Necrotising enterocolitis is a common acquired gastrointestinal emergency in neonates. The intestinal disease results in compromised gastrointestinal integrity and tissue necrosis and can lead to death, multi-organ dysfunction, and long-term morbidity such as short gut syndrome. Reference Claud and Walker1,Reference Markel, Martin and Chaaban2 The incidence has been almost unchanged in recent decades, Reference Dominguez and Moss3 suggesting that necrotising enterocolitis remains a problem alongside current diagnostic and preventative strategies.
Although necrotising enterocolitis is predominantly considered a disease of premature, low-birth-weight infants, it also occurs in term neonates, accounting for approximately 10% of cases in neonates. Reference Ostlie, Spilde and St Peter4 A preponderance of reports has found necrotising enterocolitis in full-term infants to be associated with some predisposing condition. Reference Bolisetty, Lui, Oei and Wojtulewicz5,Reference Martinez-Tallo, Claure and Bancalari6 A study conducted by Iannucci, Oster, and Mahle, Reference Iannucci, Oster and Mahle7 found term infants with definitive cases of necrotising enterocolitis were most likely to have a CHD as their predisposing factor, an association that had previously been supported in a larger study. Reference Ostlie, Spilde and St Peter4 Precisely how CHD influences the development of necrotising enterocolitis remain unclear; however, the ability to adequately distribute regional blood flow to the gastrointestinal system is often impaired in newborns with cardiac disease. Reference Ostlie, Spilde and St Peter4 Hence, the role of reduced blood flow has been postulated to play an important role in disease development. The prevalence of necrotising enterocolitis in term newborns with cardiac disease is 10-100 times greater compared to infants without CHD, suggesting a varying necrotising enterocolitis pathophysiology in this cohort compared to their preterm counterparts. Reference McElhinney, Hedrick and Bush8
Current hospital practices see that many babies with an antenatal diagnosis of CHD are delivered at an earlier gestational age. Previous studies have suggested that there is a substantial reduction in the risk of death with each week of increasing gestational age even in term-born (≥ 37 weeks) neonates with CHD. Reference Costello, Pasquali and Jacobs9 Whether this is also reflected in gastrointestinal organ maturity is not known. Second, prenatal diagnosis of CHD is often associated with birth by planned caesarean section. Reference Ge, Mahle, Burd, Jelin, Sekar and Jelin10 Caesarean delivery can lead to alteration in the normal process of acquisition, composition and development of the gut microbiota in the newborn. Altered gut microbiome has been linked with late development of gastrointestinal disorders in childhood. Reference Bentley, Roberts, Bowen, Martin, Morris and Nassar11 and could also potentially predispose to necrotising enterocolitis in newborns with CHD in the setting of deranged gut blood flow (see also Appendix Figure 1).
The complicated aetiology of necrotising enterocolitis, and clinicians and researchers continued endeavours to understand its development and tools for early detection, mean that strategies for prevention are underdeveloped. Reference Markel, Martin and Chaaban2 Hence, investigating the predisposing factors of necrotising enterocolitis remains important not only in ameliorating patient outcomes but also for empowering staff to implement factors known to reduce its risk of development. In this study, we aimed to determine the relationship between (a) gestational age and (b) mode of birth in the later development of necrotising enterocolitis in term-born newborns with CHD.
Materials and methods
Study population and setting
We studied term-born infants (gestation age ≥ 37 weeks) who were less than 28 days old at the time of surgery for CHD between 1 January, 2007 and 31 December, 2017. This was a hospital-based case–control study conducted at the Royal Children’s Hospital in Melbourne. Study information was obtained from the cardiac ICU database and hospital medical records. The study protocol was approved by the human research and ethics committee of the Royal Children’s Hospital Melbourne (Human Research Ethics Committee No: QA/53113/RCHM-2019)
Cases were neonates who developed necrotising enterocolitis, and controls were neonates who did not develop necrotising enterocolitis. A 1:3 case to control ratio was used; cases were matched with controls using the same perioperative lesion type (see below for more details) and year of admission as matching variables. If a case could not be matched with a control according to year of admission, controls were sourced in the years immediately preceding or succeeding the year in question.
