Univentricular heart defects are a group of complex CHD requiring staged correction that produces a Fontan circulation in the patient’s early childhood. Most patients undergo three operations. Reference Rao1 Stage 1 repair, ensuring blood flow to the lungs and systemic circulation, is completed in the neonatal period – e.g., via the Norwood procedure. In stage 2, a bidirectional Glenn operation is performed, typically within 6 months of birth; the superior vena cava is detached from the heart and connected to the pulmonary arteries. Finally, in stage 3, a total cavopulmonary connection is formed by connecting the inferior vena cava with the pulmonary arteries. This operation occurs at approximately 3–4 years of age. Nowadays, after Fontan completion, up to 75% of patients survive to adulthood. Reference Downing, Allen and Glatz2,Reference Pundi, Johnson and Dearani3
Patients with univentricular heart defects need imaging surveillance throughout life. Alongside echocardiography, cardiac catheterisation has traditionally served as a second-line imaging modality employed routinely during pre-and post-operative follow-up. Reference Edwards, Reddy and Kicska4 Therefore, children with univentricular heart defects commonly undergo multiple cardiac catheterisations. This is an important issue to address in these patients, in light of studies demonstrating that children exposed to ionising radiation from diagnostic imaging are at increased risk of future malignancies, Reference Brenner and Hall5 and also because the most routine pre-Glenn and pre-total cavopulmonary connection catheterisations are purely diagnostic in nature. Actually, cardiac magnetic resonance has been proposed to be effective enough for routine pre-operative imaging in low-risk patients since pre-operative pressure measurements have not been shown to improve the prediction of adverse post-operative outcomes in these patients. Reference Prakash, Khan, Hardy, Torres, Chen and Gersony6 To avoid unnecessary invasive assessments, a diagnostic risk classification algorithm has been proposed by Prakash et al. Reference Prakash, Khan, Hardy, Torres, Chen and Gersony6 Ideally, whenever applicable, CT angiography and cardiac MRI should serve as the primary screening tool for routine pre-operative assessment and reduce invasive techniques use for patients requiring interventions. Modern cardiac CT scanners provide detailed anatomical information at low doses of ionizing radiation, Reference Le Roy, Vernhet Kovacsik and Zarqane7 and cardiac magnetic resonance has proved successful in the pre-operative assessment of patients with univentricular heart defects. Reference Brown, Gauvreau and Powell8–Reference de Lange11
This report describes a tertiary care institution’s transition from a policy of routine invasive imaging to routine non-invasive imaging for patients with univentricular heart defects. We demonstrate here the safety and cost savings of non-invasive techniques in patients with single-ventricle physiology during staged reconstruction.
Methods
This study had two phases. 1) Retrospective assessment of the transition from cardiac catheterisation to CT angiography prior to the Glenn operation and 2) prospective study addressing cardiac MRI before and after the total cavopulmonary connection operation. All examinations were performed at the university hospital, which covers referrals to paediatric cardiac surgery for the nation’s population of 5.5 million. For pre-Glenn imaging, we compared the hospital’s last 21 routine cardiac hemodynamic catheterisations from years 2008–2011, with the first 20 routine CT angiography procedures performed in 2012–2015 (see Table 1). For pre-and-post-total cavopulmonary connection imaging, 89 cardiac magnetic resonance studies of the patients with univentricular heart defects (2017–11/2019) were analysed (see Table 2). The cohort included only patients classified as low-risk according to the previously published risk assessment algorithm (Appendix, Table A1). Reference Prakash, Khan, Hardy, Torres, Chen and Gersony6 Low-risk patients were studied only noninvasively by cardiac magnetic resonance, and those, classified as high-risk and excluded from the cohort, were examined by cardiac catheterization with or without cardiac magnetic resonance. Patients with cardiac pacemakers were examined only invasively.
