CVD still remains the major contributor to the global burden of disease worldwide( Reference Pedersen, James and Brouwer 1 ). Even though there has been substantial reduction in CVD mortality over the last 30 years, new reports show an increase in acute myocardial infarction among the younger population in Norway, and similar observations have been reported from other countries as well( Reference Sulo, Vollset and Nygard 2 , Reference Kostis, Deng and Pantazopoulos 3 ). Elevated plasma LDL-cholesterol is an established risk factor of CVD( Reference Taylor, Huffman and Macedo 4 ), and dietary fatty acids play a significant role in modulating plasma LDL-cholesterol, thereby influencing the risk of CVD( Reference Schwab, Lauritzen and Tholstrup 5 – Reference Stone, Robinson and Lichtenstein 8 ). In particular, there is strong evidence that replacing SFA with PUFA will reduce the risk of CVD( Reference Mozaffarian, Micha and Wallace 9 , Reference Jakobsen, O’Reilly and Heitmann 10 ). However, controversy still exists about beneficial v. potential harmful effects of n-6 PUFA as n-6 PUFA has been suggested to promote inflammation( Reference Fritsche 11 , Reference Simopoulos 12 ).
Adherence to a healthy Nordic diet based on the Nordic nutrition recommendations has previously been shown to have beneficial effects on blood lipids among subjects at risk of CVD( Reference Uusitupa, Hermansen and Savolainen 13 – Reference Adamsson, Reumark and Fredriksson 15 ). However, the extent of food changes needed to achieve these effects is less explored. In order to increase compliance to dietary fat intake recommendations in the general population, it is important that one can achieve this with relatively small dietary changes, leading to improved lipid profile. A few, if any, double-blind, randomised controlled trials have investigated the effects of exchanging a combination of a few commercially available food items with similar food items with improved fat quality on blood lipids and inflammatory markers. The aim of the present study was therefore to investigate the effect of exchanging regularly consumed commercially available key food items with similar food items with improved fat quality (replacing SFA with PUFA, mostly n-6 fatty acids but also n-3 fatty acids) in the daily diet for 8 weeks in a double-blind, randomised controlled trial, on serum total cholesterol, LDL-cholesterol and inflammatory markers among non-statin-treated healthy subjects with moderate hypercholesterolaemia.
Methods
Subjects
Healthy adults aged 25–70 years were recruited by advertisements in local newspapers and local area community flyer postings. Inclusion criteria were high-sensitive C-reactive protein (hs-CRP)<10 mg/l and serum total cholesterol of 5–7·8 mmol/l (for those between 50 and 70 years), 5·0–6·9 mmol/l (for those between 30 and 49 years) and 5·0 and 6·1 mmol/l (for those between 25 and 29 years). As we wanted to include subjects with cholesterol values at the upper range of normal plasma cholesterol values and as these ranges vary among age groups, we used the age-specific cholesterol concentrations that were set by the commercial laboratory we used for analysis to define the normal range. Furthermore, all subjects had to have LDL-cholesterol≥3·5 mmol/l and fasting TAG≤2·6 mmol/l, a stable body weight during the last 3 months (±5 %), BMI between 20 and 35 kg/m2, willing to eat all the study products daily during the study and not take fish oil or other dietary supplements for the last 3 weeks before study start and during the study. Stable use of medications against high blood pressure was allowed. Exclusion criteria were use of lipid-lowering, anti-inflammatory (except drugs against allergy), anti-diabetes or weight-reduction drugs or planned weight reduction. In addition, subjects with chronic illnesses such as kidney, liver and other endocrine diseases and CHD during the last 3 months were excluded. Further exclusion criteria were increased thyroid hormones (T3>6·5 pmol/l and T4>23 pmol/l) or thyroid-stimulating hormone (TSH) (TSH>4 mU/l), fasting blood glucose>6·0 mmol/l, increased alanine aminotransferase (ALAT) and aspartate aminotransferase (ASAT) (>3× upper reference value), hormonal treatment (except stable dose of thyroxin and oral contraceptives), pregnancy and lactation, use of plant sterols within 3 weeks before the study start and during the intervention, and high alcohol intake (>40 g/d).
