Introduction
Habitat loss represents the principal driver of the decline of biodiversity in Canada (Venter et al. Reference Venter, Brodeur, Nemiroff, Belland, Dolinsek and Grant2006) and elsewhere (Maxwell et al. Reference Maxwell, Fuller, Brooks and Watson2016), but assessing its effects depends on good baseline information. Knowledge from relatively unaltered habitats is key; it provides insight into biogeographic processes and enables us to quantify the effects of habitat change. Yet, our understanding of species distributions in areas with unaltered habitats remains inadequate for many groups (Whittaker et al. Reference Whittaker, Araújo, Jepson, Ladle, Watson and Willis2005; Lomolino et al. Reference Lomolino, Riddle and Whittaker2017) and is often short-term (Mihoub et al. Reference Mihoub, Henle, Titeux, Brotons, Brummitt and Schmeller2017). This Wallacean shortfall (the paucity of knowledge of species distributions) is particularly acute for insects, especially in geographically isolated areas (Lomolino et al. Reference Lomolino, Riddle and Whittaker2017; Vergara-Asenjo et al. Reference Vergara-Asenjo, Alfaro and Pizarro-Araya2023).
The situation is exemplified by ground beetles (Coleoptera: Carabidae, Latrielle), one of the largest families of insects, with more than 40 000 species worldwide (Lövei and Sunderland Reference Lövei and Sunderland1996) and more than 2000 in North America (Bell Reference Bell and Dindal1990). Despite being well known and extensively studied across Canada (e.g., Lindroth Reference Lindroth1969; Bousquet Reference Bousquet2010, Reference Bousquet2012; Bousquet et al. Reference Bousquet, Bouchard, Davies and Sikes2013; Ernst and Buddle Reference Ernst and Buddle2015; and many others), there is almost no knowledge of carabids in many remote and difficult-to-access regions, even though these regions offer the best opportunities to study relatively unaltered native community assemblages.
One such remote area is Akimiski Island in the Qikiqtaaluk Region of Nunavut, Canada. The island, located in James Bay, the southernmost part of the Arctic Ocean, is a postglacial rebound island between 3500 and 4000 years old (Martini Reference Martini1981), with no permanently occupied human dwellings, only seasonal residents, and almost no human-altered habitat. Akimiski Island has a history of biological research. The island’s birds (Nguyen et al. Reference Nguyen, Nol and Abraham2003; Pollock et al. Reference Pollock, Abraham and Nol2012; Richards and Gaston Reference Richards and Gaston2018; Brook et al. Reference Brook, Leafloor, Abraham, Ankney and Patton2019, Reference Brook, Pollock, Abraham and Brown2021; Gan et al. Reference Gan, Abraham, Brook and Murray2019), mammals (Kolenosky and Prevett Reference Kolenosky and Prevett1983; Peacock et al. Reference Peacock, Derocher, Lunn, Obbard, Ferguson, Loseto and Mallory2010; Obbard and Middel Reference Obbard and Middel2012), and plants (Blaney and Kotanen Reference Blaney and Kotanen2000; O et al. Reference O, Kotanen and Abraham2005) have been well documented. Although some insect species have been inventoried (e.g., Dytiscidae; DeGasparro et al. Reference DeGasparro, Brown, Alarie and Beresford2018), little is known about most of the island’s ground beetles.
In this paper, we report the results of a seven-year survey of ground beetles (excluding subfamily Elaphrinae; Fleming and Beresford Reference Fleming and Beresford2019) found on Akimiski Island. Our results almost double the number of Carabidae known from Nunavut, extend the known range of many species, fill many record gaps between western and eastern Canada, and provide an essentially complete account of the relative abundance of the ground beetle community on Akimiski. Our work also highlights the importance of multiyear studies to provide complete inventories, a common challenge in biodiversity studies (Moreno and Halffter Reference Moreno and Halffter2000).