Table 1. Patient characteristics and summary comparison of routine pre-Glenn catheterisation and CT angiography (phase 1)
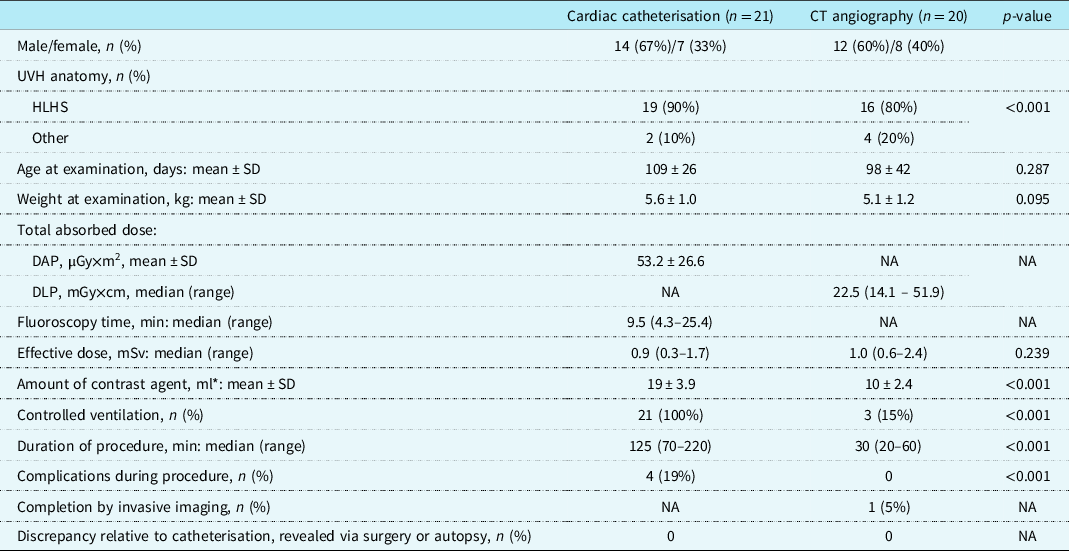
* Iomeprol 250 mg/ml or 300 mg/ml for catheterisation; iodixanol 270 mg/ml for CT angiography (2.0 ml/kg).
DAP = dose area product; DLP = dose length product; HLHS = hypoplastic left heart syndrome; UVH = univentricular heart.
Table 2. Patient characteristics and cardiac magnetic resonance examination parameters, n = 89 (phase 2)
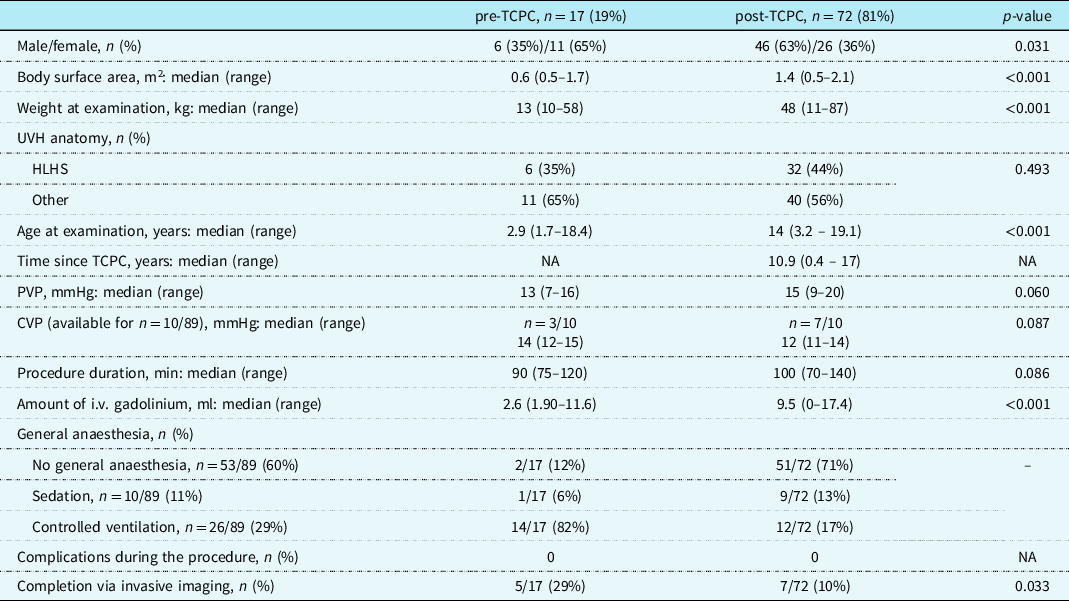
CVP = central venous pressure; HLHS = hypoplastic left heart syndrome; PVP = peripheral venous pressure; TCPC = total cavopulmonary connection; UVH = univentricular heart.
Non-gated chest CT angiography was performed with a 64-slice scanner (GE Medical Systems Discovery CT750). The dose of the contrast medium iodixanol 270 mg/ml was 2 ml/kg. The effective radiation dose was calculated from the absorbed dose in line with International Commission on Radiological Protection Publication 103 guidelines, and simulations were performed via the CT-Expo software application (v. 2.0.2).
