With the advancement of catheter materials and imaging techniques related to interventional procedures, cardiac catheterisation has become a routine treatment method for alleviating or treating specific CHD patients in neonates. Reference Barry, Bouhout, Turner, Petit and Kalfa1 Pulmonary atresia with intact ventricular septum and critical pulmonary stenosis usually have to be treated in the neonatal period, as their pulmonary blood flow depends on the patent ductus arteriosus. For neonates with pulmonary atresia with intact ventricular septum and critical pulmonary stenosis, percutaneous balloon pulmonary valvuloplasty and percutaneous pulmonary valve perforation can establish and expand the right ventricular pulmonary artery connection, increase stable pulmonary blood flow, and promote right ventricular development. For pulmonary atresia with intact ventricular septum and critical pulmonary stenosis without right ventricular-dependent coronary circulation, treatment with percutaneous pulmonary valve perforation and percutaneous balloon pulmonary valvuloplasty can avoid surgical intervention and significantly improve prognosis. Reference Mortezaeian, Khorgami and Omidi2 Ductal stenting can provide acceptable short-term relief for neonates with unstable pulmonary blood flow. Compared with the Blalock-Taussig shunt, ductal stenting can lead to a higher mid-term survival rate, fewer complications, lower cardiac ICU, and total hospital stays. Reference Tseng, Truong and Peck3 Therefore, cardiac catheterisation has become an alternative to traditional surgical procedures for neonates with pulmonary atresia with intact ventricular septum or critical pulmonary stenosis. Reference Cheung, Mastropietro and Flores4
Although the trauma of neonatal cardiac catheterisation is lower than that of surgical procedures, some complications may occur during or after the procedure. For pulmonary atresia with intact ventricular septum and critical pulmonary stenosis neonates undergoing cardiac catheterisation, it is necessary to maintain sufficient volume and myocardial contractility to avoid a decrease in systemic vascular resistance and an increase in pulmonary vascular resistance, as these can further reduce pulmonary blood flow and hypoxia. In addition, complete or partial occlusion of the ductus arteriosus during the perioperative period can lead to refractory hypoxaemia and life-threatening haemodynamic instability. Other possible complications include arrhythmia (mostly temporary), vascular trauma, cardiac or large vessel perforation, cardiac valve injury, blood transfusion, hypothermia, and drug allergic reactions. Reference Alakhfash, Jelly and Almesned5 This study retrospectively analysed the clinical data of neonates with pulmonary atresia with intact ventricular septum and critical pulmonary stenosis who underwent cardiac catheterisation in our hospital from January 2015 to October 2022 and summarised our anaesthesia regimen and complications during these procedures. We present the following article in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting checklist.
Methods
Participants
All clinical data were collected from all newborns with pulmonary atresia with intact ventricular septum and critical pulmonary stenosis who were hospitalised in our hospital from January 2015 to October 2022. The inclusion criteria for this study were as follows: (I) the first treatment was based on cardiac catheterisation; (II) no complicated CHD with other types; and (III) no right ventricular-dependent coronary circulation. The inclusion criteria for critical pulmonary stenosis were as follows: diagnosis by echocardiography or angiocardiography, that is, severe stenosis of the pulmonary valve orifice, right ventricular systolic pressure greater than the left ventricle, accompanied by patent ductus arteriosus, bidirectional shunt or right to left shunt through the oval foramen (atrial septal defect), and moderate to severe tricuspid regurgitation. The inclusion criteria for pulmonary atresia with intact ventricular septum were as follows: (I) diagnosis of pulmonary atresia with intact ventricular septum by echocardiography; (II) the right ventricular inflow tract, outflow tract and trabecular part all exist, and tricuspid valve annulus Z-value > −3; and (III) pulmonary valve is membranous atresia.
Preoperative preparation
Before the procedure, selective infusion of prostaglandin E1 was performed according to the patent ductus arteriosus status of the patient to maintain patent ductus arteriosus patency to obtain stable pulmonary blood flow. Supplementation of the patient with low-concentration oxygen to maintain the necessary pulse oxygen saturation and active radiation heating was performed as needed to maintain normal body temperature. Percutaneous balloon pulmonary valvuloplasty was first performed using a coronary balloon, followed by a balloon of (1–1.3) pulmonary valve cannula. Percutaneous pulmonary valve perforation was performed using a specialised guide wire for chronic total occlusion, which had a higher success rate than radiofrequency perforation. Reference Kamalı, Tanıdır, Erdem, Sarıtaş and Güzeltaş6
Anaesthesia technique
Intraoperative monitoring included electrocardiogram, heart rate, systolic blood pressure, diastolic blood pressure, pulse oxygen saturation, urine output, and body temperature. Anaesthetic induction was achieved by low-concentration oxygen inhalation (<40%), intravenous injection of midazolam 0.2 mg/kg, fentanyl 0.5 μg/kg, cisatracurium 0.1 mg/kg, and ketamine 1 mg/kg, followed by insertion of a 3.5 or 4.0 uncuffed endotracheal tube under direct laryngoscopy. If the neonate arrived at the catheterisation laboratory (Cath Lab) and was intubated, induction with sevoflurane was performed through endotracheal tube, with intravenous injection fentanyl 0.5 μg/kg and cisatracurium 0.1 mg/kg. The endotracheal tube was confirmed above the level of the tracheal carina by auscultation of breath sounds. Sevoflurane (0.8–1.0 MAC) was used to maintain anaesthesia. For newborns with pulmonary atresia with intact ventricular septum, the left radial artery was selected for ultrasound-guided arterial catheterisation and invasive blood pressure monitoring. For patients without a central venous catheter before the procedure, the right internal jugular vein was chosen for ultrasound-guided central venous catheterisation. The calculated dose of prostaglandin E1 was connected to the intravenous line for continuous infusion in pulmonary atresia with intact ventricular septum patients with finer patent ductus arteriosus and for emergency use in other patients. For puncture of the femoral artery and vein, ultrasound guidance can be chosen according to physician preference. The 5 French sheath was fixed in the femoral vein, and the 4 French sheath was fixed in the femoral artery, with heparin given at 0.5 mg/kg. Haemodynamics and pulse oxygen saturation were closely monitored throughout the procedure, especially during manipulations that may affect the pulmonary blood flow.
