Background
Fontan surgery was described by two surgical groups in the late 1960s, one led by François Fontan in France and the other by Guillermo Kreutzer in Brazil. Initially, it was performed on patients with tricuspid atresia. Still, later it was indicated for patients with other defects with univentricular physiology, such as double-inlet to a single-ventricle hypoplastic left ventricular syndrome, mitral atresia with standard aortic root, and septal defects. Complete unbalanced atrioventricular septal defects, pulmonary atresia with an intact septum, severely hypoplastic right ventricle, or any heart defect with a single functional ventricle. Reference Cazzaniga, Fernández Pineda and Villagrá1,Reference Rao2
Fontan surgery is the last procedure planned within the palliative pathway. It seeks to ensure flow to and from the single ventricle, separating systemic and pulmonary venous drainage, restoring serial circulation, and preventing hypoxaemia and pulmonary vascular disease. In the last 30 years, the technique has undergone changes which have managed to reduce morbidity and mortality and have improved survival. Reference Rao2
In 1977, doctors Chussat and Fontan published 10 commandments to choose the ideal candidate for the procedure. In recent years, these commandments have been refined in light of the available evidence in an attempt to prevent as many complications as possible. In this order of ideas, patients between 4 and 5 years of age are taken into account, who have sinus rhythm, normal drainage of the vena cava, normal volume of the right atrium, competent mitral valve, left ventricular ejection fraction) greater than or equal to 60%, absence of alterations in the pulmonary arteries, mean pulmonary artery pressure less than or equal to 15 mmHg, resistance of the pulmonary arterioles less than 4 U/Wood/m2, pulmonary artery diameter ratio/aorta greater than or equal to 0.75, McGoon greater than 2, Nakata greater than 250 mm2/m2, systemic vascular resistance index 20 U/m2, pulmonary vascular resistance index 1–3 U/m2, absence of valvular regurgitation, end-diastolic pressure less than 12 mmHg, in addition to ventricular morphology, taking into account that right-sided and/or indeterminate morphology are poor prognostic factors both in terms of mortality and morbidity. Likewise, a weight of less than 15 kg usually increases the risk of surgically treating CHD. Reference Book, Gerardin, Saraf, Marie and Rodriguez3,Reference Giannico, Hammad and Amodeo4
The physiology of this procedure establishes deleterious effects on both the pulmonary and systemic circulation, leading to long-term morbidity. One of them is Fontan failure, which occurs in 4% of patients. It is defined as a clinical syndrome in which the circulation cannot meet the metabolic demands of the organism. Reference Book, Gerardin, Saraf, Marie and Rodriguez3 This can be classified into three categories: ventricular dysfunction, systemic physiology complications, and chronic Fontan failure. Risk factors described with failure include heterotaxy, extracardiac Fontan, no fenestration, elevated ventricular pressure, balloon atrial septostomy, pulmonary hypertension, systolic dysfunction, common atrioventricular canal, and atrioventricular valve regurgitation. Reference Rogers, Glatz and Ravishankar5–Reference Podzolkov, Chiaureli and Yurlov7
A study in Colombia Reference Vargas, Vargas, Castilla, Rodriguez and Martinez8 showed that the most common causes of early failure are pleural effusion (52.4%), myocardial dysfunction, and surgical wound infection (19%). Regarding late complications, arrhythmias (38%), protein-losing enteropathy (8%), plastic bronchitis, liver disease, and cerebral thromboembolic events (1%), among others, have been documented. Pundi et al,9 in their work on 40-year follow-up after Fontan surgery, observed overall survival at 10, 20, and 30 years in 1,052 patients of 74, 61, and 43%, respectively.
Factors associated with decreased overall or late survival included pre-operative use of diuretics, increased cardiopulmonary bypass time, procedures performed before 1991, atrioventricular valve replacement at the time of surgery, increased circuit pressure (20 mmHg) or left atrial pressure (13 mmHg), prolonged chest tube drainage (21 days), post-operative ventricular arrhythmias, renal failure, and protein-losing enteropathy. Reference Pundi, Johnson, Dearani, Pundi, Li and Hinck9
Therefore, the objective of the study is to describe the characteristics and risk factors associated with Fontan failure in a reference centre for paediatric cardiology and cardiovascular surgery. This is over 21-year period.
