The Fontan procedure is a palliative surgery for patients with a single functional ventricle. Since its first description in 1971, it has evolved from the atrio-pulmonary to the total cavo-pulmonary connection Fontan, with the first total cavo-pulmonary connection developed being the intra-atrial lateral tunnel procedure, followed by the alternative extra-cardiac conduit procedure. Reference Khairy, Poirier and Mercier1–Reference de Leval, Kilner and Gewillig3 As a result, survival rates have improved such that a child born today with a univentricular heart who undergoes a successful total cavo-pulmonary connection can expect to live well into adulthood, provided appropriate care is given. Reference Khairy, Poirier and Mercier1,Reference Pundi, Dearani and Zhuo4
It is now possible to observe long-term outcomes of Fontan palliation, and this is crucially important given the difficulties in managing this rare and heterogeneous condition. The Australian and New Zealand Fontan registry of more than 1400 patients has provided valuable insights into the outcomes of all Fontan patients in the 2 countries. Reference Iyengar, Winlaw and Galati5 However, the evidence from large-scale, multicentre, randomised, placebo-controlled clinical trials involving Fontan survivors is limited, with two key studies to date: the “Treatment With Endothelin Receptor Antagonist in Fontan Patients, a Randomized, Placebo-Controlled, Double-Blind Study Measuring Peak Oxygen Consumption” (TEMPO) study (75 patients from Denmark and Sweden) Reference Hebert, Mikkelsen and Thilen6 and the “Fontan Udenafil Exercise Longitudinal” (FUEL) trial (400 patients from North America and the Republic of Korea). Reference Goldberg, Zak and Goldstein7
The Patient Registry for Adolescents and Adults with Stable Fontan Circulation aimed to describe the clinical characteristics, at enrolment, of “stable” patients at least 10 years following modern Fontan surgery, with the ultimate aim of identifying a global cohort of patients who could potentially be eligible for recruitment into a large, placebo-controlled trial such as the Clinical Study Assessing the Efficacy and Safety of Macitentan in Fontan-Palliated Subjects (RUBATO) trial (NCT03153137). 8
Materials and method
Study design
The PREpArE-Fontan registry is an international, multicentre, cross-sectional, non-interventional study (Supplementary Table 1). The study was conducted in compliance with Good Pharmacoepidemiology Practice guidelines 9 and the Declaration of Helsinki as revised in 2013, 10 and ethical approvals were obtained as required by local legislation in each participating country.
The registry enrolled patients aged ≥12 years who underwent lateral tunnel or extra-cardiac conduit Fontan surgery at least 10 years prior to enrolment. Patients were “stable”, meaning they are not in acute decline. As such, major exclusion criteria included NYHA functional class IV, re-intervention/reoperation of the Fontan circulation, severe stenosis in the Fontan circulation (>50% of the cross-sectional area), protein-losing enteropathy, plastic bronchitis, hepatic encephalopathy, or severe hepatic impairment at enrolment. Full eligibility criteria are provided in Table 1. Patients were invited to enrol by participating physicians at face-to-face clinic visits and patients and/or their legal guardians gave written informed consent prior to study enrolment.
Table 1. Full eligibility criteria
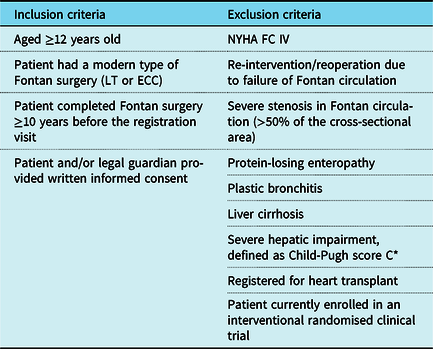
ECC = extra-cardiac conduit; LT = lateral tunnel; n = number of patients; NYHA FC = New York Heart Association functional class
* Based on a measurement of total bilirubin, serum albumin, and international normalised ratio (or prothrombin time for patients treated with anticoagulants), as well as presence/severity of ascites and hepatic encephalopathy
%, percentage of patients out of those excluded from the analysis population
Patient and public involvement
There was no patient or public involvement in the research/design of the study.