Materials and methods
Study area
We conducted our surveys on Akimiski Island, Nunavut, from 2008 to 2014. Akimiski Island has an area of about 3000 km2, with habitat characteristics of both tundra and boreal forest (Martini Reference Martini1981; Blaney and Kotanen Reference Blaney and Kotanen2000). The island is 13.7 km east of Ontario’s nearest coastline, with three islands in the strait between Akimiski and Ontario. Akimiski Island emerged from the ocean 3500–4000 years ago, after the retreat of the glaciers (Martini Reference Martini1981). With no permanent inhabitants, Akimiski Island is part of the Omushkego Cree’s territory (Tsuji et al. Reference Tsuji, General, Tsuji, Powell, Latychev, Clark and Mitrovica2020) and is a breeding site for waterfowl, shorebirds, and passerines (Brook et al. Reference Brook, Pollock, Abraham and Brown2021). Human activity is largely restricted to wildlife harvesting and scientific research, and there is almost no human-altered habitat.
A low-lying island, Akimiski Island has tidal mudflats, marshes, and gravel beach ridges along the coast and fens and bogs in the interior. The most common tree is black spruce, Picea mariana (Miller) (Pinaceae). The coastal marshes are dominated by two grasses, Puccinellia phryhanodes (Trinius) Scribner and Merrill, and Festuca rubra Linnaeus (Poaceae) (Blaney and Kotanen Reference Blaney and Kotanen2000; Kotanen and Abraham Reference Kotanen and Abraham2013). The coastal sand and gravel ridges contain gooseberry, Ribes oxyacanthoides Linnaeus (Grossulariaceae), and juniper, Juniperus sp. Linnaeus (Cupressaceae) shrubs, with willow, Salix spp. Linnaeus (Salicaceae), occupying the lower areas (Martini and Glooschenko Reference Martini and Glooschenko1984). The nearby coastal area of Ontario, directly west of Akimiski Island, is essentially the same habitat extending for about 40 km inland, which is characteristic of the Hudson Bay Lowlands (Blaney and Kotanen Reference Blaney and Kotanen2000; Crins et al. Reference Crins, Gray, Uhlig and Wester2009).
Fieldwork for the present study was conducted at two sample sites on the island’s north coast (Fig. 1). The first site was located on the coast at an Ontario Ministry of Natural Resources research station (53° 06′ 18″ N, 80° 57′ 25″ W); the second site was located in the island’s interior, on a dry ridge of gravel and shallow soil 2 km southwest of the research station (53° 06′ 00″ N, 80° 58′ 00″ W). The interior site was a small area (∼ 30 m2) of open canopy and young poplar trees that was surrounded by wooded bog and fen.

Figure 1. Inset map of Akimiski Island, Nunavut, Canada. The circle represents two sampling sites, 2008–2014. The eastern two-thirds of the island is the Akimiski Island Bird Sanctuary, the border of which is denoted by the meridian at approximately 80° W.
Collecting methods
Specimens were collected by pitfall traps, modified pitfall-malaise traps, and individual hand capture from mid-July to the first of August each year except 2008, when sampling was conducted from mid-June to the end of August. During 2008 and 2009, we used 10 modified pitfall-malaise traps (International Polar Year, or IPY, traps; McKinnon et al. Reference McKinnon, Bety, Smith, Morrison and Bolduc2008), which acted as both intercept traps for flying insects and pitfall traps for crawling insects (Fig. 2). The pitfall part of the trap was constructed using plastic trays (38 cm long × 7 cm wide and × 5 cm high) set with the top edge level with the ground. The trap was surmounted by a collecting bottle to capture flying insects. Specimens that flew into the screen would be directed either into the collecting bottle at the top or fall into the tray at the bottom (Gan et al. Reference Gan, Jumean, Beresford and Abraham2009; Bolduc et al. Reference Bolduc, Casajus, Legagneux, McKinnon, Gilchrist and Leung2013). The trays and collecting bottles were partially filled with soapy water to drown trapped insects. We deployed IPY traps 20 m apart along two transects parallel to the coast. One transect comprised five traps in supratidal habitat; the second transect comprised five traps in the intertidal habitat.

Figure 2. Photograph of International Polar Year (IPY) trap set on the coast of Akimiski Island, Nunavut, Canada. The pitfall portion of this trap is set below the interception screen at ground level. The white cone acts as a rain shield to prevent the pitfall trough from filling with rainwater and as a funnel to direct climbing insects up into the collection head at the top. Photo by Lisa Pollock.