All subjects were advised to maintain their usual lifestyle habits throughout the study, without changing their physical activity habits, alcohol consumption and dietary habits, except for the inclusion of study products. All subjects were weighed during the study (every 2nd week), and if there was a change a registered dietitian gave advice to maintain weight. The study was conducted according to the guidelines laid down in the Declaration of Helsinki. All subjects gave their written informed consent, and the Regional Ethics Committee for Medical Research in South East Norway approved the study. The study was registered at ClinicalTrials.gov (ClinicalTrials.gov Identifier: NCT 01679496).
Study design
An 8-week, double-blind, randomised, controlled, parallel-designed trial was conducted at the Oslo and Akershus University College of Applied Sciences and the University of Oslo, Norway, from July 2012 until April 2014. A telephone interview and a screening visit were performed to check eligibility criteria of the participants entering the study. After screening 701 subjects, 115 were randomised, 108 received allocated interventions, 100 completed the study and ninety-nine were analysed. Subjects lost during follow-up and the numbers of subjects included in the statistical analysis are outlined in the flow chart (Fig. 1). The fifteen dropouts did not differ from the subjects who completed the study with respect to age and lipid profile; however, BMI was slightly higher among the subjects who dropped-out (27·1 and 25·0 kg/m2, respectively) and more men than women dropped out (nine and six, respectively).

Fig. 1 Flow chart of the participants. C group, control diet group; Ex group, experimental diet group.
Before the baseline visit, all subjects participated in a 2-week run-in period (starting 1–4 weeks after the screening visit) in which all subjects had to include the control food items in their daily diet. A registered dietitian delivered the food items and gave them instructions on how to introduce the food items in their regular diet, and reminded them to eat at least the minimum amount of each product (Table 1). At baseline, subjects were randomly assigned and stratified by sex and age into one of two intervention groups (1:1 ratio), where the control diet group (C-diet group) continued with the control food items and the experimental diet group (Ex-diet group) received experimental food items. The experimental food items were the same type of food products as the control food items but with a different fat composition (SFA replaced with mostly n-6 PUFA) (Table 1). Clinical and blood laboratory assessments were performed at baseline and after 8 weeks of follow-up. Every 2nd week, the participants received food items at the study centre, and body weight was recorded.
Table 1 Minimum daily intake of food items and the PUFA and SFA content

C-diet group, control diet group; Ex-diet group, experimental diet group.
* Two types of food items were delivered to the participants, and they could choose which among them they wanted to eat daily.
† The products have exchanged the dairy fat with vegetable oils (sunflower and rapeseed).
‡ The participants could include either of these products in their daily diet, in total 120 g.
Study products
In the present study, we wanted to investigate the effect of consumption of commercially available food items with different fat quality by exchanging regular food items used habitually by the population in Norway. The control food items were chosen based on Norwegian sales statistics and were among the most sold products within each food category (Nielsen market trends 2011, Norway). The minimum amount of intake of each food item to be consumed was based on data from the National nutrition survey in Norway( 16 ). The experimental food items were similar, commercially available food items (Mills DA, Oslo, Norway) with an improved fatty acid composition( Reference Muller, Kirkhus and Pedersen 17 ). In these products, a part of the saturated fat was replaced by sunflower and rapeseed oils. Rapeseed oil is rich in n-6 PUFA, n-3 PUFA and MUFA and is a typical Nordic product. In combination with sunflower oil, rapeseed oil is known to have an effect on cholesterol based on the equation developed by Muller et al.( Reference Muller, Kirkhus and Pedersen 17 ). The experimental food items were therefore particularly rich in n-6 PUFA, but also contained n-3 PUFA and MUFA, thereby increasing the total unsaturated fatty acid content. The study products included liquid margarine, margarine-based spread, oil, liver pate, cheese, bread, bun or baguette, muesli cereals or flakes, cream and crème fraîche, and mayonnaise. The cereals and bread/baguettes/buns were included as they also had an improved fatty acid composition (higher amount of n-6 PUFA) because of the presence of added plant oil and ingredients (e.g. oat and sunflower seeds). In general, the products were mainly used for breakfast, lunch and supper, and oils, butter/margarine, cream and crème fraîche were used for cooking. An overview of the products, the SFA and PUFA contents and the daily minimum amount of each product that the participants were instructed to consume is shown in Table 1. In addition, in the food items of the C-diet group, the fibre content in the bread, buns and baguettes was 5·1 g/100 g, and in the muesli cereals and flakes the fibre content was 7 g/100 g and 3 g/100 g, respectively. In the food items of the Ex-diet group, the fibre content of the bread, bun and baguettes was 10 g/100 g and of cereals was 18 g/100 g. In addition, the amount of β-glucan was estimated to be 1·83 g/d in the Ex-diet group based on minimum intake of bread/baguettes/buns and cereals.