Data collection and statistical analysis
Heart rate, systolic blood pressure, diastolic blood pressure, and pulse oxygen saturation were collected at the following time points: before the start of the procedure (T0), at percutaneous balloon pulmonary valvuloplasty (T1), after percutaneous balloon pulmonary valvuloplasty (T2), and when the patient was transferred out of the cath lab (T3). Clinical outcomes included procedure time and anaesthesia time. Severe cardiovascular adverse events during the perioperative period, including arrhythmia, vascular trauma, cardiac perforation, and cardiac arrest, were recorded. All analyses were performed using SPSS 26 software (IBM, Armonk, New York, USA). The Kolmogorov-Smirnow test was performed to determine the conformity of the data to normal distribution. If the measurement data of a group conformed to a normal distribution, the mean ± standard deviation was used to describe the difference between groups. If the data were not normally distributed, the median (interquartile range) is provided. The comparison between groups was performed using the Mann–Whitney U test. Repeated measurement analysis of variance was used for statistical processing. The count data are expressed as frequencies (percentages). Bivariate comparisons of variables between pulmonary atresia with intact ventricular septum and critical pulmonary stenosis were performed using χ2 and Fisher exact tests. p < 0.05 was considered statistically significant.
Results
Patient demographics, clinical data, and results of the catheterisation procedure
This study included 71 patients, of which six patients underwent surgery directly due to severely underdeveloped right ventricle, and two patients had other complex CHDs; thus, a total of 63 patients met the inclusion criteria (Figure 1). All patients survived the intervention. Central venous catheter and invasive arterial blood pressure monitoring were provided for 26 neonates during the perioperative period. Among the patients with critical pulmonary stenosis, 40 successfully received percutaneous balloon pulmonary valvuloplasty, while three patients received ductal stenting due to moderate right ventricular dysplasia at the same time. For patients with pulmonary atresia with intact ventricular septum, 17 of the 23 patients successfully underwent percutaneous pulmonary valve perforation and percutaneous balloon pulmonary valvuloplasty. Of these, five patients underwent ductal stenting due to unstable patent ductus arteriosus blood flow. Three patients only underwent ductal stenting. In addition, three patients received hybrid therapy. Compared with critical pulmonary stenosis, the procedural time and anaesthesia time of pulmonary atresia with intact ventricular septum were significantly prolonged, as were the numbers of patients using prostaglandin E1 and vasoactive substances in the perioperative period, and postoperative mechanical ventilation time > 24 h was also significantly increased (Table 1).