Materials and methods
This is a retrospective analytical study that included all patients diagnosed with Fontan failure during a 21-year follow-up at the CardioVID Clinic, a reference centre for paediatric cardiology and cardiovascular surgery in Medellin, Colombia. The study was approved by the CardioVID Clinic ethics committee.
Patients
All patients diagnosed with Fontan failure who met the inclusion criteria during the period from January 1, 2001 to June 1, 2022 were included. Data were obtained from medical records and tabulated in a chart format. Data collection in Microsoft Excel by the researchers from the time of admission to the last consultation or hospitalisation was recorded.
Variables
Variables such as age, sex, weight, height, body surface area, and history of previous surgical procedures such as systemic–pulmonary fistulae, pulmonary artery banding, or bidirectional cardiopulmonary bypass were considered. In addition, variables related to the procedure such as Fontan type (extracardiac and fenestration), failure diagnosis time, failure type (protein-losing enteropathy, plastic bronchitis, univentricular dysfunction, liver injury, arrhythmias, and kidney injury), death, and transplant were included,. The variables associated with failure were defined as those variables associated with a higher risk of developing it. These variables were pulmonary artery mean pressure, McGoon, Nakata, pulmonary vascular resistance index, end-diastolic pressure, valvular regurgitation, ventricular morphology, and weight.
Analysis
A descriptive analysis was carried out for the quantitative variables, and normality verification was made using the Shapiro–Wilk test, according to which the measures of central tendency present are defined (median and average), position measures (quartiles, deciles, and percentiles), and their respective measures of dispersion (standard deviation and interquartile range). For qualitative variables, absolute and relative frequencies were determined. Statistical analysis was performed using the Jamovi program, free distribution software.
Results
During the study period, 212 patients were identified in the institution, of which 108 are in the Fontan stage. Of these patients, 17 met Fontan failure criteria (15.7%). Among the identified patients, 14 were men (82.4%) with a median age of 4.3 years (IQR 3.4–5.1). Five patients had Ebstein anomalies (29.4%) and four had hypoplastic left ventricular syndrome (23.5%). All patients underwent Fontan with an extracardiac tube. In 13 (76.5%) of them, it was decided to leave the fenestration open. The Fontan size was 18 mm (IQR 16–18), with a mean pressure of 16.1 (SD ± 4.73), as shown in Table 1.
Table 1. Characteristics of the patients with the Fontan procedure who presented failure (n = 17).
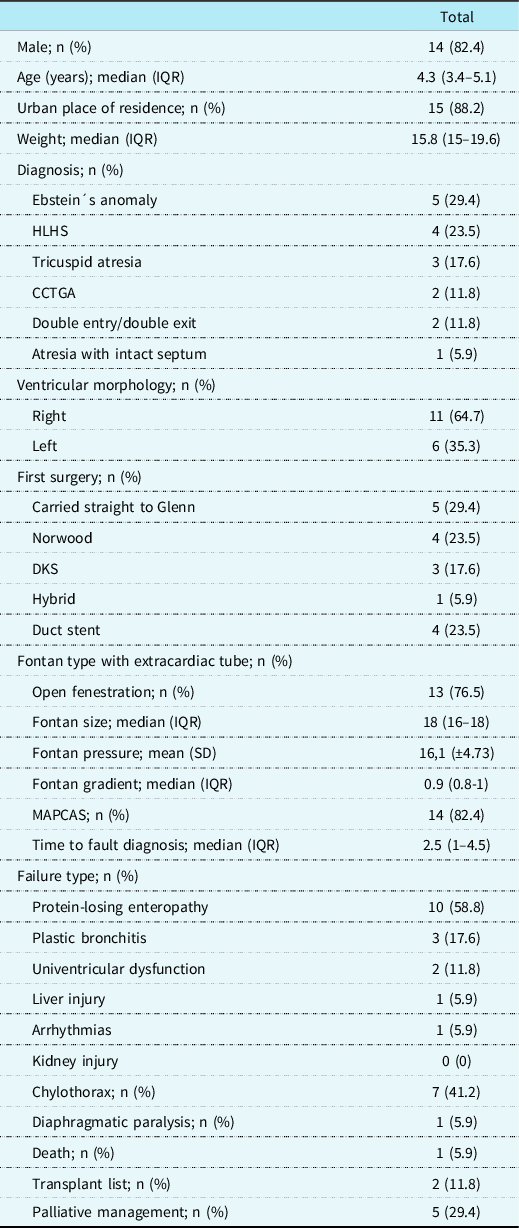
CCTGA = congenitally corrected transposition of the great arteries; DKS = Damus–Kaye–Stancel; HLHS = hypoplastic left heart syndrome; IQR = interquartile range; MAPCAS = major aortopulmonary collateral arteries.