In 2010–2013, we used pitfall traps constructed from 500-mL cups (12 cm high × 9.2 cm diameter) set in the ground with the top of the cup level with the soil to allow crawling beetles to fall in. The traps were partially filled with about 200 mL of nontoxic propylene glycol (nontoxic RV antifreeze) and did not contain any attractant or bait. Our sampling varied by year: four traps in 2010 (all at the research station), eight traps in 2011 (six at the research station and two at the interior site), 12 traps in 2012 (five at the research station and seven at the interior site), and seven traps in 2013 (all at the interior site). In 2014, sampling was done by hand capture only.
Finally, in all years, we collected beetles by hand ad hoc when specimens were spotted.
Specimen preparation and identification
We preserved collected specimens in vials containing 80% ethanol. For identification, specimens were pinned, labelled, and identified using dichotomous keys (Lindroth Reference Lindroth1969; Bousquet Reference Bousquet2010). Our identifications were confirmed by experts Dr. David Maddison (Oregon State Arthropod Collection, Oregon State University, Corvallis, Oregon, United States of America) and Dr. James Liebherr (Cornell University Insect Collection, Cornell University, Ithaca, New York, United States of America). Pinned specimens are housed in the entomology reference collection at Trent University (Peterborough, Ontario, Canada), except for reference specimens of Bembidion, which are deposited in the Oregon State Arthropod Collection, and of Agonum, which are deposited in the Cornell University Insect Collection.
Analysis
We determined existing known ranges for each species using records from publications and databases (Lindroth Reference Lindroth1969; Bousquet Reference Bousquet2010, Reference Bousquet2012; Bousquet et al. Reference Bousquet, Bouchard, Davies and Sikes2013; Ernst and Buddle Reference Ernst and Buddle2015; Canadian National Collection of Insects, Arachnids and Nematodes (CNC) 2022; Fleming et al. Reference Fleming, Schaefer and Beresford2022; Global Biodiversity Information Facility 2024). We categorised any species with records from northeastern Ontario or northern Québec as “expected.” Species with records from further afield – that is, from western or southern Ontario or Québec – were categorised as new range records.
Given the importance of flight in carabid dispersal (Den Boer Reference Den Boer1970; As Reference As1984; Venn Reference Venn2016), we calculated the proportion of flight-capable versus flightless species in our collection. Both morphologies are well represented by species of Carabidae (Lindroth Reference Lindroth1969; Bousquet Reference Bousquet2010).
Diversity
We estimated the number of species of ground beetles using Chao-1 and iChao-1 numbers (Gotelli and Colwell Reference Gotelli, Colwell, Magurran and McGill2010). Chao-1 estimates the expected number of species from sampled abundances using the proportion of species represented by only one or two specimens (singletons and doubletons) in the total catch (Gotelli and Colwell Reference Gotelli, Colwell, Magurran and McGill2010); iChao1 is similar but also includes species with three or four specimens (Chiu et al. Reference Chiu, Wang, Walther and Chao2014). We also assessed diversity using Hill’s numbers (Jost Reference Jost2006). Hill’s numbers are interpreted as the number of species (H 0), the number of abundant species (H 1), and the number of very abundant species (H 2; Ludwig and Reynolds Reference Ludwig and Reynolds1988) – that is, H 0 is species richness, and H 1 and H 2 are algebraically related to Shannon’s and Simpson’s indices (Jost Reference Jost2006). Finally, we used the rarefaction method to assess how many specimens needed to be caught to adequately inventory the Carabidae community. Rarefaction returns the mean expected number of species that would have been captured at smaller sample sizes (Krebs Reference Krebs1989). These analyses were performed using PAST 4.0 (Hammer et al. Reference Hammer, Harper and Ryan2001).
Results
We collected 1368 specimens, which were identified to 31 species from 15 genera. Nineteen species (853 specimens) have Nearctic distributions, and 12 species (515 specimens) have Holarctic distributions. Of the 31 species caught, 29 are first records for the territory of Nunavut (Table 1). Fourteen of the species are able to fly, whereas 17 species do not fly (Lindroth Reference Lindroth1969; Bousquet Reference Bousquet2010; Table 1).