Data collection
Data were extracted from patients’ medical charts during a single routine clinic visit, which served as the registration visit/study enrolment. The following data were collected as part of routine clinical care at the time of enrolment or, if not available at study enrolment, within the previous 2 years: presence/occurrence of comorbidities, medications, laboratory parameters (haemoglobin level, iron deficiency (serum ferritin saturation <20%), platelet count, creatinine clearance, serum albumin and protein), and cardiopulmonary assessments, including vital signs (resting heart rate, systolic and diastolic blood pressure, and oxygen saturation at rest and during exercise), arrhythmia, physician assessment of NYHA functional class, and invasive haemodynamics (inferior and superior caval vein pressures, right and left pulmonary artery pressures, pulmonary artery wedge pressure, cardiac index, and pulmonary vascular resistance). The percentage of patients with normal haemoglobin levels (13–17 g/dl for males; 12–15 g/dl for females), platelet counts (150–400 × 109/L), creatinine clearance (97–137 ml/min for males and 88–128 ml/min for females), serum albumin (35–50 g/L), and protein (60–80 g/L) were estimated based on collected data. The following data were collected where available at study enrolment: demographic characteristics (height and weight at enrolment or latest measurement within the previous year), relevant medical history, historic details of the Fontan operation, and relevant ongoing or initiated treatments. For women of childbearing potential, additional specific information was also collected, such as whether they were currently pregnant and the outcomes of any previous pregnancies. The events or conditions listed in Supplementary Table 2 were defined as relevant medical history and any instances of these, as judged by the investigator, were recorded in the electronic case report forms. For the majority of events/conditions, any instances that were reported in the patients’ medical records within the previous 2 years were also recorded in the electronic case report forms (Supplementary Table 2). Participating physicians also recorded whether relevant tests, including cardiopulmonary exercise testing, had been performed within the previous year.
This study also calculated the percentage of registrants who could potentially be eligible for the RUBATO trial, which aims to assess the effect of macitentan on exercise capacity through cardiopulmonary exercise testing. 8 Not all of the information required to fully determine patients’ eligibility for RUBATO was collected in the electronic case report form as part of the PREPARE registry. Moreover, some information was not taken into account for determining potential eligibility since some criteria (e.g., NYHA functional class, oxygen saturation, and ascites) can change or occur before screening for the clinical trial. Hence, the estimate provided is the percentage of patients who could be potentially eligible for the RUBATO clinical trial.
Statistical analyses
Statistical analyses were performed using SAS® version 9.2 (SAS Institute, Cary, NC, United States of America), and listings and tables were formatted using AdClin® version 3.3.1 (AdClin, Paris, France). Missing observations were recorded as such and assessments that were not undertaken by the physician were recorded as “not done”. Data were summarised by descriptive statistics for the overall study population. Parameters of interest were summarised by descriptive statistics for patients stratified by region (Europe, USA), Fontan surgical method (extra-cardiac conduit, lateral tunnel), and age (12–17, 18–25, 26–35, >35 years).
Post hoc exploratory analyses were performed to examine correlations and associations between clinical characteristics and renal dysfunction, stratified by creatinine clearance (<90, ≥90 ml/min). Analyses included unpaired t-test (or Mann–Whitney–Wilcoxon test when assumptions were not met) for continuous variables, the chi-squared test (or Fisher’s exact test when assumptions were not met) for categorical nominal variables, and the Mann–Whitney–Wilcoxon or Cochran–Mantel–Haenszel test for categorical ordinal variables. The multivariate relationship between creatinine clearance and clinical parameters (patient and treatment characteristics) that were associated with creatinine clearance in univariate regression (p < 0.25) was assessed using logistic regression, applying a backward/forward algorithm to select parameters. Diagnostic tests for collinearity were performed. All statistical tests were two-sided at the 5% significance level, unless otherwise stated.
Results
The PREpArE-Fontan registry screened 266 patients at 7 sites across Denmark, Germany, Switzerland, the UK, and the USA between February, 2016 and April, 2017 (Supplementary Table 1). Of the 266 patients, 254 were included in this analysis. Reasons for exclusion were missing NYHA functional class (n = 6), other Fontan surgery type (n = 3), or Fontan surgery <10 years before the registration visit (n = 3).
Patient demographics and clinical characteristics
Patient characteristics by region are shown in Tables 2 and 3. The most common underlying diagnoses were double inlet left ventricle, tricuspid valve atresia, and pulmonary valve atresia. Overall, 136 (53.5%) patients underwent extra-cardiac conduit and 118 (46.5%) underwent lateral tunnel, and a greater time had elapsed since Fontan procedure completion if a lateral tunnel procedure was performed compared with an extra-cardiac conduit (median of 22.0 and 15.1 years, respectively). In total, 130 patients experienced at least 1 concomitant procedure: of those, 89 patients (68.5%) had fenestration, 16 (12.3%) had a systemic-to-pulmonary shunt, 12 (9.2%) had pulmonary artery banding, and 30 (23.1%) had other concomitant procedures (Table 3).