The modified Bell staging criteria (Appendix Table 1) was used to diagnose and stage the severity of necrotising enterocolitis. The type of congenital cardiac lesion was divided into one of six types based on the perioperative lesion types: left ventricular outflow tract lesion, right ventricular outflow tract lesion, shunt lesion, conotruncal lesion, single ventricle lesion, and miscellaneous lesions. The cardiac surgeries were classified using the Risk Adjusted Congenital Heart Surgery (RACHS-1) scoring system. Reference Jenkins, Gauvreau, Newburger, Spray, Moller and Iezzoni12 Gestational age was modelled as a continuous variable, and birth mode was recorded as either vaginal or caesarean. The following variables were included in the analysis: sex, preoperative enteral feeds (yes/no), preoperative mechanical ventilation (yes/no), cardiac arrest during admission, and presence of non-cardiac congenital anomaly (yes/no). Non-cardiac congenital anomaly includes both genetic diagnoses and non-cardiac malformations.
Conditional logistic regression analysis was used to study the association between study variables of interest (gestational age and mode of delivery) and development of necrotising enterocolitis. The unadjusted model reports the association between (a) gestational age and necrotising enterocolitis and (b) model of delivery and necrotising enterocolitis separately. The adjusted model 1 reports the estimates for gestational age and mode of delivery after adjusting for sex and non-cardiac congenital anomaly (all included in one model). In the adjusted model 2, in addition variables in model 1, three additional variables (preoperative mechanical ventilation, preoperative enteral nutrition, and cardiac arrest) were also included. The study estimates are reported as unadjusted and adjusted odds ratio with 95% confidence interval. Analyses were performed using STATA-IC (version 16.1; StataCorp, College Station, TX).
Results
Patient characteristics