Table 1. Annual and total numbers of specimens of each species of Carabidae captured on Akimiski Island, Nunavut, Canada, in 2008–2014. Flight capability is from Bousquet (Reference Bousquet2010), except where indicated *

*Based on Lindroth (Reference Lindroth1969); either not listed (B. bimaculatum, B. graphicum) or listed as flightless (B. morulum) in Bousquet (Reference Bousquet2010)
† Species previously recorded from Nunavut
Of the 31 species (H 0 = 31), seven were determined to be abundant (H 1 = 7.1) on Akimiski Island and four were determined to be very abundant (H 2 = 4.1). These four were Bembidion bimaculatum (Kirby) (571 specimens; 41.7% of the total catch), Carabus maeander Fischer von Waldheim (316 specimens; 23.1%), Amara torrida (Panzer) (132 specimens; 9.6%), and Pterostichus punctatissimus (Randall) (59 specimens; 4.3%). Two of the four, C. maeander and A. torrida, have Holarctic distributions (Table 1). Four species were represented by a single specimen each, and seven species were represented by two specimens each (Table 1). The Chao-1 and iChao-1 estimates of richness were 31.8 and 32.1, respectively, implying that our sampling likely captured the full complement of species in the study area.
Based on the rarefaction analysis, each year of sampling produced fewer species than expected from the number of captured specimens (Fig. 3). This slow accumulation of species continued for the first three years of data collection (Fig. 3).

Figure 3. Rarefaction analysis of the mean number of Carabidae species expected based on the number of collected specimens, Akimiski Island, Nunavut, Canada, 2008–2014. The dashed lines are the upper and lower 95% confidence limits. White symbols represent individual years; black symbols represent cumulative years. The values for 2008–2012 and 2008–2014 are coincident.
Discussion
The arrival of species to islands is a long-standing topic in biogeography (Lomolino et al. Reference Lomolino, Riddle and Whittaker2017). As a postglacial rebound island, Akimiski Island is a tabula rasa, or blank slate, for new colonists (Coope et al. Reference Coope, Moore and Gibbs1986; Buckland and Dugmore Reference Buckland, Dugmore, Maizels and Caseldine1991). Interestingly, our inventory contained a near-equal mix of flight-capable and flightless species (Table 1). Although the 14 flight-capable species could have arrived by air, the 17 flightless species almost certainly were transported by rafting on floating ice, vegetation, or freshwater slicks (Coope et al. Reference Coope, Moore and Gibbs1986). Rafting may have carried the flight-capable species, too (As Reference As1984). The distance from the mainland to Akimiski Island, although not insurmountable, poses a considerable barrier to insect species dispersal, emphasising the importance of both aerial and rafting mechanisms in colonisation (Heatwole and Levins Reference Heatwole and Levins1972; Kotze et al. Reference Kotze, Niemelä and Nieminen2000).
Carabid beetles, in general, exhibit a wide range of flight capabilities, which are highly variable between and even within species (Den Boer Reference Den Boer1970; Thiele Reference Thiele1977; Desender Reference Desender2000). Although most carabids can fly short distances well, the prevailing winds in the region could assist weak fliers travelling to Akimiski Island (As Reference As1984). Additionally, the Akimiski Strait islands would likely act as stepping stones, facilitating colonisation by providing rest points for migrating beetles (MacArthur and Wilson Reference MacArthur and Wilson2001). The establishment of founding populations on Akimiski Island further underscores the importance of these varied dispersal strategies. Overall, the mix of flight-capable and flightless species highlights the complex dynamics of species dispersal to this isolated island (Venn Reference Venn2016).
Dispersal to Akimiski by flight
Flight facilitates carabid dispersal (Den Boer Reference Den Boer1970, Reference Den Boer1977). In our study, eight species found on Akimiski Island are undocumented in watersheds in the eastern half of northern Ontario that empty into James Bay: Amara lacustris LeConte, Amara lindrothi Hieke, Bembidion bimaculatum, Bembidion graphicum Casey, Bembidion morulum LeConte, Patrobus lecontei Chaudoir, Pelophila rudis (LeConte), and Sericoda obsoleta (Say). Only one of these species is flightless, A. lindrothi, which was only recently described and is consequently underrepresented in published records. The other seven species are known to fly. The lack of records of these seven flight-capable species from the northeastern Ontario watersheds is consistent with these species not depending on flotsam as a transport mechanism (Buckland Reference Buckland1988; Fleming et al. Reference Fleming, Schaefer, Abraham, Smith and Beresford2021).