Table 2. Demographics by region
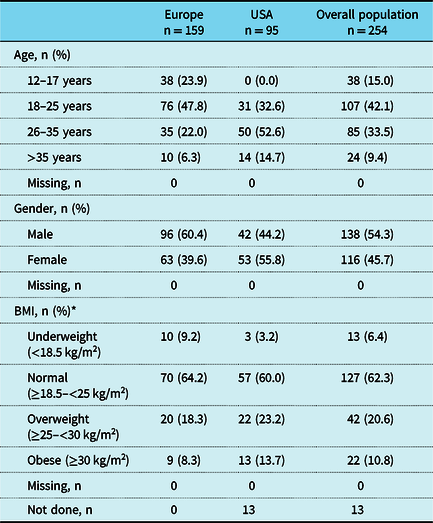
BMI = body mass index
* Total number of patients were n = 109 (Europe), n = 95 (USA), and n = 204 (overall) for BMI
The denominator for percentages is the number of patients with data available; patients with missing measurements or measurements not done are not included in percentage calculations
Table 3. Clinical characteristics by region

ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; ECC = extra-cardiac conduit; LT = lateral tunnel
* Defined as an ACE inhibitor, ARB, beta-blocker, diuretic, anti-arrhythmic medication, or medication for pulmonary hypertension
** A patient may have had more than one procedure
The denominator for percentages is the number of patients with data available; patients with missing measurements or measurements not done are not included in percentage calculations
Fontan completion and assessments in the previous year by region
The mean (SD) age of patients at the time of Fontan completion was 6.1 (5.5) years for patients from Europe and 7.9 (8.3) years for patients from the USA. The assessments performed within the year prior to or at the registration visit are summarised for patients by region in Figure 1. The largest differences between Europe and the USA were the percentages of patients who underwent any quality of life questionnaire (18.4% versus 0.0%) or an abdominal ultrasound scan (35.2% versus 50.5%).
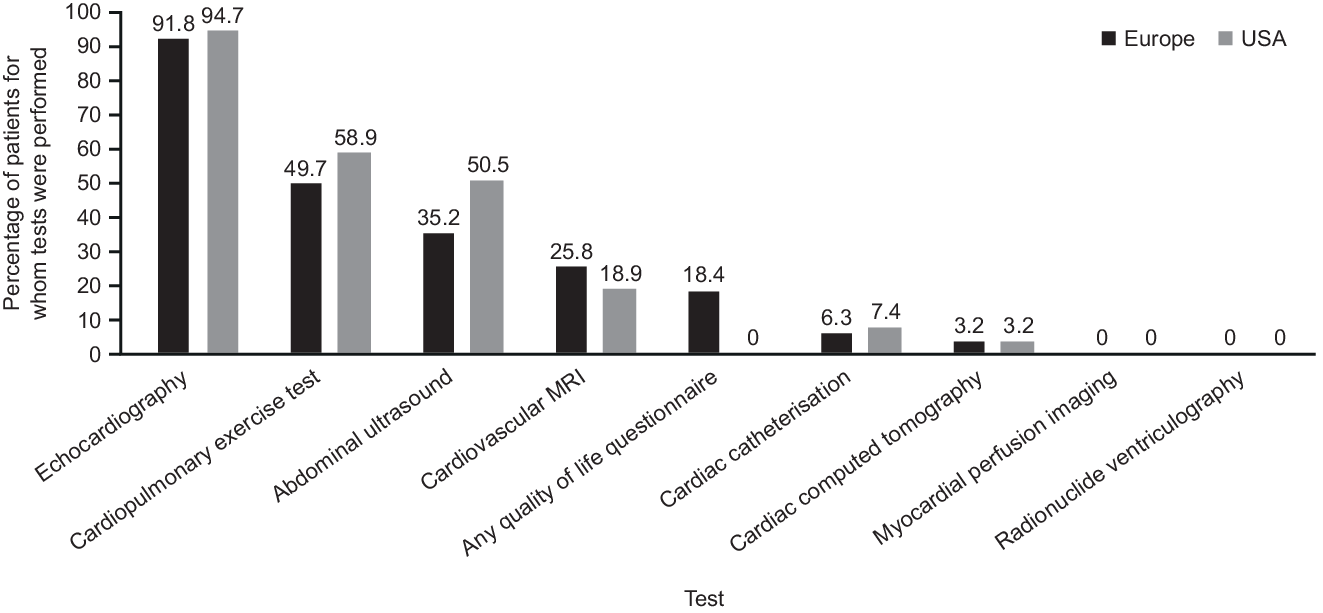
Figure 1. Tests performed within the previous year or at registration visit.