The fatty acid composition of the control food items and the experimental food items differed particularly with respect to PUFA and SFA contents (Table 2). The total content of PUFA was 5·1 and 14·4 g/d, in the control food items and in the experimental food items, respectively, based on the minimum intake described in Table 1. In the control food items, the total amount of n-6 fatty acids was 4·2 g/d, whereas the content in the experimental food was 12·9 g/d. The total amounts of SFA in the control food items and the experimental food items were 19·2 and 5·7 g/d, respectively.
Table 2 The fatty acid composition of food items delivered to the subjects based on minimum daily intakeFootnote *
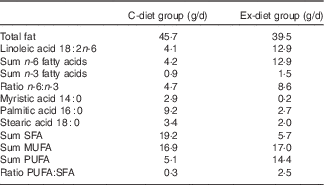
C-diet group, control diet group; Ex-diet group, experimental diet group.
* The data are given as mean values of the two analysis for each food product and added together with all food items in each food group based on the minimum daily intake of each product.
Blinding and randomisation
The randomisation list was created by an external statistician (LINK Medical, Norway), using four strata – females younger than 50 years, females aged 50 years or older, males younger than 50 years and males aged 50 years or older – and a block size of 6. The SAS system (R) was used to generate the list. The randomisation allocations, selected consecutively, were sent to the food packaging personnel on demand, according to strata information of newly recruited subjects.
All food items were packed in boxes outside the study centre and only the people packing the boxes knew who would be allocated to which group. Each box was labelled with an ID number, and the closed boxes with the food items were delivered to the study centre. At the study centre, the subjects received the ID-labelled boxes. Thus, the study was double-blinded as neither the subjects nor the nutritionist who handed out the food boxes knew which group the subjects were assigned to.
Dietary assessments and compliance
All subjects who received allocated intervention completed a four-day, pre-coded food diary at the beginning and at the end of the intervention period. The diary used was originally developed for use among Norwegian children and adolescents( Reference Lillegaard, Loken and Andersen 18 – Reference Lillegaard and Andersen 20 ). The diary included >270 food items grouped together according to the typical Norwegian meal pattern( Reference Lillegaard, Loken and Andersen 18 – Reference Lillegaard and Andersen 20 ). Each food group was supplemented with open-ended alternatives. Along with the food diary, each participant received a validated photography booklet that contained thirteen series of coloured photographs, each with four different portion sizes ranging from small to large. Food amounts were estimated in predefined household units (e.g. glasses, pieces or tablespoons) or from photographs. We included specific pre-coded questions about the control/experimental food items used in the intervention and the participants had to mark when they used the control/experimental food items and how much was eaten.
The diaries were scanned using the Teleform program, version 6.0 (InfoShare Solutions AS). Daily intake of energy was computed using the food database and software system KBS (KBS, version 7), developed at the Department of Nutrition, University of Oslo( Reference Rimestad, Løken and Nordbotten 21 ). Compliance was assessed by using a checklist where all the products consumed each week were registered. Each food group was ranked equally, and the percentage (%) of intake based on the minimum amounts to be consumed per day was calculated during the whole study period. A person was considered compliant to the protocol if the average of the percentage compliance of all food items eaten during the study was 80 %. Only three of the subjects were not compliant to the protocol. The overall compliance in both groups was similar (123 and 112 %; Ex-diet group and C-diet group, respectively). The compliance to the different food items was also quite similar in all food categories between the groups and is described in detail below: bread/baguettes/buns (114 and 110 %), muesli cereals or flakes (102 and 78 %), cheese (107 % in both groups), liver pate (220 and 197 %), butter-based spread (104 and 100 %), frying fat (104 and 90 %), oil (95 and 93 %), mayonnaise (129 and 108 %), cream (124 and 110 %) and crème fraîche (131 and 129 %), for the Ex-diet group and C-diet group, respectively.