According to the type of failure, 10 (58.8%) patients presented protein-losing enteropathy and 3 (17.6%) had plastic bronchitis. During follow-up, 1 (5.9%) patient died, as shown in Table 1.
Ten patients (58.8%) were between 4 and 15 years old, 3 (35.3%) had a mean pulmonary artery pressure greater than 15 mmHg, 5 (71.4%) had a McGown less than 2, and 5 (62.5%) had a Nakata less than 250 mm2/m2. End-diastolic pressure was greater than 12 mmHg in 11 (73.3%), as shown in Table 2. Fifty per cent of the patients presented with Fontan failure at 75.3 months (IQR 27.8–158), as shown in Figure 1
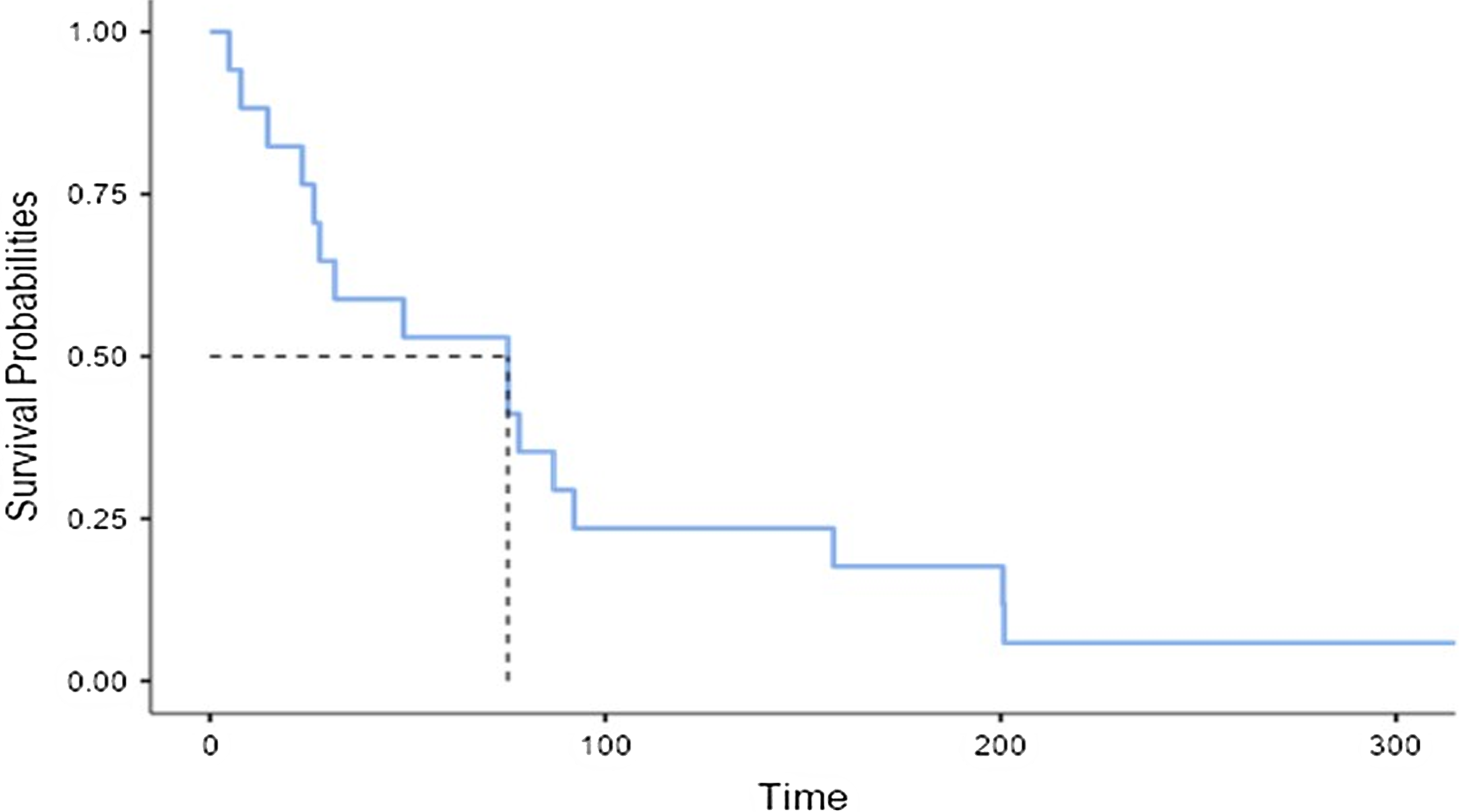
Figure 1. Time of month between surgery and Fontan failure diagnosis. *The dotted line refers to the median in months.