MRI = magnetic resonance imaging.
Patient characteristics by age group
Parameters of interest are summarised by age group in Tables 4–6. Median body mass index was within the normal range overall, and increased with age, as did the percentage of patients classed as either overweight or obese. Nearly, all patients were NYHA functional class I (60.6%) or II (34.6%). Mean (SD) oxygen saturation was 93% (4) at rest and 89% (6) with exercise in the overall population, but decreased with age. Across age groups, laboratory parameters were mostly within normal ranges (haemoglobin: 55%–71%; platelet count: 40%–88%; serum albumin: 72%–94% of patients with available data normal, depending on age group), whereas creatinine clearance rates were abnormal in a greater proportion of patients (46%–58% of patients with available data abnormal, depending on age group).
Table 4. Clinical characteristics by age group
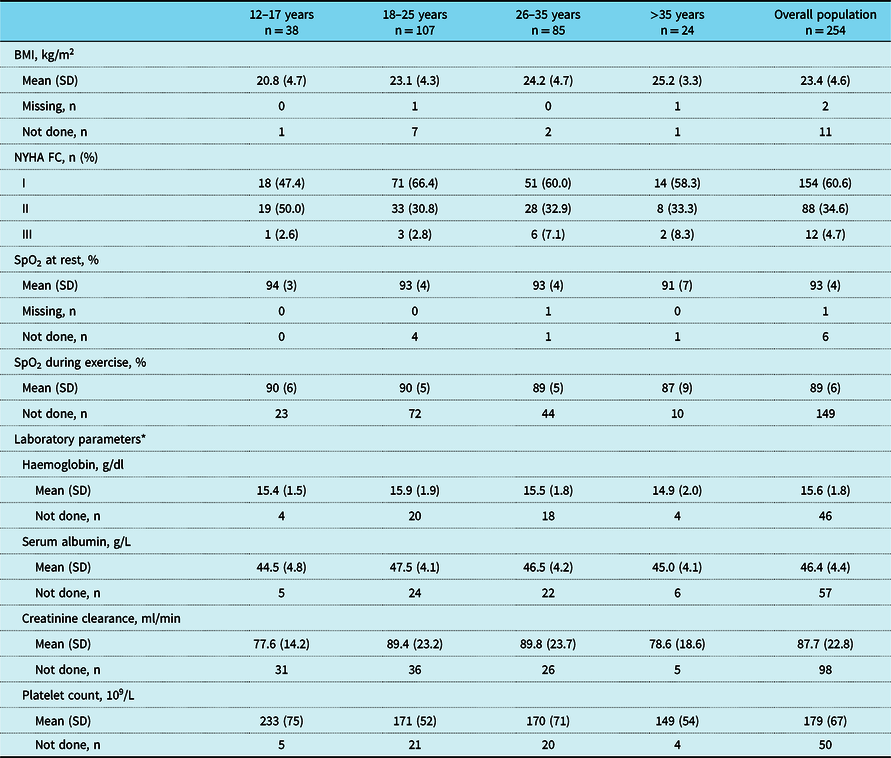
BMI = body mass index; SpO2 = oxygen saturation; NYHA FC = New York Heart Association functional class
* Overall, two patients had iron deficiency (serum ferritin saturation <20%)
Data are the most recent measurement recorded within the previous 2 years or at registration visit. N – numbers shown in column headings are the total numbers of patients in these age groups, but not every variable was measured in every patient. Missing data are only presented for parameters where data are missing for at least one patient in the overall population. The denominator for percentages is the number of patients with data available; patients with missing measurements or measurements not done are not included in percentage calculations
Table 5. Relevant medical history by age group
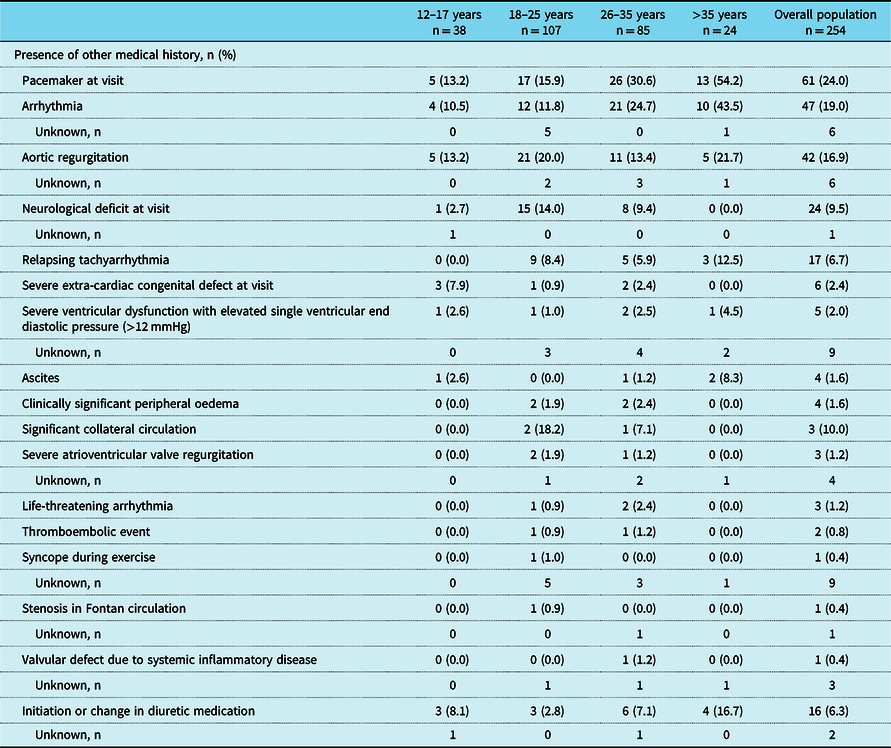