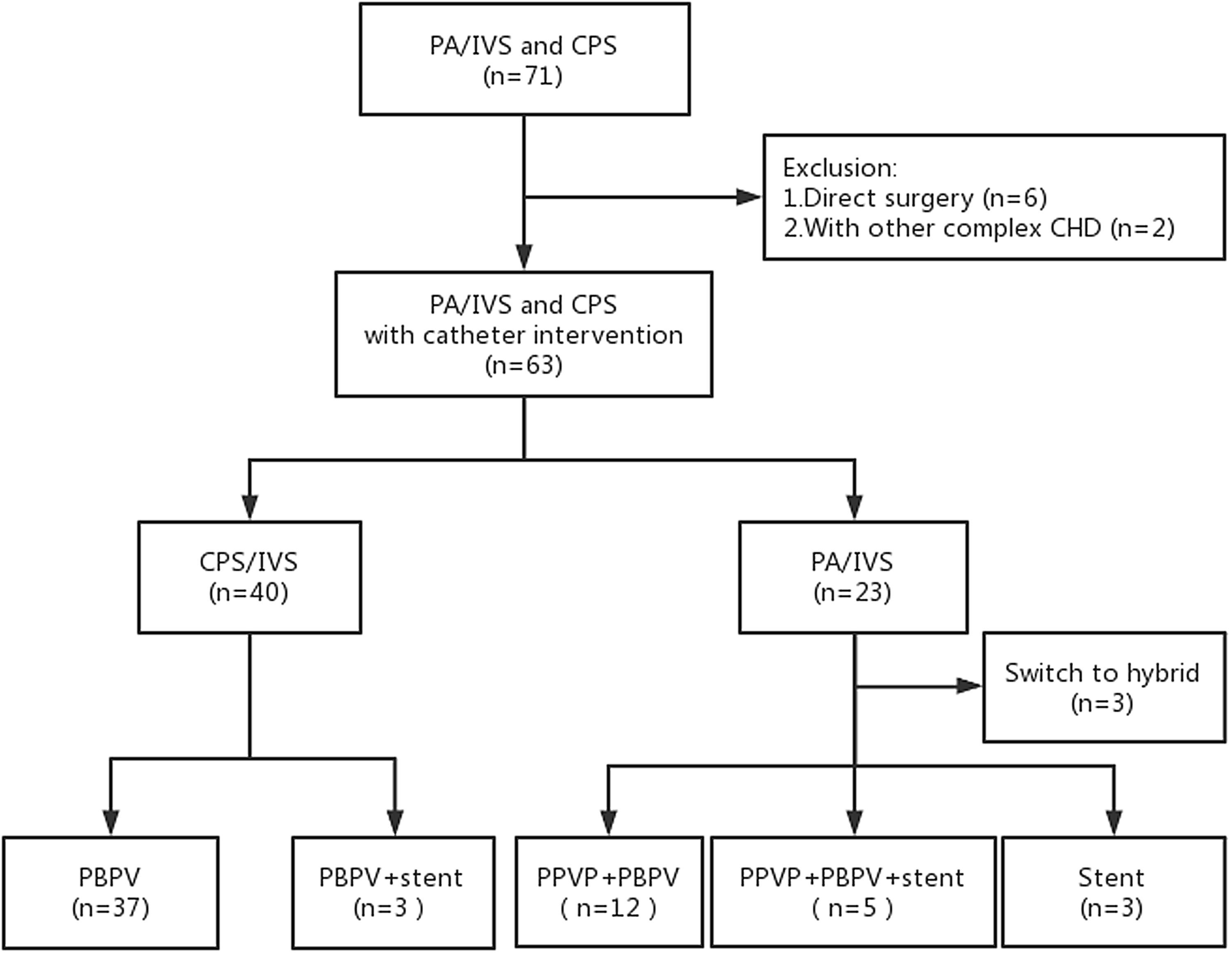
Figure 1 Study flow chart. PA/IVS, pulmonary atresia with intact ventricular septum; CPS, critical pulmonary stenosis; CHD, congenital heart disease; PBPV, percutaneous balloon pulmonary valvuloplasty; PPVP, percutaneous pulmonary valve perforation
Table 1. Demographics and procedural data
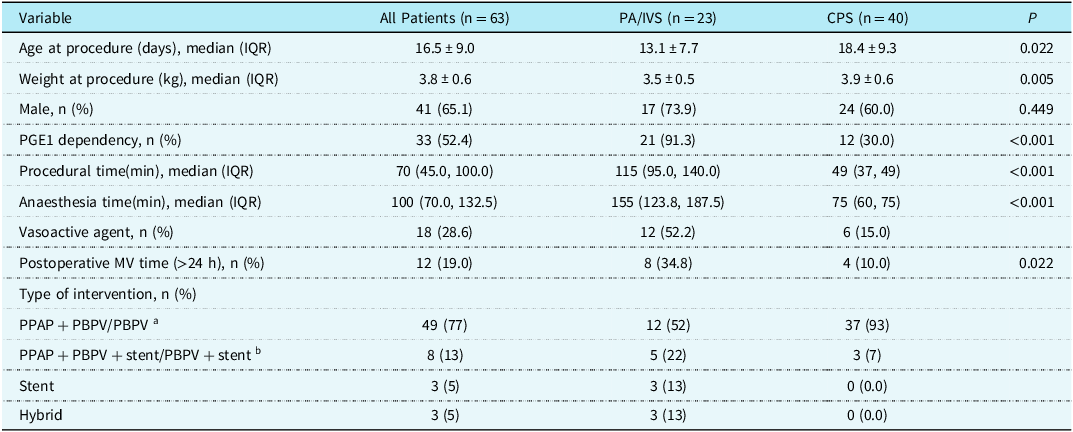
PA/IVS = pulmonary atresia with intact ventricular septum; CPS = critical pulmonary stenosis; PGE1 = prostaglandin E1; PPVP = percutaneous pulmonary valve perforation; PBPV = percutaneous balloon pulmonary valvuloplasty; MV = mechanical ventilation; PDA = patent ductus arteriosus.
a PA/IVS patients received PPAP + PBPV, while CPS patients received PBPV.
b PA/IVS patients received PPAP + PBPV + stent, while CPS patients received PBPV + stent.