During the 11-year study period (2007–2017), 1175 newborns underwent cardiac surgery for CHD, of whom 223 were less than 37 weeks gestational age and were excluded (Fig 1). Of the 952 remaining neonates, 60 cases of necrotising enterocolitis were identified (6.3%); 43 developed necrotising enterocolitis postoperatively (72%); and 17 (28%) were preoperatively diagnosed. In total, 62% (n = 37) of cases were in the modified Bell stage 2 or more (Table 1). Neonates with a left-ventricular outflow tract lesion or a single ventricle lesion accounted for 55% (n = 33) of the cases. The median (IQR) age at diagnosis of necrotising enterocolitis was 8.5 (5.5–15) days.
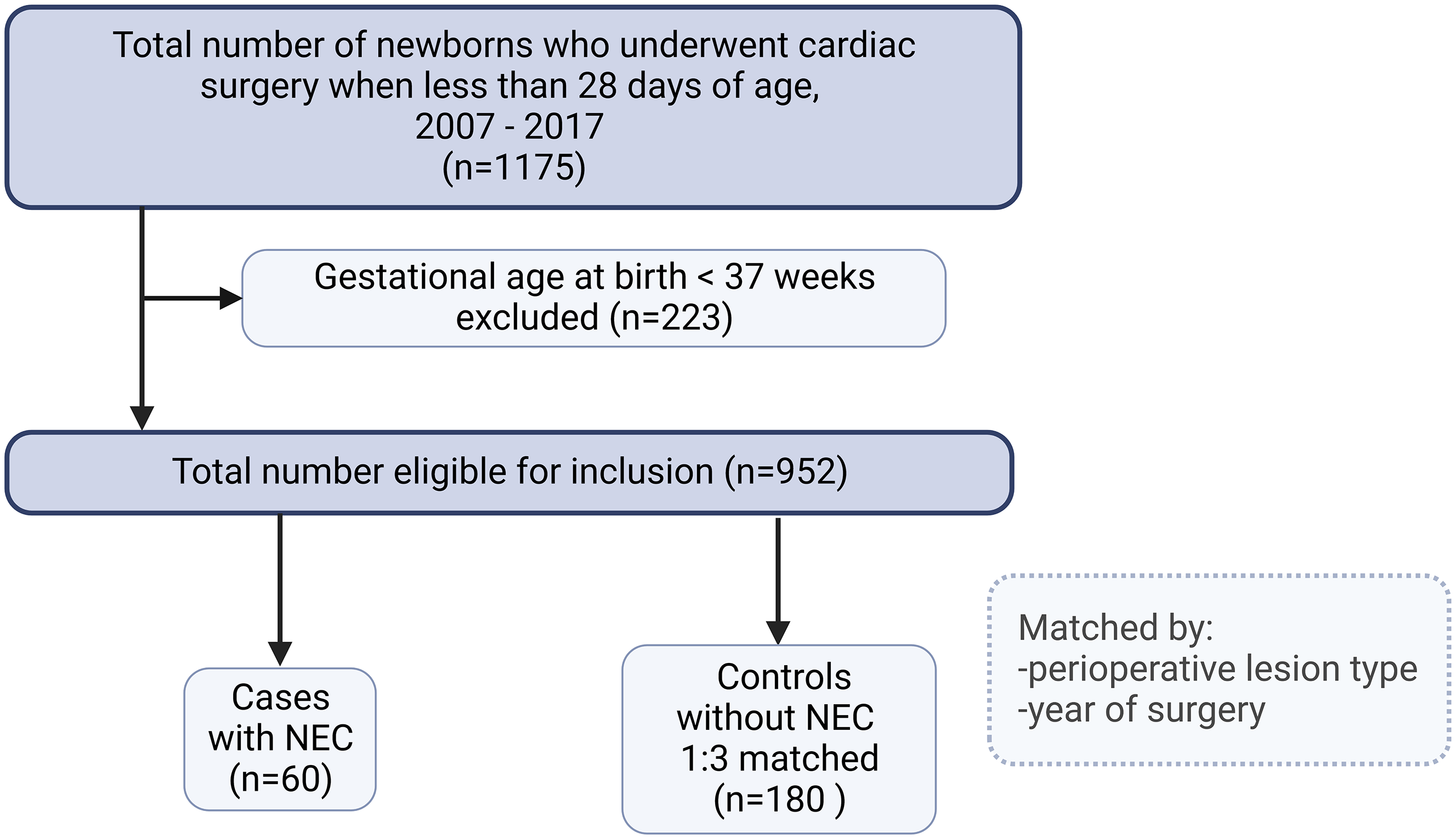
Figure 1. Study flow chart. Two controls who had missing information on one the study variable (preoperative enteral feeding) were not included in the final analysis. So, the final outcome analysis was performed on 238 neonates (60 cases and 178 controls) who had complete information.
Table 1. All cases of necrotising enterocolitis (n = 60) by modified Bell staging and perioperative lesion type.
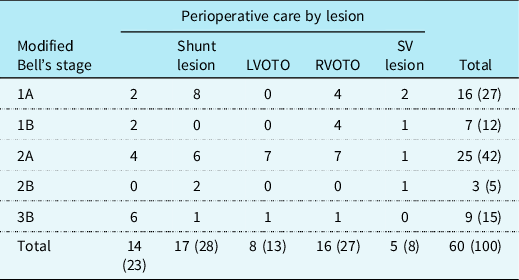
LVOTO = left ventricular outflow tract obstruction; RVOTO = right ventricular outflow tract obstruction; SV = single ventricle.
Reported as no (%); percentages do not add up to 100 due to rounding.
Each of the 60 cases of necrotising enterocolitis was matched with 3 controls (1:3) giving a total of 180 controls. The full set of study information was available for all cases and for 178 controls resulting in a final study sample of 238. The clinical characteristics of the cases and controls are shown in Table 2. More than 50% of the cases and controls were fed preoperatively. The cardiopulmonary bypass time and cross-clamp time were similar in the cases and controls, and a high proportion of cases had experienced a cardiac arrest during the admission. Both death in hospital and death at follow-up were higher in cases.