Dispersal to Akimiski by rafting
The direction of flow of waters in James and Hudson bays is affected by tides, water currents, and wind direction and could be important to the arrival of colonists to the island. The current in southern Hudson Bay flows eastwards along the north shore of Ontario, with some current entering the east side of James Bay (Hachey Reference Hachey1935). In James Bay, water flows south along the western side of the bay past Akimiski Island towards the southern coast of the bay (Martini Reference Martini1981; Stewart and Lockhart Reference Stewart and Lockhart2005) and then follows the coastline, flowing east and then north along the eastern side (Stewart and Lockhart Reference Stewart and Lockhart2005). The surface water generally flows almost due east (St-Laurent et al. Reference St-Laurent, Straneo, Dumais and Barber2011); however, wind changes can reverse the direction of surface water movements (Prinsenberg Reference Prinsenberg1978).
The closest distance between Akimiski Island and the mainland is approximately 14 km. Akimiski is situated east of Akimiski Strait, opposite the mouth of the Attawapiskat River (Déry et al. Reference Déry, Mlynowski, Hernández-Henríquez and Straneo2011), south of the outflows from the Ekwan River, and north of the outflow from the Albany and Moose River systems (Déry et al. Reference Déry, Mlynowski, Hernández-Henríquez and Straneo2011). Debris floating downstream from the Ekwan and Attawapiskat rivers (among others) into James Bay could arrive on the shore of Akimiski Island. Due to the northward current on the east side of James Bay, it is less likely that debris from Québec rivers would end up on Akimiski Island. However, debris in the eastern or southern parts of James Bay could also be carried by surface water to Akimiski Island (Barber Reference Barber1972).
New records and abundance
Our study substantially augments the list of carabid beetles in Nunavut. Indeed, Akimiski Island is at the southernmost reaches of this jurisdiction, and species richness is generally higher at lower latitudes (Lomolino et al. Reference Lomolino, Riddle and Whittaker2017). Many of these new territorial records are artefacts of political boundaries – a reminder that jurisdiction boundaries, although important to listing and conservation responsibility, are not synonymous with geographical or ecological boundaries.
At present, 41 species of Carabidae (excluding Cicindelidae; Duran and Gough Reference Duran and Gough2020) are recognised from Nunavut: 34 are listed in Bousquet et al. (Reference Bousquet, Bouchard, Davies and Sikes2013) and seven Elaphrinae Nunavut records appear in Fleming and Beresford (Reference Fleming and Beresford2019). The addition of our 29 territorial records for Nunavut increases this total to 70 Carabidae species. All are native species. Moreover, our inventory of the Carabidae on Akimiski Island reveals both similarities and differences with the carabid communities found on the adjacent mainland. For example, many more species are known in Ontario (517), Québec (474), and the Northwest Territories (212; Table 2). Nunavut and Yukon are the only regions in Canada with no known adventive species (Bousquet et al. Reference Bousquet, Bouchard, Davies and Sikes2013). This pattern is consistent with the global tendency for polar regions to have lower proportions of alien species than elsewhere (Alsos et al. Reference Alsos, Ware and Elven2015).
Table 2. Number of Carabidae species in Nunavut and adjacent jurisdictions. Cicindelidae, although listed in Bousquet (Reference Bousquet2010) and Bousquet et al. (Reference Bousquet, Bouchard, Davies and Sikes2013) as subfamily Cicindelinae, is now considered as a distinct family (Duran and Gough Reference Duran and Gough2020)
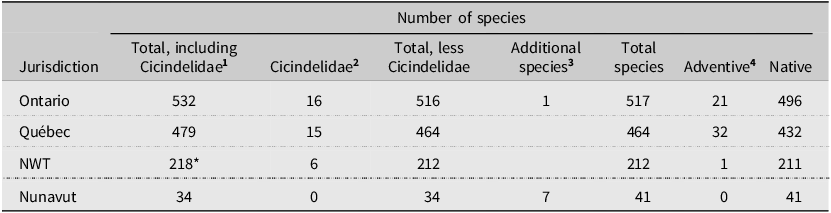
1 Bousquet et al. (Reference Bousquet, Bouchard, Davies and Sikes2013, Table 1).
2 Bousquet (Reference Bousquet2010, inferred from pp. 43 and 35).
3 Fleming and Beresford (Reference Fleming and Beresford2019).
4 Bousquet (Reference Bousquet2012, Table 5).
* 218 is from Bousquet (Reference Bousquet2012); note: Bousquet et al. (Reference Bousquet, Bouchard, Davies and Sikes2013) reported this as 217.