Data and comparison of vital signs
The data and comparison of vital signs at different time points are shown in Figure 2. Both pulmonary atresia with intact ventricular septum and critical pulmonary stenosis patients experienced changes in vital signs during balloon dilation, manifested by a slowed heart rate, decreased blood pressure, and decreased pulse oxygen saturation. Compared to critical pulmonary stenosis patients, pulmonary atresia with intact ventricular septum patients exhibit more unstable perioperative haemodynamics and pulse oxygen saturation. After balloon dilation, the pulse oxygen saturation of critical pulmonary stenosis patients significantly increased. However, towards the end of the procedure, their pulse oxygen saturation indicated a slight reduction without statistical significance. The reason for the significant increase in pulse oxygen saturation in pulmonary atresia with intact ventricular septum patients after surgery is that some patients received ductal stenting, which increased pulmonary blood flow.
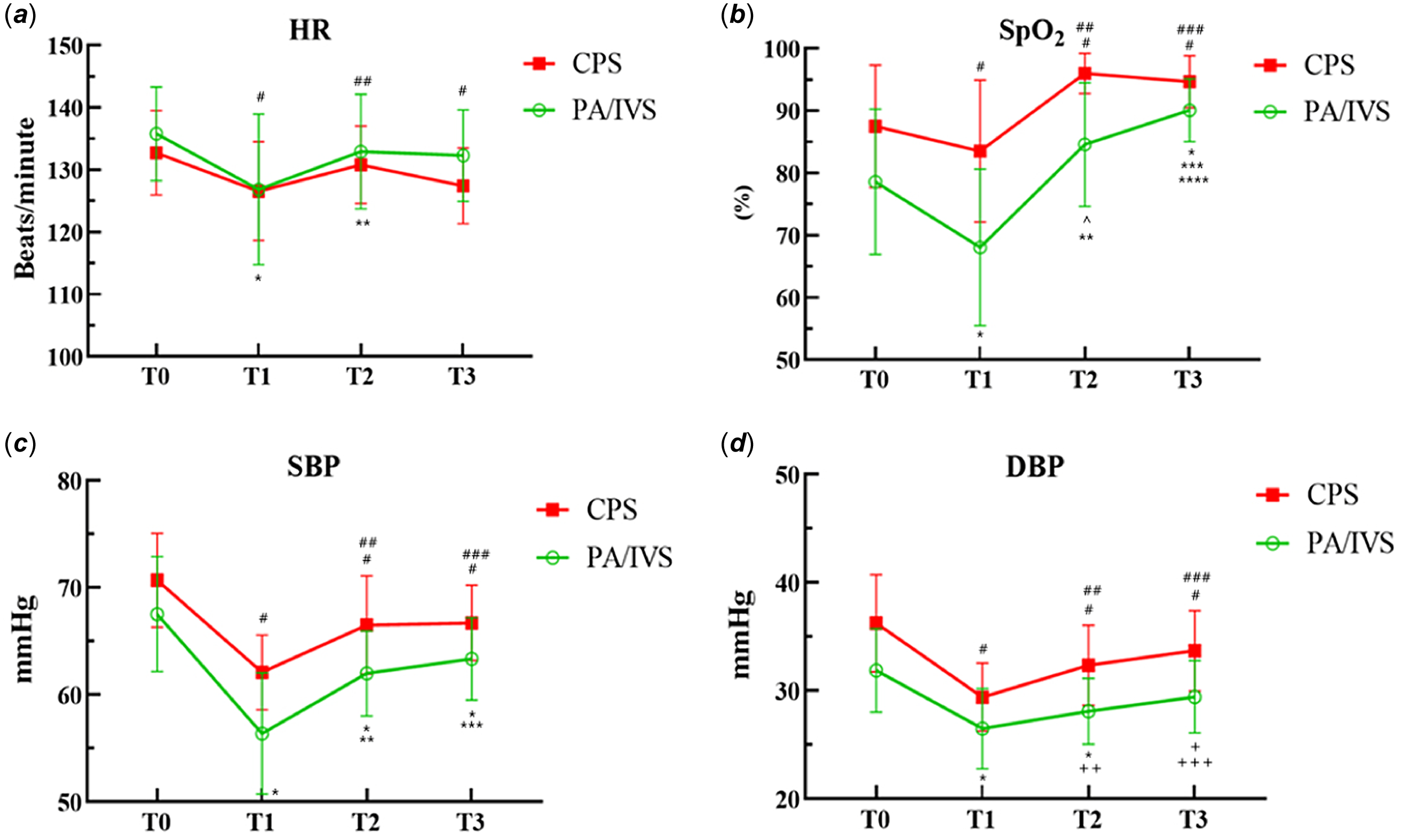
Figure 2 Vital signs measured at each time point. Heart rate (HR, A), pulse oxygen saturation (SpO2, B), systolic blood pressure (SBP, C), and diastolic blood pressure (DBP, D) were recorded at T0 (before the start of the procedure), T1 (at perform PBPV), T2 (after the PBPV), and T3 (when the patient was transferred out of the cath lab). Values shown are means, and error bars indicate standard deviation. In the CPS group: #, represents comparison with T0, P<0.0001; ##, T1 vs. T2 P<0.0001; ###, T1 vs. T3 P<0.0001. In the PA/IVS group: *, represents comparison with T0, P<0.0001; **, T1 vs. T2 P<0.0001; ***, T1 vs. T3 P<0.0001; ^, T0 vs. T2 P=0.001; +, T0 vs. T3 P=0.007; ++, T1 vs. T2 P=0.008; +++, T1 vs. T3 P=0.004.