Blood sampling and laboratory analysis
The day before blood sampling, subjects were asked to refrain from alcohol consumption and vigorous physical activity. Venous blood samples were drawn after an overnight fast (≥12 h). Serum was obtained from silica gel tubes (Becton Dickinson Vacutainer Systems) and kept at room temperature for at least 30 min, until centrifugation (1500 g , 15 min). Plasma was obtained from EDTA tubes (BD Vacutainer; Becton Dickinson Vacutainer Systems), immediately placed on ice and centrifuged within 10 min (2000 g , 4°C, 15 min). Fasting serum hs-CRP, total cholesterol, LDL-cholesterol, HDL-cholesterol, lipoprotein (a) (Lp(a)), apoA and apoB, TAG, glucose, insulin, ALAT and ASAT were measured by standard methods at a routine laboratory (Fürst Medical Laboratory). Serum IL-6 was measured using Quantikine high sensitivity and soluble TNF (sTNF) receptor 1 was measured by Quantikine ELISA kit from R&D Systems according the manufacturer’s instructions. Interferon (IFN) γ was measured by high-sensitivity ELISA from eBioscience according the manufacturer’s instructions.
Total plasma fatty acid analysis
A sample of 40 µl EDTA plasma was added to 100 µl internal standard (1,2-diheptadecanoyl-sn-glycero-3-phosphatidylcholine) and 800 µl 3 n-methanolic HCl, mixed and heated at 80°C for 120 min. The resulting fatty acid methyl esters were added to 500 µl hexane and 300 µl 3 m-KOH in water. GC analysis of the hexane phase was performed directly from this solution according to the method described below. Analyses were performed using a 7890 n GC with a split/splitless injector, a 7683B automatic liquid sampler and a flame ionisation detector (Agilent Technologies). Separations were performed with a SP-2380 (30 m×0·20 mm i.d.×0·25-µm film thickness) column from Supelco. The concentration of the individual fatty acids was measured as µg fatty acid/ml plasma (Vitas Analytical Service) and presented as percentage of total fatty acids.
Clinical assessment
Body weight (kg) was measured on a digital scale in light clothing without shoes. Blood pressure was measured in a sitting position on the right arm after 15 min of rest. Two measurements were obtained with a minimum 3-min interval, and the average value was calculated. If the two values differed >5 mmHg, a third measurement was obtained and the average of the two most similar values was used.
Fatty acid analysis in food items
A duplicate of all test food items distributed to the participants was collected at three time points during the study. All duplicates were kept at −20°C until the end of the study, and fatty acid composition was measured at a routine food analysis laboratory (Eurofins). The mean value of two measurements (start and end of study) was calculated.
Statistical analysis
Power calculations estimated that 180 subjects (including a 20 % dropout rate) were required for obtaining 80 % power with a type I error of 5 % to detect a difference between the two groups of 8 % in LDL-cholesterol at the end of the study. Data are presented as mean values and standard deviations if normally distributed or as medians (25th–75th percentile) if not normally distributed. Paired t test was used to assess change within groups and independent t tests to compare changes between groups when the data were normally distributed. Wilcoxon’s signed-rank test was used to assess change within groups, and the Mann–Whitney U test was used to compare changes between groups when the data were not normally distributed for continuous variables. χ 2 Test and Fischer’s exact test were used for categorical variables depending on the expected cell frequencies. P<0·05 was regarded as significant. IBM SPSS Statistics, version 20 was used for statistical analysis.
Results
Of 115 randomised subjects, 108 subjects received allocated intervention, and eight subjects (five in the Ex-diet group and three in the C-diet group) were lost to follow-up. One subject was excluded from the analysis in the Ex-diet group because of missing blood analysis at the end of the study, leaving ninety-nine subjects for the final analysis (forty-one men and fifty-eight women) (Fig. 1). There were no significant differences between the C-diet group and the Ex-diet group at baseline (Table 3).
Table 3 Baseline characteristics after randomisation to diets of those who completed the study (Mean values and standard deviations; numbers and percentages; medians and 25–75th quartiles)

C-diet group, control diet group; Ex-diet group, experimental diet group; Lp(a), lipoprotein (a); hs-CRP, high-sensitive C-reactive protein; ASAT, aspartate aminotransferase; ALAT, alanine aminotransferase.
apoB; n 46 (Ex-diet group); hs-CRP; n 51 (C-diet group).
Dietary changes
The dietary registrations showed no change in dietary intake within each group during the intervention period, except for fibre intake, which increased in the Ex-diet group from the first to the last week (data not shown). The mean dietary intake during the intervention for each group showed that 6·5% energy (E%) of SFA was replaced by PUFA in the Ex-diet group compared with the C-diet group (Table 4). The E% from fat was similar in both groups; however, there was a difference in the E% of protein and carbohydrates between the groups. The E% from protein was slightly higher and the E% from carbohydrates was slightly lower in the Ex-diet group.
