan and Arie21). A possible mechanism by which LFC–LFA exerts the effects observed in the present study is that the fusion of LFC with LFA enhances antimicrobial ability.
The structure of the villus–crypt architecture of the small intestine can reflect the health of the small intestine. After weaning, the height of gut villi in piglets is reduced and the depth of the crypt is increased(Reference Pluske, Hampson and Williams1). The present study showed that LFC–LFA can increase the height of the villi in the jejunum and ileum along with the villus height:crypt depth ratio in the jejunum and ileum. This suggests that LFC–LFA can promote the development of villus–crypt architecture of the intestinal mucosa. Humphrey et al. (Reference Humphrey, Huang and Klasing37) reported that the addition of rice that expressed the LF gene to a broiler diet increased the height of villi in the duodenum(Reference Humphrey, Huang and Klasing37). A toxin produced by ETEC in the gut can cause inflammation of the intestinal mucosa and diarrhoea(Reference Swidsinski, Ladhoff and Pernthaler38). Morphological changes in the small intestine, such as shortening of the villi and an increase in crypt depth, are closely related to the presence in the gut of the toxin produced by ETEC(Reference Gislason, Iyer, Hutchens and Lonnerdal39). The fact that dietary LFC–LFA increased the height of the gut villi in piglets may be related to the fact that LFC–LFA can decrease the concentration of E. coli and increase those of lactobacilli and bifidobacteria in the gut.
Oxidative stress is characterised by: (a) depletion of intracellular antioxidants (largely glutathione) and free-radical scavengers (vitamins E and C); (b) inhibition of the activity of various enzymes that contribute to the metabolism and detoxification of reactive oxygen species, such as GPx, glutathione reductase, glutathione transferase, catalase and superoxide dismutase; (c) increased production of reactive oxygen species (superoxide anion radical, H2O2, peroxyl radical, hydroxyl radical, NO, peroxynitrite radical, etc)(Reference Alessia, Paola and Alessandro40). Changes in the activities of antioxidant enzymes (GPx, NOS, POD and superoxide dismutase) can be considered as biomarkers of the antioxidant response(Reference Sies41). The present study showed that dietary LFC–LFA increased serum antioxidant enzyme activities (GPx, POD and T-AOC) and liver antioxidant enzyme activities (GPx, POD, superoxide dismutase and T-AOC) in piglets. LFC–LFA exerts antioxidant activity by binding Fe2+, which can activate oxygen free radicals. Therefore, the binding of LF and its peptides with Fe2+ in the gut can prevent lipid oxidation and the production of free radicals caused by Fe2+Reference Lindmark-Månsson and Åkesson42). The binding of LFC with Fe2+ can effectively decrease the transformation of peroxide to oxygen free radicals, and LFC can also reduce the oxidation of ascorbic acid and tryptophan(Reference Bihel and Birlouez-Aragon43). The enzymes work together to eliminate reactive oxygen species and small deviations of their physiological concentrations could have a dramatic effect on the resistance of cellular lipids, proteins and DNA to oxidative damage. These effects imply that the bioactive peptides of the fusion protein under study are taken up by the intestinal mucosa, a point which remains to be demonstrated.