Table 2. Clinical and haemodynamic characteristics associated with Fontan failure (n = 17).
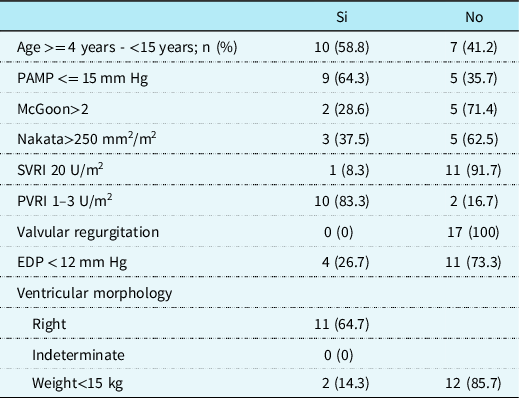
EDP = end-diastolic pressure; PAMP = pulmonary artery mean pressure; SVRI = systemic vascular resistance index; PVRI = pulmonary vascular resistance index.
In patients who presented with Fontan failure and unfavourable haemodynamic characteristics, haemodynamic interventions were required during follow-up. Among these, eight patients underwent embolisation of systemic–pulmonary collaterals using MReye coils (one patient) and Amplatzer vascular plugs (seven patients). Two patients underwent venovenous collateral embolisation using the Amplatzer vascular plug II device.
Regarding the fenestration, one of the patients required stent placement to reopen the fenestration due to protein-losing enteropathy and hypertension in the system. This patient needed new stent dilations on two occasions, but these attempts were not successful. Four patients underwent closure of the fenestration using an Amplatzer-type device. However, during the follow-up, one of them required the creation of a new fenestration and its subsequent reopening.
Single-chamber pacemaker insertion was performed in two patients due to sinus dysfunction. Two of the patients experienced severe univentricular dysfunction in the immediate post-operative period, leading to their cannulation on veno-arterial ECMO for 4 and 5 days, respectively. One of the patients with protein-losing enteropathy, who had been experiencing multiple crises, underwent thoracic duct ligation. This was followed by lymphangiography and chemical pleurodesis to address the condition.
Discussion
The present study presents the results of 17 patients in a 21-year follow-up where the factors associated with failure in Fontan surgery are described, which occurred in 15.7% of the patients. 82.4% of the patients were male. This is an association that has been reported in several series, such as Vargas et al Reference Vargas, Vargas, Castilla, Rodriguez and Martinez8 where two out of three patients were male. The reported median age in years corresponds to the current series due to the age of choice for performing the procedure. Reference Calderón-Colmenero, Ramírez, Viesca and Ramírez10
Ebstein’s anomaly was the most frequent diagnosis (29.4%), followed by hypoplastic left heart syndrome and tricuspid atresia. Other studies have reported different distributions such as Ortiz et al Reference Ortiz-Vázquez, Espinoza-Blanco, Ramírez-Marroquín, Calderón-Colmenero, García-Montes Jose and Cervantes-Salazar11 where tricuspid atresia was the most frequent diagnosis (56%).
For their part, Calderón et al Reference Calderón-Colmenero, Ramírez, Viesca and Ramírez10 reported 65.4% of patients diagnosed with tricuspid atresia, 13.6% with pulmonary atresia with an intact septum, and only 3.7% with Ebstein anomaly. The ventricular morphology was predominantly right at 64.7%, different from series such as Correína-Costa Reference Correia-Costa12 where patients with Fontan failure presented with left morphology.