Cardiovascular adverse events
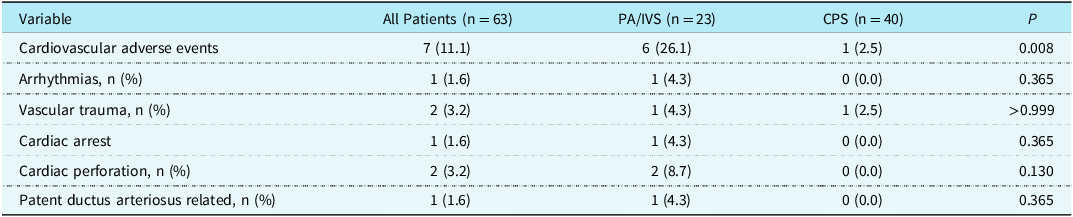
The most common cardiovascular adverse event is transient arrhythmia during the manipulation of wires or catheters. These arrhythmias are usually benign and self-limiting, but one patient develops persistent supraventricular tachycardia that requires medication treatment. In addition, differences in bilateral femoral artery pulsations after surgery are common, but there were no cases of femoral artery pulse disappearance. For pulmonary atresia with intact ventricular septum patients, one patient developed femoral arteriovenous fistula. Confusingly, a critical pulmonary stenosis patient was found to have femoral vein occlusion and collateral formation during the second procedure. In addition, there were severe cardiovascular adverse events, including one case of cardiac arrest in which brief chest compressions were performed and timely recovery was achieved. There were also two cases of cardiac or main pulmonary artery perforation, one with mild pericardial effusion, and the other underwent hybrid surgery. Blood transfusion during the perioperative period is necessary for hybrid surgery due to increased bleeding after heparin administration and thoracotomy. All three patients who underwent hybrid surgery received blood transfusion treatment. Overall, the incidence of cardiovascular adverse events in the neonatal perioperative period was higher in the pulmonary atresia with intact ventricular septum group than in the critical pulmonary stenosis group (Table 2).
Table 2. Severe cardiovascular adverse events
Discussion
In this retrospective study, we analysed 63 patients with pulmonary atresia with intact ventricular septum and critical pulmonary stenosis. All patients survived the intervention. Compared with critical pulmonary stenosis, the procedural time and anaesthesia time of pulmonary atresia with intact ventricular septum were significantly prolonged, as were the numbers of patients using prostaglandin E1 and vasoactive substances in the perioperative period, and postoperative mechanical ventilation time > 24 h was also significantly increased. During catheter intervention procedures, pulmonary atresia with intact ventricular septum patients exhibit more unstable perioperative haemodynamics and pulse oxygen saturation. In addition, the incidence of cardiovascular adverse events during the perioperative period was higher in the pulmonary atresia with intact ventricular septum group than in the critical pulmonary stenosisgroup.
Among the procedural risks of congenital cardiac catheterisation, neonatal atretic valve perforation with or without valvuloplasty is at level 5, which is the most dangerous level. Reference Quinn, Yeh and Gauvreau7 In addition to the potential risks associated with the intervention itself, pulmonary atresia with intact ventricular septum and critical pulmonary stenosis newborns are characterised by low age, low weight, cyanosis, pulmonary hypertension, and low cardiac output. which may increase their risks. For interventional treatment of pulmonary atresia with intact ventricular septum and critical pulmonary stenosis neonates, the incidence of adverse events reported by different research centres ranges from 14 to 35%. Reference Mortezaeian, Khorgami and Omidi2,Reference Melekoglu and Baspinar8,Reference Petit, Qureshi and Glatz9 In the latest multicenter cohort from the United States, the incidence of adverse events was 25.7% for pulmonary atresia with intact ventricular septum newborns who received catheter intervention during their first hospitalisation. Coronary artery stenosis and low weight during the first intervention were important factors associated with adverse events. In our pulmonary atresia with intact ventricular septum cohort, the incidence of perioperative adverse events was 26.1%. Compared to pulmonary atresia with intact ventricular septum newborns, the results of critical pulmonary stenosis percutaneous balloon pulmonary valvuloplasty were very good, with an overall success rate of 100% and an incidence of adverse events of only 2.5%, similar to the research results of Alakhfast and colleagues. Reference Alakhfash, Jelly and Almesned5
Newborns with pulmonary atresia with intact ventricular septum and critical pulmonary stenosis depend on patent ductus arteriosus to obtain sufficient pulmonary blood flow, requiring prostaglandin E1 to maintain patent ductus arteriosus patency. Identifying right ventricular-dependent coronary circulation through transesophageal echocardiography and CT before surgery is very important and affects the next treatment plan and surgical decision. Reference Gleich, Latham, Joffe and Ross10 Patients with wider patent ductus arteriosus and unrestricted atrial shunting can be placed in regular wards with continuous or discontinuous low-dose prostaglandin E1 treatment depending on the condition. However, regular wards must be able to perform echocardiography examinations and quickly transfer to the cardiac ICU in case of changes in condition. In addition, parents should be informed to pay attention to the occurrence of apnoea events and other complications of prostaglandin E1. Newborns with hypoxia and unstable patent ductus arteriosus blood flow should be