Relevant medical history is defined as any of the above-listed events or conditions being either ongoing at registration visit or reported within the 2 years prior to the registration visit. Exceptions to this are severe extra-cardiac congenital defect, neurological deficit, and use of a pacemaker, which were only included if they were ongoing at registration visit. N – numbers shown in column headings are the total numbers of patients in these age groups, but not every variable was measured in every patient. Missing data are only presented for parameters where data are missing for at least one patient in the overall population. The denominator for percentages is the number of patients with data available; patients with missing measurements or measurements not done are not included in percentage calculations
Table 6. Treatment and medications initiated or ongoing at registration visit by age group.
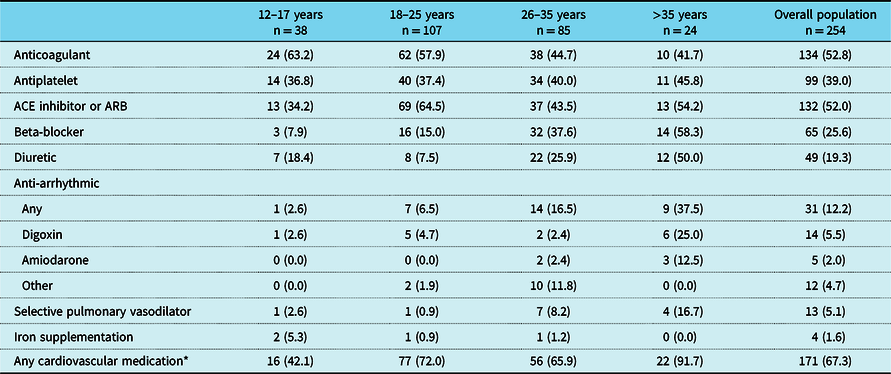
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker
* Defined as an ACE inhibitor, ARB, beta-blocker, diuretic, anti-arrhythmic medication, or medication for pulmonary hypertension
A total of 19% of patients had a history of an arrhythmia within the previous 2 years and 24% had a pacemaker, both with increasing prevalence with older age (Table 5). Mean (SD) time since pacemaker implantation was 12.5 (4.0), 9.6 (5.9), 15.3 (8.2), and 13.9 (6.9) years for the age groups 12–17, 18–25, 26–35, and >35 years, respectively. In general, there was a trend towards higher medication use with increasing age (e.g., anti-platelet therapy and anti-arrhythmic medication such as beta-blockers), but the use of oral anticoagulants decreased with age (Table 6).
Patient characteristics by surgical method
A greater percentage of patients who underwent lateral tunnel had experienced arrhythmias within the 2 years prior to the registration visit compared with those who underwent extra-cardiac conduit (23.9% versus 14.5%, respectively). Similar percentages of patients were NYHA functional class I/II between the two surgical procedures (61.0% and 33.1% for lateral tunnel versus 60.3% and 36.0% for extra-cardiac conduit, respectively). Pacemaker prevalence was similar between the surgery types: 26.3% and 22.1% for lateral tunnel and extra-cardiac conduit, respectively.