Two of the 31 species caught in the present study, A. lacustris LeConte, 1855 and Pterostichus brevicornis (Kirby), had been previously recorded in mainland Nunavut. Amara lacustris is transcontinental, but its distribution is predominantly in northern North America (Lindroth Reference Lindroth1969). It is flight-capable and is not known from Québec or Ontario. The two specimens in our collection represent an eastward range extension of more than 1000 km. Pterostichus brevicornis is flightless, with a Holarctic distribution across northern Eurasia, Alaska, and northern Canada, including Ontario (Fleming et al. Reference Fleming, Schaefer and Beresford2022) and Québec (Bousquet Reference Bousquet2012).
We anticipated that northern species documented elsewhere might be part of our collection. Nineteen of the 29 species (comprising 710 specimens) with new records in this study are known from similar latitudes and habitats in the adjacent provinces of Ontario and Québec (Lindroth Reference Lindroth1969; Ernst and Buddle Reference Ernst and Buddle2015; Fleming et al. Reference Fleming, Schaefer and Beresford2022). The species documented at similar latitudes are Agonum affine Kirby, Agonum gratiosum (Mannerheim), Agonum superioris Lindroth, Amara latior (Kirby), Amara torrida, Calathus ingratus Dejean, Carabus maeander, Chlaenius alternatus Horn, Cymindis cribricollis Dejean, Loricera pilicornis pilicornis (Fabricius), Miscodera arctica (Paykull), Notiophilus aquaticus (Linnaeus), Notiophilus semistriatus Say, Patrobus foveocollis (Eschscholtz), Platynus mannerheimii (Dejean), Pterostichus adstrictus Eschscholtz, Pterostichus brevicornis, Pterostichus patruelis (Dejean), and Pterostichus punctatissimus. All 19 species were expected from existing records, and our results, of which 18 are first records for Nunavut (P. brevicornis having been previously reported in that territory), do not fill substantial range gaps for these species or extend the ecological range of these species. All 19 are described as widespread in northeastern North America by Bousquet (Reference Bousquet2010) and as circumpolar, transcontinental, or transamerican by Lindroth (Reference Lindroth1969; Lindroth (Reference Lindroth1969) reports P. mannerheimii as Agonum mannerheimi Dejean, 1828). Of these, 13 species are flightless (Table 1). This life-history feature, plus the species’ presence in northeastern Ontario or northern Québec, is consistent with the hypothesis of these species being transported on debris to Akimiski Island.
Three other species have southern Ontario and Québec records: Amara quenseli (Schönherr), Amara sinuosa (Casey), and Patrobus stygicus Chaudoir. These three species are also widespread in northeastern North America (Bousquet Reference Bousquet2010), and although our records fill range gaps between southern Ontario and Québec, we also expected to find these species on Akimiski Island because of their widespread distributions.
Surprisingly, the list of Carabidae on Akimiksi Island that we anticipated based on nearby occurrences (above) does not include three of our seven most abundant species: Bembidion bimaculatum (571; 42% of the specimens captured), Patrobus stygicus (40; 2.9%), and Carabus taedatus agassii LeConte (31; 2.3%). We expected that more abundant species would have nearby records and that any range extensions would be from those species that were rare in our collections – a reasonable expectation, but wrong in this case. These species’ abundance on Akimiski Island may represent a case of ecological release (Lomolino et al. Reference Lomolino, Riddle and Whittaker2017) – that is, the filling of vacant ecological space where insular species are free of competition from mainland species. We discuss these and other individual species below.
Species accounts
The remaining eight species caught during the present study are either new range records or gap infills. All are Nearctic species, and only two are flightless: A. lindrothi and C. taedatus agassii.