closely monitored in the cardiac ICU to identify evolving physiological changes and monitor the dosage effect or side effects of prostaglandin E1. Neonates receiving prostaglandin E1 experience apnoea events, which may require selective intubation and mechanical ventilation. If cyanosis was severe, it may be necessary to supplement low-concentration oxygen. Neonates with pulmonary atresia with intact ventricular septum and critical pulmonary stenosis suffer from persistent worsening hypoxaemia or acidosis without obvious pulmonary problems, which may indicate patent ductus arteriosus closure and require emergency echocardiography. After confirming the patency of the patent ductus arteriosus, sustained hypoperfusion or acidosis indicates a limited and insufficient atrial level shunt, which may require emergency intervention. Reference Toganel11
For patients with ductal-dependent pulmonary blood flow, haemodynamic management during anaesthesia induction and maintenance should focus on maintaining appropriate pulmonary circulation and systemic circulation blood flow ratio, maintaining sufficient systemic vascular resistance to ensure adequate pulmonary blood flow. Anaesthesia usually uses composite anaesthesia induction and maintenance techniques, including opioid drugs, inhalation anaesthetics, and muscle relaxants, as well as inhalation of low-concentration oxygen. Usually, there is no need to use preanaesthesia medication. Anaesthesia induction can be achieved by inhalation or intravenous injection. Sevoflurane and propofol cause greater myocardial inhibition than fentanyl, etomidate, or ketamine, although all drugs can cause significant hypotension. When patients use nondepolarised muscle relaxants for intubation, the maintenance of anaesthesia may be the combination of opioids and inhalation anaesthesia. Reference Smith-Parrish, Vargas Chaves and Taylor12 In the absence of intravenous injection, sevoflurane inhalation induction without airway stimulation can be used. Immature myocardium is very sensitive to the inhibitory effect of volatile anaesthetics, and sudden inhalation of high concentrations should be avoided. Cardiac catheterisation usually causes minimal pain stimulation when placing vascular access sheaths. The infiltration of local anaesthetics at the puncture site can be used to alleviate the pain caused by these interventions, but surgeons may not like it and may affect the pulsation of touching the puncture vessel. Reference Tierney and Kenny13 The prepared liquid tube for liquid therapy should be carefully handled. The use of an infusion pump can accurately input the amount of liquid, and it is important to prevent hyperglycaemia and hypoglycaemia. When a neonate experiences tachycardia and hypotension, anesthesiologists should pay attention to whether it is caused by low blood volume. Because the operation time of critical pulmonary stenosis newborns is short, it is generally unnecessary to insert a urinary catheter. Pulmonary atresia with intact ventricular septum newborns should always rule out insufficient capacity, as intravascular volume consumption can increase cyanosis, but smaller urine volumes (0.5–2 mL/kg/h) may be difficult to measure. Therefore, diuretics are rarely used. According to the severity of cyanosis, a dose of 10–30 mL/kg should be used as the challenge dose. Reference Subramaniam14 Additionally, attention should be given to anticoagulation monitoring. Activated coagulation time can be used to guide heparin targeting for > 200 s. Reference Taggart, Gordon, Morgan and Goldstein15
Due to the high position of the glottis in newborns, it may be difficult to intubate. If necessary, inhalation of sevoflurane can be attempted to check the exposure of the glottis before administering muscle relaxants. A stable venous pathway is necessary, usually with central venous catheterisation. Ultrasound-guided arteriovenous puncture and catheterisation can reduce puncture time and vascular damage. Invasive pressure measurement should be performed as much as possible. Invasive blood pressure can reflect early changes in pulmonary blood flow earlier than pulse oxygen saturation. Reference Zhang, Zhang, Song and Ren16 Radial artery puncture should be carried out after anaesthesia induction to reduce the discomfort of children and avoid ductal spasm. Generally, the left upper limb radial artery is selected to avoid the ipsilateral systemic pulmonary shunt side. The establishment of a central venous catheter is necessary for pulmonary atresia with intact ventricular septum, as it can ensure a stable venous channel and the infusion of vasoactive drugs, including prostaglandin E1, norepinephrine, dobutamine, and fluid infusion. Of course, ultrasound guidance can be used to reduce the time and local trauma of arterial and central venous catheterisation. Reference Abdelbaser, Mageed, Elmorsy and Elfayoumy17 Ultrasound can improve the success rate of arterial puncture and catheterisation, which is related to the ultrasound equipment and operating doctors in local hospitals. If radial artery cannulation is unsuccessful, invasive blood pressure monitoring can be intermittently monitored by connecting the arterial pressure monitoring device after surgical femoral artery cannulation.