Patient characteristics by creatinine clearance category
Overall, median (interquartile range) creatinine clearance was 89.0 (75.5; 90.0) ml/min. Exploratory analyses of renal dysfunction (using creatinine clearance categories of <90 and ≥90 ml/min at registration) were undertaken in 156 patients with available information. Patients with a creatinine clearance value <90 ml/min (n = 81) were less likely to have normal serum albumin or protein levels at enrolment (χ2 p < 0.001 and p = 0.072, respectively) compared with those with a creatinine clearance of ≥90 ml/min (n = 75). Of the medications that were ongoing or initiated at registration, angiotensin-converting enzyme inhibitors, anticoagulants, antiplatelet therapy, and anti-arrhythmic medications were found to be associated with creatinine clearance category (Supplementary Table 3). Patients with creatinine clearance <90 ml/min were more likely to use angiotensin-converting enzyme inhibitors (χ2 p = 0.007), anticoagulants (χ2 p = 0.002), and anti-arrhythmic medication (χ2 p = 0.028), but less likely to receive antiplatelet therapy (χ2 p = 0.004), than those with creatinine clearance ≥90 ml/min. Patients with creatinine clearance <90 ml/min were, on average, more likely to have Fontan procedure completion at an older age (Wilcoxon p = 0.007). The majority of patients in the <90 ml/min creatinine clearance category had undergone extra-cardiac conduit procedure (65.4%), whereas the majority of patients in the ≥90 ml/min creatinine clearance category had undergone lateral tunnel procedure (65.3%) (χ2 p < 0.001). The two creatinine clearance categories were similar with respect to other laboratory and cardiopulmonary parameters (Supplementary Table 3). The results of a logistic regression analysis assessing the multivariate relationship between creatinine clearance categories and clinical variables are shown in Supplementary Table 4.
Patients eligible for enrolment in a clinical trial at registration
Of the 266 patients in the PREpArE-Fontan registry, 142 (53.4%) had clinical characteristics that indicated clinical stability and could be potentially eligible for enrolment in the RUBATO clinical trial. The main medical reasons for being ineligible were having a pacemaker (49.2%), relapsing tachyarrhythmia within the 3 months preceding the registration visit (8.9%), and known valvular defects (7.3%).
Discussion
The PREpArE-Fontan registry characterises “stable” Fontan patients who could potentially be eligible for recruitment into a placebo-controlled clinical trial, such as the RUBATO trial. The overall clinical characteristics are consistent with the study selection criteria being designed to identify “stable” Fontan patients: the majority were NYHA functional class I or II patients, with no recent history of arrhythmias and laboratory values mostly within the normal range. Notably, however, their renal function was mildly impaired. Subgrouping this cohort according to their age, region, and type of Fontan repair revealed some differences that warrant further investigation.
The prevalence of arrhythmias, a known complication of Fontan palliation, Reference Pundi, Johnson and Dearani11 is slightly lower in this registry than previously reported. Reference Zheng, Li and Li12 Notably, fewer patients who had undergone the extra-cardiac conduit procedure reported a history of arrhythmia compared with the lateral tunnel in our study, and this concurs with the results of two recent meta-analyses that demonstrated a lower risk of late arrhythmias with the extra-cardiac conduit procedure. Reference Zheng, Li and Li12,Reference Li, Fan and Hirata13 Overall, in this study of “stable” (and, consequently, quite young) Fontan patients, the majority (81%) had no record of arrhythmia within the 2 years prior to registration.
Pacemaker use in this study (24.0%) is lower than a previous single-centre study including only adult Fontan patients, in which 53 (43.1%) of patients had a pacemaker in situ. Reference Elder, McCabe and Veledar14 Our results suggest that pacemaker implantation could be occurring at a younger age than previous decades, given that time since pacemaker insertion is only slightly less in the youngest age group compared with the oldest age group (150 versus 167 months, respectively).
Medication use tended to increase with age; however, the use of angiotensin-converting enzyme inhibitors in this study was surprisingly high overall (123 [48%] patients), and highest in the 18–25-year-old age group. This is slightly higher than findings from the much larger Australian and New Zealand Fontan registry, in which 36% of 1268 surviving patients were being treated with an angiotensin-converting enzyme inhibitor in 2015, Reference Wilson, Iyengar and Winlaw15 despite a lack of evidence on the safety and efficacy in single-ventricle patients. Reference Wilson, Iyengar and Winlaw15,Reference Wilson, Iyengar and d’Udekem16 Reasons for prescription were not captured, so it is difficult to interpret the use of angiotensin-converting enzyme inhibitors by age group, and there was little variation between regions (51.6% versus 46.5% of patients in the USA and Europe, respectively).