Table 4 Dietary intake during the intervention (Mean values and standard deviations, average of two dietary registrations; median and 25th–75th percentile)
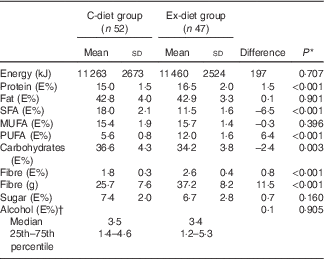
* Independent t test was used for normally distributed variables.
† Mann–Whitney U test was used for not normally distributed variables.
Total plasma fatty acids
After 8 weeks of intervention, the inclusion of Ex-diet food items caused a significant change in total plasma fatty acids compared with the C-diet group (Table 5). In particular, C12 : 0, C14 : 0 and C16 : 0 fatty acids were significantly reduced in the Ex-diet group compared with the C-diet group (P<0·001 for all). In addition, the n-6 PUFA, linoleic acid (LA, C18 : 2n-6) and arachidonic acid (AA, 20 : 4n-6), significantly increased in the Ex-diet group compared with the C-diet group (P<0·001 for LA and P=0·001 for AA). Oleic acid (C18 : 1n-9) and DPA (C22 : 5n-3) were significantly reduced in the Ex-diet group compared with the C-diet group (P<0·001 for both).
Table 5 Proportion of plasma fatty acids (% of total fatty acids) at baseline and end of the intervention (Mean values and standard deviations)

C-diet group; control diet group; Ex-diet group, experimental diet group.
* P1: baseline v. end of study C-diet group; paired t test.
† P2: baseline v. end of study Ex-diet group; paired t test.
‡ P3: between group change: independent t test.
§ Wilcoxon’s signed-rank test was used as the data were not normally distributed.
|| n 41 in the Ex-diet group and n 48 in C-diet group due to samples below detection limit.
Effects on total cholesterol and LDL-cholesterol
The inclusion of food items with improved fat quality in the Ex-diet group caused significant lowering of serum total cholesterol (P<0·001), LDL-cholesterol (P<0·001), HDL-cholesterol (P=0·006) and apoB (P<0·001) after 8 weeks, compared with the C-diet group (Table 6). The percentage change in total cholesterol and LDL-cholesterol within the Ex-diet group was −8 and −9 %, receptively, and the percentage change between the two groups was −9 and −11 % in total cholesterol and LDL-cholesterol, respectively. No significant changes in serum total cholesterol, LDL-cholesterol, HDL-cholesterol, apoA and apoB were observed within the C-diet group. The intervention did not affect plasma levels of Lp(a).
Table 6 Clinical and biochemical values at baseline and at the end of the study (Mean values and standard deviations; medians and 25th–75th percentiles)

C-diet group, control diet group; Ex-diet group, experimental diet group; Lp(a), lipoprotein (a); hs-CRP, high-sensitive C-reactive protein; sTNFR1, soluble TNF receptor 1; IFN, interferon; BP, blood pressure.
* P1: baseline v. end of study C-diet group; paired t test or Wilcoxon’s signed-rank test.
† P2: baseline v. end of study Ex-diet group; paired t test or Wilcoxon’s signed-rank test.
‡ P3: between group changes: independent t test or Mann–Whitney U test.
§ n 31 in C-diet group and n 25 in Ex-diet group.
Effects on inflammatory markers
Increased intake of n-6 PUFA in the diet had no negative effect on plasma levels of the inflammatory markers hs-CRP, IL-6, soluble TNF receptor 1 (sTNFR1) and IFN-γ neither within nor between groups (Table 6).
Effects on other cardiovascular risk factors
No changes in fasting serum glucose, insulin, HbA1c and blood pressure were observed either within groups or between groups (Table 6). We did, however, detect a small but significant increase in body weight of 0·4 kg within the C-diet group (P=0·006), leading to a significant difference between the two groups (P=0·044), even though no effect on body weight was seen in the Ex-group (P=0·885). In addition, we observed a slight increase in fasting serum TAG in the C-diet group (P=0·004) leading to a significant difference in the change in serum TAG between the groups (P=0·002).