The infusion of prostaglandin E1 during the perioperative period should be based on the specific situation of the patient and the planned intervention plan. Connect the calculated dose of prostaglandin E1 to the venous pathway. For pulmonary atresia with intact ventricular septum patients with finer patent ductus arteriosus, continuous infusion is needed, while other patients are prepared for emergency use. In addition, when stent implantation is needed, the infusion of prostaglandin E1 needs to be suspended to obtain the best catheter contraction, reduce the occurrence of catheter stent-related complications, and may also help to minimise the problems related to pulmonary circulation excess. Reference Haddad, Hanna, Charbel, Daou, Chehab and Saliba18 The combination of norepinephrine and high-dose prostaglandin E1 can increase systemic afterload, reduce pulmonary vascular resistance, and improve lung perfusion. It should be considered that the use of catecholamines is accompanied by an increase in oxygen consumption (heart rate) and can offset the benefits of improved pulse oxygen saturation, even leading to metabolic acidosis. Reference Khalil, Jux, Rueblinger, Behrje, Esmaeili and Schranz19
The crisis events in haemodynamics mainly arise from the impact of the intervention procedure on pulmonary blood flow. Anaesthesiologists should closely monitor changes in vital signs during the procedural process. This monitoring becomes even more crucial during the stage where the use of wires and catheters could reduce pulmonary blood flow. For patients with ductal-dependent pulmonary blood flow, risks and injury to the femoral artery could be significantly reduced if the procedure only requires access through the femoral vein and does not involve catheterisation through the patent ductus arteriosus. According to our observations, haemodynamic crisis events during the process of cardiac catheterisation mainly concentrate on three stages: (I) In newborns, the space in the ventricle is small, and mechanical stimulation from guide wires or catheters during procedures can cause arrhythmia, leading to reduced cardiac output and decreased pulmonary blood flow. (II) When guide wires or catheters pass through the arterial duct and enter the main pulmonary artery, they can obstruct part of the patent ductus arteriosus and even cause temporary spasms, resulting in decreased pulmonary blood flow. (III) When a balloon is used to dilate the pulmonary valve, the inflated balloon can obstruct the right ventricular outflow tract, particularly in critical pulmonary stenosis patients without patent ductus arteriosus, leading to a significant reduction in pulmonary blood flow.
The general risks associated with neonatal cardiac catheterisation include arrhythmia, difficulty in establishing vascular access, vascular injury, myocardial perforation, and bleeding. For patients who require ductal stenting support, complications also include stent thrombosis and displacement.
The most common cardiovascular adverse event during the perioperative period is transient arrhythmia, with an incidence rate of 10% -29.2%, mostly caused by the manipulation of guide wires or catheters. Reference Loureiro, Cardoso, Gomes, Martins and Pinto20,Reference Yucel, Bulut, Kucuk, Balli and Celebi21 This may be due to the mechanical stimulation of cardiac nerves or direct irritation of the myocardium by the devices used during the procedure. In most cases, cardiac arrhythmias will spontaneously subside and do not require treatment. If arrhythmia has clinical significance, the first treatment to be used is to stop the catheter procedure. In cases where arrhythmia cannot be resolved through catheterisation, treatment should be carried out according to neonatal arrhythmia guidelines, including medication, electrical cardioversion, and even cardiopulmonary resuscitation. Reference Kasar, Tanıdır and Öztürk22 If not handled in a timely manner, arrhythmia may lead to cardiac arrest. In a study of 7289 procedures, the researchers found that there were 70 cases of cardiac arrest, of which 38 were caused by arrhythmia, indicating that arrhythmia should not be taken for granted. Reference Odegard, Bergersen and Thiagarajan23 In our case, transient arrhythmias were relatively common, with most cases recovering sinus rhythm by pausing surgical procedures, withdrawing the catheter, and only one case developing supraventricular tachycardia that required medication maintenance treatment.
Vascular-related injuries are also common, mostly involving the femoral artery. When establishing catheter access, the blood vessels of newborns are thinner. If the puncture and catheterisation are not smooth, arterial vasospasm will occur. If the outer diameter of the indwelling arterial sheath is large and blood anticoagulation is insufficient, thrombosis may form, and even thrombotic occlusion may occur. In a study on patent ductus arteriosus stent placement reported by Agha, femoral artery thrombosis occurred in seven newborns (7.2%). Reference Agha, Abd-El Aziz and Kamel24 A study by Tadphale et al. suggested that a diameter of less than 3 millimetres in the femoral artery and a ratio of sheath outer diameter to vascular lumen diameter greater than 50% are associated with an increased risk of experiencing palpable disappearance of femoral artery pulsation at the end of surgery. Reference Tadphale, Yohannan and Kauffmann25 Multiple studies have shown that low body weight, larger arterial sheath diameter, and longer surgical time are independent risk factors for femoral artery injury. Reference Kou, Wang, Long, Tang and Li26 Alexander et al. indicated that the use of ultrasound guidance in paediatric cardiac catheterisation can reduce the incidence of loss of arterial pulse requiring treatment. Reference Alexander, Yohannan and Abutineh27 In summary, newborns with femoral artery catheterisation may experience differences in pulse intensity between the lower limbs on both sides and even develop loss of arterial pulse. Before surgery, the diameter of the femoral artery should be measured using conventional ultrasound, and a smaller guiding sheath should be used to actively avoid entering the femoral artery when the femoral artery diameter is less than 3 millimetres. When loss of arterial pulse is noted at the end of the surgery, ultrasound evaluation and Doppler pulse assessment of the dorsal foot artery should be performed, and anticoagulant therapy should be administered if necessary. In our study, neonatal vascular injury in critical pulmonary stenosis was relatively rare, as only three patients underwent femoral artery catheterisation, with two patients experiencing differences in the pulsation of both femoral arteries. Confusingly, a critical pulmonary stenosis patient was found to have femoral vein occlusion and collateral formation during the second procedure. Most pulmonary atresia with intact ventricular septum patients had differences in bilateral femoral artery pulsation, but no loss of arterial pulse occurred. One patient with pulmonary atresia with intact ventricular septum developed a femoral arteriovenous fistula after surgery.