Overall, renal function, as indicated by creatinine clearance, was mildly impaired in PREpArE-Fontan patients. Exploratory analyses showed that patients with reduced creatinine clearance were more likely to be on additional medications such as angiotensin-converting enzyme inhibitors, suggesting that they were a “sicker” population that required medication, and in whom impaired organ function was more likely. It is not possible to make a valid interpretation without a larger sample size, but the finding of mild renal impairment is consistent with recent findings. Renal dysfunction is now known to be relatively common in Fontan patients, with an estimated glomerular filtration rate <90 ml/min/1.73 m2 being reported in 10–53% of long-term survivors, in whom it is associated with poor prognosis. Reference Opotowsky, Baraona and McCausland17–Reference Patel, Kwiatkowski and Andrei20 Furthermore, a recent systematic review of the factors associated with long-term mortality after the Fontan procedure identified renal failure as the fourth most common cause of late death. Reference Alsaied, Bokma and Engel21 This is due to elevated central venous pressure and impaired cardiac output resulting in a cascade of pathophysiological consequences in Fontan patients, including a progressive decline in renal function. Reference Rychik22 It is possible to speculate on the high levels of poor renal function observed, but the results from this registry, and previous studies, suggest a need for further investigation and increased screening for signs of chronic kidney disease in Fontan patients.
There were a few substantial differences between Europe and the USA in terms of Fontan surgery type, prescriptive practices, and assessments performed within the previous year. The differences in assessments performed within the year prior to registration may be explained by differences in local guidelines, healthcare systems, and resources available to each of the sites. It is reassuring that over 90% of PREpArE-Fontan patients from both continents had received an echocardiogram in the previous year, given the recent recommendations for echocardiographic assessment to be performed annually at a minimum. Reference Baumgartner, De Backer and Babu-Narayan23–Reference Sachdeva, Valente and Armstrong26 The 2019 statement from the American Heart Association recommends regular hepatic screening, so the percentage of Fontan patients having an abdominal ultrasound may rise in the future (50.5% American and 35.2% European PREpArE registrants). As the registry design allowed for the collection of information extending only 1 year prior to registration, it should be noted that patients could have been just outside this window for assessments to be declared in this registry. For other disparities between the regions in surgery type and prescriptions, we cannot exclude the possibility that underlying age differences are partly responsible.
The percentage of PREpArE-Fontan adults who were overweight or obese (31%) was lower than that observed in the general American (70%) or European (53%) population. Reference Marques, Peralta and Naia27,28 This is relevant because being overweight is associated with poor prognosis of Fontan-palliated patients, Reference Martinez, Byku and Novak29,Reference Cohen, Zak and Atz30 and being underweight can also be a sign of poor medical condition. Reference Cohen, Zak and Atz30 However, as this registry included a “stable” survivor group, those with cardiac cachexia may have been already excluded, but “sick” survivors with Fontan failure whose BMI would be overestimated due to fluid rather than true body mass may have been incorrectly included.
Importantly, 53.4% of patients had clinical characteristics of stability and were potentially eligible for the RUBATO trial, investigating the safety and efficacy of macitentan in Fontan patients. The lack of a subpulmonary ventricle in Fontan physiology means that a low pulmonary vascular resistance is required to maintain cardiac output, hence the importance of investigating pulmonary vasodilators in Fontan patients. However, adequately powered clinical trials are difficult to conduct, given the rarity and severity of the condition, and the evidence so far is limited. Reference Hebert, Mikkelsen and Thilen6,Reference Goldberg, Zak and Goldstein7
This cross-sectional study provides real-world data on “stable” Fontan-palliated patients across two continents. These findings do not reflect the prognosis of the entire and diverse real-world Fontan population, due to the sickest patients being excluded to achieve the study’s aim. The study has limitations inherent to its observational design (clinical assessments performed as per routine clinical practice) and limitations related to its cross-sectional design (retrospective data collection and a lack of follow-up assessments). In addition, data collection was based on investigators’ holistic review of patients’ medical records and the formula used to calculate creatinine clearance was not captured. A cohort of 254 adult Fontan patients represents a reasonable size given that patients were enrolled over 14 months, but the relatively low number of patients in this study, compared with the Australian and New Zealand registry that had a much longer period of recruitment, Reference Iyengar, Winlaw and Galati5 is a limitation. However, this registry has the advantage of being international, and therefore able to highlight differences between Europe and the USA. Further work is required to better understand Fontan pathophysiology and develop novel therapeutic strategies.