Amara lindrothi
Amara lindrothi was first described in 1990 (Bousquet Reference Bousquet2012) and is not commonly found; we collected only six individuals. Records are scarce and widespread; most are from the southwestern United States of America. In Canada, the species has been found in the Yukon, northern Alberta, northern Manitoba, and Labrador; it is not known from either Ontario or Québec. In the United States of America, it has been found in Colorado, New Mexico, Nevada, Utah, and Wyoming (Bousquet Reference Bousquet2012; Bousquet et al. Reference Bousquet, Bouchard, Davies and Sikes2013; Global Biodiversity Information Facility 2024). The habitat preferences of A. lindrothi are not known other than that the beetle occurs in the Arctic bioclimatic zone (Bousquet Reference Bousquet2010). The few records may, in part, be attributed to having been described only 34 years ago. Our record marks the species’ presence in the vast distributional gap between Manitoba and Labrador.
Bembidion bimaculatum
We caught 571 B. bimaculatum, our most abundant species. It is a predominantly western species (Lindroth Reference Lindroth1969), and Bousquet (Reference Bousquet2012) describes the northern part of its range as “north-central Ontario (CNC 2022) to the Arctic Plains of Alaska.” Current distribution records are scarce east of Manitoba. Ontario records are from Geraldton, about 100 km north of Lake Superior (CNC 2022), as well as Moosonee and the Ottawa area (Global Biodiversity Information Facility 2024). There are also records from New Hampshire (Global Biodiversity Information Facility 2024) and Greenland (Lindroth Reference Lindroth1969). We could not find any habitat data for this species; our records add to knowledge of the eastern distribution of this species, and the abundance of our collections suggests the species may prefer gravel ridge or coastal habitats.
Bembidion graphicum
Bembidion graphicum was common in our study: 21 specimens were collected. It is a western North American species (Bousquet Reference Bousquet2012). In 2010, two specimens were collected on Manitoulin Island, Ontario, the easternmost records for this species (Paiero Reference Paiero2017). Our collection of 21 specimens of B. graphicum from Akimiski Island is 800 km north of Manitoulin Island and is a large range extension in the eastern part of the range. Bembidion graphicum prefers habitats along the margins of pools and lakes and saline environments (Lindroth Reference Lindroth1969), habitats common on Akimiski Island.
Bembidion morulum
Bembidion morulum has been collected in Churchill, Manitoba, and in northern Ontario near the Manitoba border near Fort Severn (CNC 2022). Records exist from Newfoundland (Bousquet Reference Bousquet2010, Reference Bousquet2012). Records are scarce (only 27 sites are listed in the Global Biodiversity Information Facility 2024 database, and three sites are listed in CNC 2022). With so few records, the present study provides important additional records for this species and partially fills a record gap from Fort Severn to Newfoundland, a stretch of some 2100 km.
Carabus taedatus agassii
Carabus taedatus agassii is known from Newfoundland in the east to the Yukon Territory in the northern part of North America (Bousquet Reference Bousquet2010, Reference Bousquet2012), with intermediate records from Québec (Bousquet Reference Bousquet2010). Lindroth (Reference Lindroth1969) described the range as remarkably disjunct. Ontario records exist for about 100 km north of Lake Superior (Global Biodiversity Information Facility 2024) and at three sites near the Hudson Bay coast (Fleming et al. Reference Fleming, Schaefer and Beresford2022; Global Biodiversity Information Facility 2024). The record from the present study fills a gap between the Ontario and Québec reports.
Patrobus lecontei
Patrobus lecontei has an extensive west-to-east distribution across much of southern Canada, with a record gap in Ontario (Bousquet Reference Bousquet2012; Global Biodiversity Information Facility 2024). The species is found along standing water among sedges but never in sphagnum moss (Lindroth Reference Lindroth1969). Our two specimens are at the northern edge of its known range, 620 km from the closest eastern record in northern Québec, and fill a large record gap between Manitoba and Québec.
Pelophila rudis
The range of P. rudis was described by Lindroth (Reference Lindroth1969) as “a rare and local species, restricted to Canada.” It is known from Newfoundland in the east, with no records until Fort Severn and Cape Henrietta Maria on the Hudson Bay coast in Ontario (Lindroth Reference Lindroth1969), and records westward in Manitoba. Our record of this uncommon species helps fill the vast known range gap, placing P. rudis east of Cape Henrietta Maria.