The first stage of the palliation pathway was mostly Glenn (29.4%), followed by Norwood (23.5%) and DKS (17.6%). Vargas et al reported bidirectional Glenn in 100%, accompanied by DKS in 38.1%. Ortiz et al Reference Ortiz-Vázquez, Espinoza-Blanco, Ramírez-Marroquín, Calderón-Colmenero, García-Montes Jose and Cervantes-Salazar11 reported the Glenn procedure as the first stage of palliation (50%), followed by DKS in 42.3%, frequencies that vary depending on diagnosis, haemodynamic variables, and preferences in surgical groups.
Extracardiac Fontan was performed in 100% of the patients per institutional protocol, with open fenestration in 76.5% of the procedures. Vargas et al reported that 95.2% of the patients were taken to extracardiac Fontan. Ortiz et al Reference Ortiz-Vázquez, Espinoza-Blanco, Ramírez-Marroquín, Calderón-Colmenero, García-Montes Jose and Cervantes-Salazar11 extracardiac Fontan was performed in 90% of cases, while fenestration was used in 10%. There is a growing preference for this technique due to better safety rates and results. However, there are still controversies at this point.
The presence of collaterals was evidenced in 82.4% of patients at the time of the procedure. Weber found that 43% of patients after Fontan surgery developed decompression collaterals, associated with an increased transpulmonary gradient. Reference Weber13
The time from surgery to Fontan failure was 2.5 years (IQR 1–4.5), which agrees with Mertens et al. Reference Mertens, Hagler, Sauer, Somerville and Gewillig14 A multicentre study of 3029 patients with Fontan surgery estimated that the time from surgery to Fontan failure was 2.7 years (0.1–16.4). In addition, an incidence of protein-losing enteropathy of 3.7% in survivors was determined. In the present study, protein-losing enteropathy was the most common type of failure (58.8%), followed by plastic bronchitis (17.6%) and univentricular dysfunction (11.8%). Schumacher et al Reference Schumacher, Stringer and Donohue15 described the factors associated with protein-losing enteropathy and plastic bronchitis, including chylothorax (OR 2.96 CI 1.65–5.31) and hypoplastic left heart syndrome (OR 2.81 CI 1, 43–5,53). In the present series, chylothorax occurred in 41.2% of patients and may represent a condition associated with failure risk.
Haemodynamic risk variables for Fontan surgery were described, including end-diastolic pressure > 12 mmHg (73.3%), McGoon < 2 (71.4%), Nakata < 250mm2/m2 (62, 5%), and pulmonary artery mean pressure > 15 mmHg in (35.7%). These have been evaluated in previous series such as Calderón et al where it was found that pulmonary artery mean pressure > 20 mmHg, left atrial pressure > 10 mmHg, pulmonary artery resistance > 2U*W, systemic ventricular dysfunction, systemic ventricular end-diastolic pressure > 10 mmHg, Nakata < 250 mm2/m2, and/or McGoon < 2 were associated with failure and death. Reference Calderón-Colmenero, Ramírez, Viesca and Ramírez10,Reference Perrin, Dore, van de Bruaene, Mongeon and Mondésert16,Reference Chowdhury, George and Sankhyan17
Death occurred in 5.9% of patients, less than reported in other historical series. Reference Podzolkov, Chiaureli and Yurlov7–Reference Calderón-Colmenero, Ramírez, Viesca and Ramírez10 More recent studies such as Vargas et al Reference Vargas, Vargas, Castilla, Rodriguez and Martinez8 have shown mortality of less than 10%, related to the correct selection of patients at the time of the procedure.
Regarding the study’s limitations, they are the limited number of patients. This is due to the fact that these types of pathologies and procedures are treated in highly complex institutions. This limits the number of subjects included in the study. Another limitation is the type of study carried out, with a secondary source, where information bias can be incurred. This could be controlled with data verification by researchers. For these reasons, it is necessary to conduct studies that include primary, prospective sources and with an increased number of patients in order to obtain information with better external validity.
Conclusions
Fontan surgery at our centre is an option for patients with univentricular physiology. Its performance requires the correct patient selection to mitigate failure and complication risks. In the institutional experience with extracardiac Fontan and strict selection criteria, only 15.7% of patients failed, the most common being protein-losing enteropathy, with an estimated mortality of 5.9%.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors
Competing interests
None.
Ethical standard
The CardioVID Clinic ethics committee approved this study