According to anatomical variations, newborns with pulmonary atresia with intact ventricular septum who undergo percutaneous pulmonary valve perforation have a relatively high risk of surgical complications, especially right ventricular or main pulmonary artery perforation. A multicenter study by the Congenital Catheterisation Research Collaboration showed that the risk of cardiac perforation was 10.5%, with a higher risk of using radiofrequency catheterisation compared to wire catheterisation. Reference Petit, Qureshi and Glatz9 In a study by Lawley et al., out of 12 initial treatment cases of pulmonary atresia with intact ventricular septum pulmonary valve perforation, two patients developed pericardial tamponade requiring emergency extracorporeal membrane oxygenation support due to pulmonary artery perforation. Reference Lawley, Hockey, Yeo, Liava'a and Roberts28 A study on interventional cardiac catheterisation of newborns: results from the Italian multicenter experience. In 1423 neonatal cardiac catheterisation procedures, a total of 47 cases of pericardial effusion occurred, of which 12 cases required drainage tube placement. A higher Catheterisation RISk Score for Pediatrics lower age, and surgical category (percutaneous pulmonary valve perforation) are independent predictors of cardiac perforation. Reference McCrossan, Karayiannis, Shields and etal29 In this study, two patients had cardiac or main pulmonary artery perforation, one patient had mild pericardial effusion that was not treated, and another patient underwent open chest hybrid surgery.
Ductal stenting is highly correlated with severe adverse complications. Ductal spasm and dissections can occur in rare cases. Reference Chen, Chen and Wang30 When using a guide wire to pass through patent ductus arteriosus, extreme caution should be exercised. In the case of ductal spasm, the wire and catheter should be withdrawn, and prostaglandin E1 should be continuously infused. If not relieved, a stent or Blalock-Taussig needs to be urgently inserted. In a few cases, stent migration or displacement may occur, and reducing the chance of this complication can be accomplished by fully constraining the ductus arteriosus by ceasing prostaglandin E1 administration. Reference Bahaidarah, Al-Ata and Alkhushi31 In rare cases, it may be necessary to urgently remove the stent in the operating room and switch to a Blalock-Taussig shunt. Ductal stenting can also artificially cause severe low diastolic blood pressure or compress the left main bronchus. Reference Faccini and Butera32,Reference De Decker, Comitis Smith, Saul, Goldfarb, Biko and O.'Byrne33 In our case, one child experienced spasms and partial occlusion when the guide wire passed through the patent ductus arteriosus, resulting in the urgent implantation of a ductal stent.
Although the risks in neonatal cardiac catheterisation can be reduced, they cannot be avoided. Some researchers have reported improved surgical techniques or intervention tools, most of which use coronary guide wires, coronary artery balloons, and various types of catheters. In addition to intervention methods and techniques, future research should aim to develop and produce intervention tools suitable for newborns. Smaller catheters, lower contour balloons and stents, and creative vascular access techniques make percutaneous intervention possible for the smallest newborns. For example, both current radiofrequency drilling and wire tip drilling pose a risk of myocardial perforation. Developing a device similar to the three-dimensional mapping for the treatment of arrhythmia and applying it to the anchoring target of the central position of the pulmonary valve is also a research direction. Reference Fukuda, Haramitsu, Yazu, Higashimori, Shiotani and Yokoi34
This study has several limitations. First, this study reviewed the immediate complications during the perioperative period. Due to follow-up issues, the long-term prognosis and complications were not statistically analysed. Second, a multicenter, large-sample observational study is needed to further classify the surgery in detail, determine the risk factors for cardiovascular adverse events, and develop an individualised perioperative management plan. Finally, due to sample size limitations, it is difficult to include more factors for multivariate analysis.
Conclusions
Due to the complexity and low incidence of diseases in newborns with pulmonary atresia with intact ventricular septum and critical pulmonary stenosis, prospective research on perioperative management measures is limited. Continuous innovation in interventional cardiology will lead to smaller and more severe patients receiving minimally invasive cardiac catheterisation treatment. All team members should fully understand the pathological and physiological changes of each patient, prepare for the procedure, including possible surgical tools and medication preparation, notify surgeons, and describe possible surgical procedures. The surgeon should pay attention to meticulous operation and may neglect the vital signs of the patient due to their concentration on the surgery. Anaesthesiologists should closely monitor and promptly remind and cooperate with the surgeon for treatment.
Acknowledgements
None.
Financial support
None.
Competing interests
None.
Ethical standard
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. This retrospective study was approved by the Institutional Review Board of Qingdao Women and Children’s Hospital affiliated with Qingdao University.