Table 2. Demographic and clinical characteristics of study participants, by case and control status.
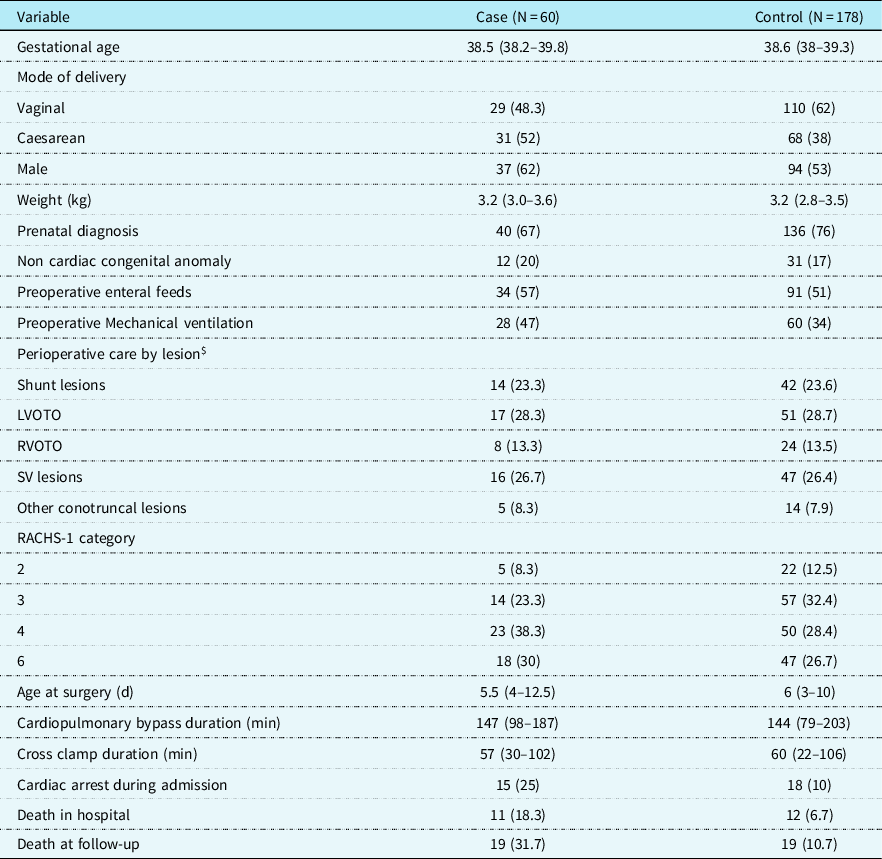
LVOTO = left ventricular outflow tract obstruction; RVOTO = right ventricular outflow tract obstruction; SV = single ventricle; PIM2 = paediatric index of mortality.
$ Matched variable.
RACHS-1: risk adjusted classification for congenital heart surgery-1.
RACHS-1 classification taken from Jenkins KJ et al.., J Thorac Cardiovasc Surg 2002; 123(1):110-8.
The final multivariable analysis results are shown in Table 3. Birth by caesarean section was significantly associated with increased risk of necrotising enterocolitis: adjusting for sex, gestational age, and non-cardiac congenital anomaly (Model 1), the adjusted odds ratio (95% confidence interval) was 2.00 (1.04–3.86), and this was further strengthened in model 2 (further adjusting for preoperative mechanical ventilation and cardiac arrest): adjusted odss ratio (95% confidence intervaI): 2.64 (1.31–5.29). No difference was found in the gestational age at birth: adjusted odds ratio per week increase (95% confidence interva): 1.20 (0.90-1.60)] and the risk of necrotising enterocolitis. Preoperative enteral feeding was not associated with increased risk of necrotising enterocolitis [1.35 (0.68–2.68)]. The results of the full multivariable model are shown in appendix table 3.