Conclusions
The PREpArE-Fontan registry characterises a sizeable cohort of “stable”, long-term survivors of modern Fontan surgery who could potentially be eligible for recruitment in a placebo-controlled clinical trial, such as the RUBATO trial. Moreover, some patients presented with functional limitations and subtle clinical signs suggestive of early Fontan failure, highlighting the importance of considering renal function in clinical practice. Data from this registry demonstrate that further study is needed to better understand the issues Fontan-palliated patients experience following modern surgery.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951121002791.
Acknowledgements
The authors would like to thank the patients and physicians who participated in the study. Medical writing and editorial support were provided by Victoria Atess and, Richard McDonald of Watermeadow Medical, an Ashfield Company, funded by Actelion Pharmaceuticals Ltd, a Janssen Pharmaceutical Company of Johnson & Johnson.
Contributors
All authors have made substantial contributions to all of the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted.
Financial support
The PREpArE-Fontan registry was sponsored by Actelion Pharmaceuticals Ltd, a Janssen Pharmaceutical Company of Johnson & Johnson.
Conflicts of interest
LS has nothing to disclose. JA has received institutional research funding from Janssen Pharmaceutical Companies of Johnson & Johnson. Yd’U has received consultant fees from Janssen Pharmaceutical Companies of Johnson & Johnson and MSD, and travel fees from Berlin Heart. Yd’U is a Clinician Practitioner Fellow of the NHMRC (1082186). Murdoch Children’s Research Institute is supported by the Victorian Government’s Operational Infrastructure Support Program. CF is an employee of Mapi (an ICON Plc company). Actelion (a Janssen Pharmaceutical Company of Johnson & Johnson) contracted Mapi to conduct this study. WJF is a consultant for Actelion US, a Janssen Pharmaceutical Company of Johnson & Johnson. AH is a consultant for Bayer. In the last 5 years, he has received speaker honoraria from Janssen Pharmaceutical Companies of Johnson & Johnson, OMT, AOP Orphan, Pfizer, GlaxoSmithKline, Medtronic, and Schiller. He is shareholder (<25,000 EUR) of Johnson & Johnson, Gilead, and Merck & Co. Inc. (MSD), as well as of funds investing in healthcare companies. His institution has participated in studies for Janssen Pharmaceutical Companies of Johnson & Johnson, Lilly, Bayer, Bristol Myers Squibb, Medtronic, Novartis, Pfizer, Edwards, and Occlutec. His institution has received unrestricted grants for investigator-initiated trials from Janssen Pharmaceutical Companies of Johnson & Johnson and Medtronic. YK has received institutional research funding from Janssen Pharmaceutical Companies of Johnson & Johnson. EM was an employee of Actelion Pharmaceuticals Ltd, a Janssen Pharmaceutical Company of Johnson & Johnson, at the time of the submitted work and is now an employee of F. Hoffmann-La Roche Ltd. DR is an employee of Actelion Pharmaceuticals Ltd, a Janssen Pharmaceutical Company of Johnson & Johnson. MS has received institutional research funding from Janssen Pharmaceutical Companies of Johnson & Johnson. PC is a paid consultant for Actelion Pharmaceuticals Ltd, a Janssen Pharmaceutical Company of Johnson & Johnson.
Ethical standards
The necessary ethical approvals were obtained for each site before the initiation of the site. Approval of the protocol was given by the following local ethics committee review boards: Ethics Committee of the Faculty of Medicine of the University of Munich for German sites (approval reference: 365/16 S), central approval from Cantonal Ethics Committee Bern in Swiss sites (2016-00078), South Central – Oxford C Research Ethics Committee for the UK sites (approval reference: 15/SC/0770), and the University of Pennsylvania institutional review board (approval reference: 823962) and the Institutional Review Board for Baylor College of Medicine and Affiliated Hospitals (approval reference: H-39462) and the UCLA office of the human research protection program (approval reference: 15-001917) for the US sites. Submission to an ethics committee was not necessary for sites in Denmark. The initial protocol was submitted to the Danish Data Protection, which confirmed that the study did not need to be reported to the Danish Data Protection Agency.
Data-Sharing statement
The data sharing policy of the Sponsor is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Social media post
This registry describes the clinical characteristics of over 100 “stable” patients for at least 10 years following modern Fontan surgery, who could be eligible for the RUBATO clinical trial, and identifies subtle signs suggestive of early Fontan failure.