Weaning stress can temporarily reduce growth(Reference Pluske, Hampson and Williams1, Reference Bruininx, van der Peet-Schwering, Schrama, Vereijken, Vesseur, Everts, den Hartog and Beynen44). The present study showed that dietary LFC–LFA can increase serum IgA, IgG and IgM levels, decrease the incidence of diarrhoea, and improve daily weight gain and daily feed intake in piglets. Debbabi et al. reported that bovine LF given orally to mice increased total IgA and IgG in intestinal secretions and LF-specific IgA and IgG in serum(Reference Debbabi, Dubarry, Rautureau and Tomé45). Prgomet et al. (Reference Prgomet, Prenner, Schwarz and Pfaffl46) also reported that calves given LF maintained a higher total IgG in serum compared with the post-colostral decline in control calves but did not affect total serum IgG by the end of the experiment(Reference Prgomet, Prenner, Schwarz and Pfaffl46). The present study showed that the changes in Ig concentrations observed with both C+L and C+CS are secondary to changes in the microbial populations, with decreased E. coli but increased lactobacilli and bifidobacteria. Shu et al. (Reference Shu, Qu and Gill47) demonstrated that feeding piglets with a probiotic Bifidobacterium lactis resulted in increased rotavirus-specific and E. coli-specific IgA, IgG and IgM in faecal supernatant fractions(Reference Shu, Qu and Gill47). This literature indicates that LF or its digestion products can influence the adaptive immune system, either directly or indirectly via alteration of the gut microflora, but the Ig responses are mainly elicited from intestinal mucosal cells, with increased secretion into the intestine and much less change, if any, in the systemic response. The improvement of daily food intake and daily weight gain by LFC–LFA was related to the fact that LFC–LFA can improve health parameters such as immune function and gut health in the present experiment. The improvement in growth performance by LFC–LFA can be attributed to the fact that LF and LFC have been shown to have antibacterial(Reference Bellamy, Takase, Yamauchi, Wakabayashi, Kawase and Tomita5, Reference Arnold, Cole and McGhee31) and antiviral activities(Reference van der Kraan, Nazmi, van't Hof, Amerongen, Veerman and Bolscher22), regulate the immune response(Reference Trif, Guillen, Vaughan, Elfer, Brewer, Roseanu and Brock48, Reference Bavaye, Elass, Mazurier, Spik and Legrand49) and improve the absorption of Fe(Reference Callesen, Halas, Thorup, Bach, Kim, Mullan, Hampson, Wilson and Pluske30, Reference Adamik and Walszczyk50, Reference Lonnerdal and Iyer51). Similarly, LFC–LFA might improve growth performance in piglets weaned at age 21 d challenged with ETEC through an antibacterial effect, the regulation of immune function, improvement of the absorption of Fe and a reduction in the incidence of diarrhoea.
Based on effect of LFC–LFA or CS on growth performance, immune function, gut flora, intestinal mucosal morphology and antioxidant activity in piglets weaned at age 21 d challenged with ETEC, the present results suggest that LFC–LFA could replace the antibiotic CS. Technology for the production of LFC–LFA has already been established. The pasteurising conditions during processing of LFC–LFA-supplemented products have also been assessed. It is now possible to supply a larger amount of LFC–LFA than the current supply. Using this product, various beneficial effects of LFC–LFA as a feed additive have been demonstrated and this has enabled us to use LFC–LFA in a large number of fields. However, regarding the safety of the consumer, possible side effects of LFC–LFA as a GM organism's product on both target animals and humans remain to be evaluated further before large-scale application. In addition, the use of LFC–LFA in combination with other additives needs to be considered.
Acknowledgements
This research was jointly supported by grants from the Program for the National Basic Research Program of China (2004CB117502), National Natural Science Foundation of China (NSFC) (30700581, 30771558 and 30671517), the Chinese Academy of Science Knowledge Innovation Project (KSCX2-YW-N-022), Fund of Agricultural Science and Technology Outcome Application (2006GB24910468), the Program for the Latest Research Field of Youth Talents in Institute of Subtropical Agriculture, the Chinese Academy of Sciences (ISACX-LYQY-QN-0701), Special Research Fund Scholarship Gainer of the Chinese Academy of Sciences (0723022110), Hunan Province Key Project (2007FJ1003), National Scientific and Technology Supporting Projects (2006BAD1 2B07-6, 2006BAD12B02-5-2), Guang Dong Province Project (2006B200330005) and Program for Changjiang Scholars and Innovative University Research Team (65 292 and IRT0540). The authors have no conflicts of interest to declare. Y. Y. was in charge of the whole trial. Y. Z. gave the original idea of testing this fusion peptide in weaned pigs. Z. T. conducted the animal experiment and wrote the whole of the paper. R. H., Z. S., L. L., T. L., W. C., X. K., M. G. and Q. T. assisted with the animal trial and chemical analyses.