Discussion
In the present study, we showed that by exchanging a combination of a few commercially available regularly consumed key food items in the daily diet, by increasing mostly n-6 PUFA intake and reducing the SFA intake, serum total cholesterol and LDL-cholesterol are reduced by 9 and 11 %, respectively, compared with a control diet. Importantly, no detectable negative effect was observed on circulating inflammatory markers.
In order to achieve long-lasting effects of dietary changes, the changes have to be easy to implement. In a recent study, it has been shown that replacing only food items included to obtain a prudent breakfast based on Nordic foods for 12 weeks was not enough to have any effect on plasma total cholesterol and LDL-cholesterol( Reference Adamsson, Reumark and Marklund 22 ). In contrast, large reductions in LDL-cholesterol were observed in two other studies where all foods were prepared and supplied throughout the study( Reference Adamsson, Reumark and Fredriksson 15 , Reference Iggman, Gustafsson and Berglund 23 ). In the SYSDIET (systems biology in controlled dietary interventions and cohort studies) study, which was a Nordic multi-centre study focusing on a healthy Nordic diet based on the Nordic Nutrition recommendations, non-HDL-cholesterol was significantly reduced in the healthy Nordic-diet group compared with the control group( Reference Uusitupa, Hermansen and Savolainen 13 ). In the present study, the participants were supplied with a few regularly consumed commercially available key food items used in a Norwegian diet, primarily used for breakfast, lunch and supper, in addition to oil, cream/crème fraîche and butter/margarine for use in dinner meals. No specific advices were given for dinner choices. All the participants in the present study had high adherence to the intervention with 97 % of all subjects complying with the intervention with respect to intake of test products. This suggests that the amount of the products used in the present study is manageable to eat on a daily basis and easy to implement. These food exchanges should therefore be possible to achieve in the general population, and shows that it is possible to make beneficial replacements of dietary fat sources in the diet without interfering too much with dietary habits. Our results are in line with the results observed in the Dietary Intervention and VAScular function study( Reference Weech, Vafeiadou and Hasaj 24 , Reference Vafeiadou, Weech and Altowaijri 25 ), where successful implementation of a food-exchange model achieved the dietary target intake for exchanging SFA with MUFA or n-6 PUFA, leading to reduced plasma total and LDL-cholesterol in a free-living population( Reference Weech, Vafeiadou and Hasaj 24 , Reference Vafeiadou, Weech and Altowaijri 25 ).
The E% from fat was similar in both groups; however, there was a difference in the E% of protein and carbohydrates between the groups. The E% from protein was slightly higher and the E% from carbohydrates was slightly lower in the Ex-diet group. The test products in the Ex-diet group contained in gram more protein and carbohydrates, particularly the bread, which contained twice the amount of protein in the Ex-diet group compared with the C-diet group. This may partly explain the differences observed in protein intake given in E%. As total fat intake (E%) was similar, this will lead to an increase in E% from carbohydrates in the C-diet group compared with the Ex-diet group.
Even though there is strong evidence that replacing SFA with PUFA will reduce the risk of CHD( Reference Mozaffarian, Micha and Wallace 9 , Reference Jakobsen, O’Reilly and Heitmann 10 ), recent data have questioned this association( Reference Chowdhury, Warnakula and Kunutsor 26 , Reference Puaschitz, Strand and Norekval 27 ), and the intake of SFA has increased in the general population( Reference Micha, Khatibzadeh and Shi 28 ). In the present study, we particularly focused on increasing the intake of n-6 PUFA by substituting SFA in food items. The largest difference in the two groups was an increased intake of PUFA by 6·5 E% with a subsequent 6·0 E% reduction in SFA in the Ex-diet group compared with the C-diet group, respectively. These changes were also reflected in the plasma fatty acid profile after the intervention. Previously it has been estimated that a 10 % (approximately 0·6 mmol/l) reduction in LDL-cholesterol is associated with a 27 % reduction in CVD risk( Reference Law, Wald and Thompson 29 ), suggesting that the observed LDL-cholesterol-lowering effect of 11 % achieved after consuming the Ex-diet in the present study is clinically relevant. If dietary changes to lower LDL-cholesterol are successfully implemented earlier in life, the CVD risk reduction is even greater than this estimate( Reference Law, Wald and Thompson 29 ). In a recent drug trial (Improve-it study) including more than 18 000 patients after acute coronary syndromes, where Ezetimibe treatment was added on to statin treatment, the difference in plasma LDL-cholesterol between the statin and statin+Ezetimibe group was 0·4 mmol/l (1·8 v. 1·4 mmol/l), leading to a significant lowered CVD event rate( Reference Cannon, Blazing and Giugliano 30 ). In the present dietary study, we found that by exchanging a few regularly consumed key food items with improved fatty acid composition, one can obtain similar reduction in plasma cholesterol, indicating that dietary changes may be as effective and easy to implement as add-on statin therapy and may be an alternative for hypercholesterolaemic subjects.