Table 3. Risk of necrotising enterocolitis associated with gestational age and mode of delivery.
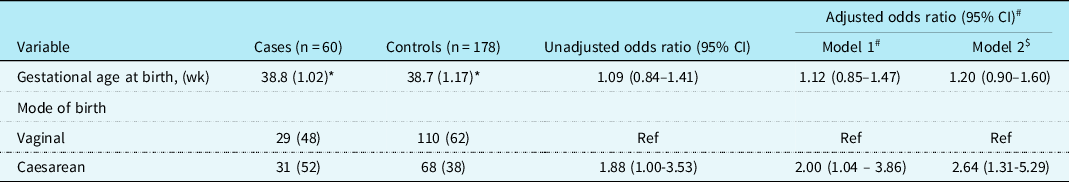
* Mean (SD); odds ratio for gestational age represents change per week increase.
# Adjusted for sex and non-cardiac congenital anomaly.
$ Adjusted for preoperative mechanical ventilation, preoperative enteral nutrition, and cardiac arrest.
Discussion
In this population of term-born neonates undergoing cardiac surgery within the first 28 days of life, we identified a 2.64 fold increased odds of developing necrotising enterocolitis, if born by caesarean delivery. This information is important given the high prevalence of caesarean deliveries among newborns with prenatally diagnosed CHD. Reference Peyvandi, Nguyen and Almeida-Jones13 While these findings need further evaluation in future studies, the mechanistic foundations for such findings can be postulated (discussed further below). We did not identify an association between increasing gestational age (linked with more developmental maturity) and a reduced risk of necrotising enterocolitis in this term-born cohort. Additionally, this study showed that preoperative enteral feeds did not increase the risk of necrotising enterocolitis.
Whilst the development of necrotising enterocolitis is multifactorial and remains largely ambiguous, this study carries with it clinical importance in identifying the risk associated with the mode of birth. Development of necrotising enterocolitis in a newborn with CHD often occurs in the setting of impaired mesenteric blood flow or cardiogenic shock, likely resulting in mesenteric hypoperfusion. Reference Siano, Lauriti, Ceccanti and Zani14,Reference Kelleher, McMahon and James15 This is unlike the classical necrotising enterocolitis seen in premature neonates, which involves immaturity of the immune system making the intestine vulnerable to necrotising enterocolitis. The implications of caesarean section versus vaginal birth must be considered when evaluating the risk of infants predisposed to necrotising enterocolitis development. There is an approximately 1.5-fold increase in the rate of primary caesarean sections associated with prenatally diagnosed CHD. Reference Ge, Mahle, Burd, Jelin, Sekar and Jelin10 The decision for planned birth is predicated on beliefs that it is safer for the baby or mother to deliver early. With increase in prenatal diagnosis rates globally, it is likely the numbers of prelabour planned caesarean deliveries will increase; in the absence of other maternal or fetal risk factors we suggest every effort should be made to allow spontaneous onset of labour.