Sericoda obsoleta
This species is found across Canada, with records from Newfoundland to the Yukon Territory. Ontario records extend from Toronto in the southern part of the province to Moose Factory in the north (Lindroth Reference Lindroth1969). It is known from the southern half of Québec. In eastern North America, our record extends the known range northwards from Moose Factory by about 200 km.
Conclusions and significance
As the rarefaction analysis reveals, four years of sampling were required to capture all 31 species (Table 1), and during the first three years, the aggregate tally of species fell somewhat short of expectations based on the tally of specimens (Fig. 3). Notably, rarefaction is based on resampling being random. Even though our traps were placed in similar areas each year, the years differed in phenology, a major determinant of insect activity. Our results underscore the potential importance of seasonal timing in surveys of insects, and of ground beetles in particular (Niemelä et al. Reference Niemelä, Haila, Halme, Pajunen and Punttila1989; Adlam et al. Reference Adlam, Despland and Beaulieu2017). For example, our mid-summer sampling captured only one Notiophilus aquaticus, whereas, in Norway, most have been caught in late August (Erikstad et al. Reference Erikstad, Byrkjedal and Kålås1989).
Transitional areas, such as Akimiski Island, are often the first to show evidence of altered species composition in response to habitat changes (Payette et al. Reference Payette, Delwaide, Caccianiga and Beauchemin2004). Although all the species in our collection from Akimiski Island were native to eastern Canada, many nonnative ground beetle species are found in the neighbouring regions of Ontario (Fleming et al. Reference Fleming, Schaefer and Beresford2022) and Québec (Bousquet Reference Bousquet2012). The absence of nonnative ground beetles highlights the island’s importance as a reference point for understanding ecological change. Akimiski Island therefore serves as an important point for comparisons both in space and time – that is, as a contrast with regions harbouring introduced species and as a baseline for shifts in the community, for example, should introduced species disperse to Nunavut and to Akimiski Island.
All ground beetles on Akimiski Island arrived in the last 3500–4000 years, after the island rebounded from the ocean following the retreat of the glaciers (Martini and Glooschenko Reference Martini and Glooschenko1984). A period of a few millennia is not long in ecological time. Ground beetle species in northern temperate regions are still recovering from the last glacial period (Baselga et al. Reference Baselga, Lobo, Svenning, Aragón and Araújo2012; Fleming et al. Reference Fleming, Schaefer, Abraham, Smith and Beresford2021), and there is no reason to assume that ground beetles have completed their dispersal to Akimiski Island. For example, in Greenland, only 17% of the Carabidae species that are able to survive in that climate and habitat are present (Coope et al. Reference Coope, Moore and Gibbs1986). Given the relative newness of Akimiski Island, novel species, including nonnative forms, likely will continue to arrive. The present study’s results provide a basis for identifying those arrivals, contingent on continued monitoring and long-term investment. In this regard, we believe that the present study may help remedy the truncated baselines that often obscure ecological change (Mihoub et al. Reference Mihoub, Henle, Titeux, Brotons, Brummitt and Schmeller2017).
Acknowledgements
We thank the many personnel of the Ontario Ministry of Natural Resources’ Far North and waterfowl programmes for their support. Funding was provided by the Ontario Ministry of Natural Resources through its Far North Branch and Wildlife Research and Development Section. We also thank the many dedicated researchers, technicians, volunteers, and pilots who helped collect the IPY and pitfall specimens and whose indefatigable cheerfulness in often harsh conditions made the time at the research station so enjoyable and productive. A special thanks to Lisa Pollock for the photograph of the IPY trap. We thank Dr. David Maddison, Director of the Oregon State Arthropod Collection (Oregon State University, Corvallis, Oregon, United States of America), for confirming the Bembidion identifications and Dr. James Liebherr, Professor Emeritus and Emeritus Curator of the Cornell University Insect Collection (Ithaca, New York, United States of America), for confirming the Agonum identifications. Finally, we thank the anonymous reviewers of an earlier draft for their careful attention and suggestions.
Competing interests
The authors declare that they have no competing interests.