In the present study, we included food items rich in rapeseed oil and sunflower oil. These oils are particularly rich in LA but contain also n-3 fatty acids and MUFA. Despite the increased intake of n-6 PUFA, we did not observe any increase in plasma levels of well-established inflammatory markers such as hs-CRP, sTNFR1, IFN-γ or IL-6 in the Ex-diet group, and our data therefore do not support evidence for any potential harmful effects of increased intake of n-6 PUFA with regard to inflammation( Reference Fritsche 11 , Reference Simopoulos 12 , Reference Johnson and Fritsche 31 ). Our data are in line with two recent RCT, which did not observe any change in inflammatory markers when intake of n-6 PUFA replaced SFA( Reference Vafeiadou, Weech and Altowaijri 25 , Reference Bjermo, Iggman and Kullberg 32 ). Moreover, a high intake of LA (8 % of total energy intake) is inversely associated with total and CHD mortality( Reference Wu, Lemaitre and King 33 ), and a recent systematic review and meta-analysis of cohort studies showed that increased LA consumption is associated with reduced risks of CHD events and CHD-related deaths, independently from other dietary factors( Reference Farvid, Ding and Pan 34 ). Our data are thus in line with these cohort studies, showing a beneficial effect of replacing SFA with PUFA and in particular LA in the diet.
In the present study, the major changes in plasma fatty acid composition were an increase in LA and a decrease in palmitic acid. However, we also observed an increase in AA, and a decrease in the plasma EPA, DPA (C22 : 5n-3) and DHA (C22 : 6n-3) in the Ex-diet group (Table 5). The increased intake of LA in the Ex-diet group may have increased desaturase and elongase enzyme activities, leading to a higher synthesis of AA. As LA and α-linolenic acid (ALA, C18 : 3n-3) compete for the same enzymes, a possible explanation could be that increased synthesis of AA from LA would lead to reduced synthesis of EPA from ALA, subsequently leading to reduced plasma levels of DPA and DHA. Indeed, the same observation with reduced DPA levels as in the present study was reported in a recent study by Weech et al.( Reference Weech, Vafeiadou and Hasaj 24 ) who developed a flexible food-exchange model to investigate the effects of substituting SFA with n-6 PUFA on CVD risk, thus supporting the findings of the present study.
The observed total cholesterol-lowering effect corresponded with the predicted total cholesterol-lowering effect calculated using an equation developed by Muller et al.( Reference Muller, Kirkhus and Pedersen 17 ) to calculate the expected change in total serum cholesterol based on the changes in fatty acids in products in the Ex-diet food group. By using this equation, an expected change in total serum cholesterol relative to control was calculated to be 0·43 mmol/l. In the present study, we observed a reduction in total cholesterol of 0·6 mmol/l in the Ex-diet group and no change in serum cholesterol in the C-diet group. This indicates that approximately 72 % of the change in total cholesterol observed in the present study may be due to the changes in fat intake. The food items in the two groups contained different amounts of fibre. The amount of β-glucan was estimated to be 1·83 g/d in the Ex-diet group based on minimum intake of bread/baguettes/buns and cereals. It has previously been shown that intake of oat β-glucan≥3 g/d reduces LDL-cholesterol and total cholesterol relative to control by 0·25 mmol/l and 0·30 mmol/l, respectively( Reference Whitehead, Beck and Tosh 35 ), which is in line with our results showing a possible additional fibre effect on plasma cholesterol (0·17 mmol/l) considering the lower intake of oat β-glucan in the present study (1·83 g/d). Unfortunately, we do not have information about the β-glucan content in the control food items. However, by using a linear multivariable regression model, changes in plasma fatty acid composition (16 : 0 and 18 : 2n-6) between baseline and end of the intervention correlated with changes in LDL-cholesterol. The regression models were adjusted for mean fibre intake during the intervention; however, adjustments for fibre intake did not change the model, suggesting that the main effect was mediated by the change in fatty acid composition (data not shown).