Mode of delivery is known to be an important factor that affects the composition of the gut microbiota throughout the neonatal period and into infancy. The role of aberrant bacterial colonisation in disease pathogenesis has long been acknowledged in literature identifying the role of microbiomes in necrotising enterocolitis development. Reference Musemeche, Kosloske, Bartow and Umland16 Caesarean delivery is followed by a considerably altered gut microbiota during the neonatal period and into infancy. Reference Korpela17,Reference Shao, Forster and Tsaliki18 While babies born vaginally are rapidly colonised by organisms from their mother and environment, babies born by caesarean are largely not exposed to the maternal flora. Reference Lawrence, Bates and Gaul19 Caesarean delivery prevents vertical transmission of gut microbes from mother to the infant. Caesarean born infants have low levels of Bifidobacteria, almost total lack of Bacteroides and have high levels of opportunistic pathogens such as Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, Enterococcus, Enterobacter, Klebsiella species, and Clostridium perfringens. Reference Korpela17,Reference Shao, Forster and Tsaliki18 Overgrowth of one or more of these pathogenic organisms (several of which produce exotoxins) creates an environment for later development of necrotising enterocolitis when the gut mucosal integrity is further compromised in cardiac neonates. The newborn gut in this setting takes up macromolecules intact and toxic products from the rapidly multiplying pathogenic bacteria may be absorbed leading to further damage, initiating necrotising enterocolitis. Reference Lawrence, Bates and Gaul19 The high risk of necrotising enterocolitis observed in our study in association with caesarean delivery could potentially be related to an increased propensity to gut inflammation, disordered immune and metabolic development related to gut microbial dysbiosis. Reference Korpela17
Our results showed a non-significant association between preoperative enteral feeds and risk of necrotising enterocolitis. A cardinal trait of necrotising enterocolitis is a compromised epithelial and endothelial barrier between the intestinal lumen and bloodstream, giving cause to associate necrotising enterocolitis with a disease of prematurity. The notion of avoiding enteral feedings preoperatively, which may further deteriorate gastrointestinal permeability, was thus founded. However, as shown previously, Reference Good, Sodhi and Yamaguchi20 the abundance of human milk oligosaccharide in expressed breast milk may be protective. These findings align with those of a study from Sweden (where it is routine practice to feed preoperatively). Among infants with ductal-dependent systemic circulation, 96% (198/206) were enterally fed before cardiac intervention, and only one infant was diagnosed with necrotising enterocolitis. Reference Nordenstrom, Lannering, Mellander and Elfvin21 It is likely that preoperative enteral feeds (particularly mother’s milk) does not contribute to the risk of necrotising enterocolitis but may also be protective against it. Human milk contains several protective bactericidal and bacteriostatic factors, many of which have been shown to reduce the necrotising enterocolitis incidence. Reference Lawrence, Bates and Gaul19 Our study did not show a meaningful association between preoperative feeds and development of necrotising enterocolitis. Previous literature Reference Nordenstrom, Lannering, Mellander and Elfvin21–Reference Willis, Thureen, Kaufman, Wymore, Skillman and da Cruz23 and results from our institution also did not show an association between preoperative enteral feeds and necrotising enterocolitis. Reference Day, Dionisio, Zannino, Brizard and Cheung24 Unless newborns preoperatively have major haemodynamic instability, enteral feeds (atleast small to moderate volumes) using mother’s milk should be encouraged routinely in cardiac neonates. Feeding guidelines to facilitate this are likely to have a meaningful impact.
The primary limitation of this study surrounds its inability to derive a direct causal relationship between necrotising enterocolitis and the variables investigated. The influence of mode of delivery on bacterial populations is not always easy to elucidate, especially when faecal samples, utilised as surrogate for the gut microbiome, were not collected. Future studies in this cohort may benefit from analysis of stool samples as has been shown previously. Reference Neu and Pammi25,Reference Jakobsson, Abrahamsson and Jenmalm26 Another limitation is that we did not capture the use of antibiotics prior to the caesarean section, although it might be reasonable to assume that that antibiotics are commonly given during caesarean delivery. Previous literature has raised the role of antibiotic use and alterations in microbiota diversity. Reference Neu and Pammi25 However, a previous study of gut microbiota diversity has also clearly shown that the microbial diversity did not differ between mothers who were given antibiotics prophylactically and who did not receive those. Reference Jakobsson, Abrahamsson and Jenmalm26
Conclusion
In conclusion, this case–control analysis has identified a strong association between caesarean delivery and the development of necrotising enterocolitis in term-born neonates with CHD. A mechanistic explanation for this association can be postulated. The findings have important implications for newborns with CHD given (a) the morbidity and mortality associated with the development of necrotising enterocolitis in this cohort and (b) the high proportion of caesarean deliveries in newborns with CHD. Further analysis of this study question (ideally using multicentre data) is important.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1047951123003128.
Acknowledgements
Siva P Namachivayam is supported by a health professional research scholarship from the National Heart Foundation of Australia (award number 101003).
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Competing interests
None.