There was a significant increase in body weight in the control group during the intervention, leading to a significant difference in body weight change between the groups. As the only change in lipid profile in the control group during the intervention was an increase in plasma TAG, we performed linear regression analysis to test whether the change in serum TAG in the control group was due to body weight changes in this group. However, we observed that the weight change in the C-group did not make a unique statistical contribution to the prediction of the change in TAG levels (β=0·066 and P=0·653).
The strengths of our study include the randomised and double-blind study design to avoid biases and obtain a high rate of compliance. A few, if any, randomised dietary studies including commercially available food items have been performed in a double-blind manner. Furthermore, the fact that the daily minimum amounts of the food items to be consumed during the intervention were based on intake data from the Norwegian dietary survey among adults( 16 ) suggests that consumption of these regular food items were manageable and seemed to be easy to include in the daily diet. The food items in both intervention groups were supplied to the participants to increase compliance. By using the equation developed by Muller et al.( Reference Muller, Kirkhus and Pedersen 17 ) to calculate the expected change in total serum cholesterol based on the changes in fatty acids in products, the equation explained 72 % of the change in total cholesterol, and the main effect therefore seems to be mediated by the change in fatty acid composition and not fibre intake. However, as the equation was developed for margarine products and the results from this equation have been extrapolated to other products, we cannot rule out that the expected change in total cholesterol may have been underestimated as we also included other food products in the present study, which have been developed based on this equation. Another limitation of the present study was the dietary registrations of ingredients in recipes of oils, butter, cream and crème fraîche in the food diaries, which may have resulted in overestimation of the amounts of fat used for cooking in both groups. Finally, a limitation was that we did not manage to include the sufficient number of subjects according to the power calculations; however, post hoc analyses showed that the number of subjects recruited were in accordance with the observed 10 % change in LDL-cholesterol between the groups (n 47 subjects in each group).
In conclusion, increasing mostly n-6 PUFA intake and reducing SFA intake by exchanging a few regularly consumed, commercially available key food items with similar food items with improved fat quality reduces serum total cholesterol and LDL-cholesterol in healthy adults with moderate hypercholesterolaemia by 9 and 11 %, respectively, with no negative effects on plasma inflammatory markers. The present study shows that a daily exchange of a few commercially available food items with improved fatty acid composition led to clinically relevant cholesterol reduction potentially affecting future CVD risk.
Acknowledgements
The authors thank Professor Emeritus Jan I. Pedersen, Department of Nutrition, University of Oslo, Norway, for valuable discussions and for reading the manuscript, and researcher Jurate Saltyte-Benth at the Division of Health Science and Research and Psychiatry, Akershus University Hospital, the University of Oslo, Norway, for help with power calculations. In addition, we thank Marit Sandvik, Ingunn Narverud, Anne Marte Wetting Johansen, all from the University of Oslo, Department for Nutrition, for technical help with blood sampling, preparation of blood samples and food diary calculations. The authors also thank all the participants of this study.
This study was supported by Akershus University College of Applied Sciences, Norway, the University of Oslo, Norway, the Throne-Holst Foundation for Nutrition Research, Oslo, Norway, and Nordplus Horizontal (NPHZ-2013/10151) and Mills DA, Oslo, Norway. Mills DA was involved in the design of the study, but S. M. U. and K. B. H. had the final responsibility of the design. Mills DA was involved in conducting the trial and provided logistical support during the trial. None of the employees at Mills DA were involved in the statistical analysis.
S. M. U., L. G., L. F. A. and K. B. H. designed the study; L. L., E. E., I. O., J. J. C., A. J. S., E. R., N. A. S., K. Torvik conducted the study; V. H. T.-H., A. L., M. G. B., K. Thyholt, L. G. provided essential material; S. M. U., L. L. and K. B. H. performed statistical analysis; S. M. U., L. L., V. H. T. H., L. F. A. and K. B. H. wrote the paper; S. M. U. and K. B. H. had the responsibility for the final content. All the authors read and approved the final manuscript.
Mills DA partially funded the study and V. H. T.-H., A. L., K. T., M. G. B. and L. G. are all employed at Mills DA. None of them own any stocks in the company. S. M. U. and K. B. H. have received research funding from TINE SA and Olympic seafood not related to the current project. No other conflicts of interest.