Cardiac magnetic resonance was performed with a 1.5 Tesla scanner (Philips Achieva or Ingenia), using the contrast agent gadoteric acid (279.3 mg/ml, 0.2 ml/kg). Imaging protocol is designed to follow recommendations issued by the Society for Cardiac Magnetic Resonance expert consensus group. Reference Fratz, Chung and Greil12 The core of the cardiac magnetic resonance protocol involves the assessment of branch pulmonary artery size and flow distribution, cardiac index, and ventricle size and function. Also, cardiac magnetic resonance data were reviewed for atrioventricular valve regurgitation and the aortic arch’s obstruction, gated MR-angiography was acquired. In cardiac magnetic resonance, the dimensions of the pulmonary arteries and descending aorta were measured from 3D balanced steady-state free precession images gated to diastole (the CT angiography scan and cardiac magnetic resonance sequence parameters are listed in the Appendix in Tables A2 and A3). Flow data was used to aid clinical decision-making and to estimate the significance of anatomical findings. Immediately prior to cardiac magnetic resonance, peripheral venous pressure was measured from a cubital venous cannula.
Catheterisation employed a Siemens Artis angiography system; iomeprol (250 mg/ml or 300 mg/ml) was used as a contrast medium. Haemodynamic analyses, including central venous pressure measurement, were performed, and vascular dimensions at systole were recorded. The effective radiation dose was calculated from the absorbed dose in accordance with International Commission on Radiological Protection Publication 103 recommendations, and simulations were carried out with the computer program PCXMC.
Costs of adopting a non-invasive-imaging strategy were estimated on the basis of average prices calculated for the hospital’s various types of paediatric cardiac imaging, with related expenses (anaesthesia, overnight hospitalisation, outpatient-clinic visits) taken into account.
Statistics
All statistical calculations were performed using “IBM SPSS Statistics v.25”. All variables were tested for normality (Shapiro–Wilk test) and reported as mean ± SD and median (range) as appropriate. Statistical significance was computed using the independent samples t-test or Mann–Whitney U-test for unpaired normal distributed and nonparametric variables, respectively. For paired groups, paired samples t-test for normally distributed variables and Wilcoxon sign test for non-normal distributed variables were calculated. The Fisher exact test and chi-square test were used for categorical variables. A p-value less than 0.05 was considered significant.
Results
Phase 1, pre-Glenn imaging
Table 1 summarises the data from CT angiography and catheterisation. Radiation exposure did not differ significantly between the groups. Amounts of iodine contrast media were greater for catheterisation than for CT angiography, with values of 19 ± 3.9 ml and 10 ± 2.4 ml, respectively (p < 0.001). The CT angiography procedure, including preparation of anaesthesia, was of markedly shorter duration than catheterisation, at 30 minutes (range: 20–60) rather than 125 minutes (range: 70–220) (p < 0.001). While controlled ventilation was used for all 21 patients during catheterisation, only 3 of the 20 CT angiography patients received it (p < 0.001). One CT angiography was performed with the “feed and wrap” technique, and the remaining 16 CT angiography procedures employed free-breathing sedation.
Complications and inconsistencies
Four of the 21 patients subject to catheterisation (19%) experienced complications during the procedure: two had arrhythmia (supraventricular tachycardia and atrial flutter, with the latter treated by cardioversion), and the other two had a transient complete AV block (with one needing cardiopulmonary resuscitation). All catheterisations showed agreement with the bidirectional Glenn intra-operative findings.
No complications occurred during CT angiography. The findings from all CT angiography studies were consistent with the Glenn operation or autopsy. One CT angiography examination was completed with the catheterization, and the CT angiography finding of narrowed pulmonary arteries was confirmed.
Phase 2, pre-and post-total cavopulmonary connection imaging
Table 2 presents an overview of the patients’ details. Most of the cardiac magnetic resonance examinations, 81% (72/89) were performed in the post-total cavopulmonary connection phase, and the remaining 17 (19%) were pre-total cavopulmonary connection studies. The cardiac magnetic resonance was carried out without sedation for 53 patients (60%), general anaesthesia with controlled ventilation for 26 patients (29%), and with sedation for 10 of them (11%).
Complications and inconsistencies
No complications occurred; all studies were successful. According to the institutional guidelines additional imaging was needed for 13% (12/89) patients to confirm the cardiac magnetic resonance finding and/or to perform intervention; this entailed 11 interventional catheterization and one further CT angiography study. In all patients, cardiac magnetic resonance showed good agreement with catheterization, CT angiography, or findings in total cavopulmonary connection operation.
Peripheral venous pressure measurements were available in 97% of the cases (86/89), showing a median pressure of 15 mmHg (range: 7–20).
Costs
The institutional prices of the different cardiac imaging modalities (catheterisation, CT angiography, and cardiac magnetic resonance) in the years following the policy change (2017–2020) are presented in Figure 1. Cardiac catheterisation was the most expensive of these modalities and is associated with additional expenses: pre-procedure outpatient-clinic visits and an overnight hospital stay. Hence, non-invasive imaging resulted in savings of €2500–4000 per patient, at minimum. The amount saved was greater when non-invasive imaging was performed without sedation or general anaesthesia.
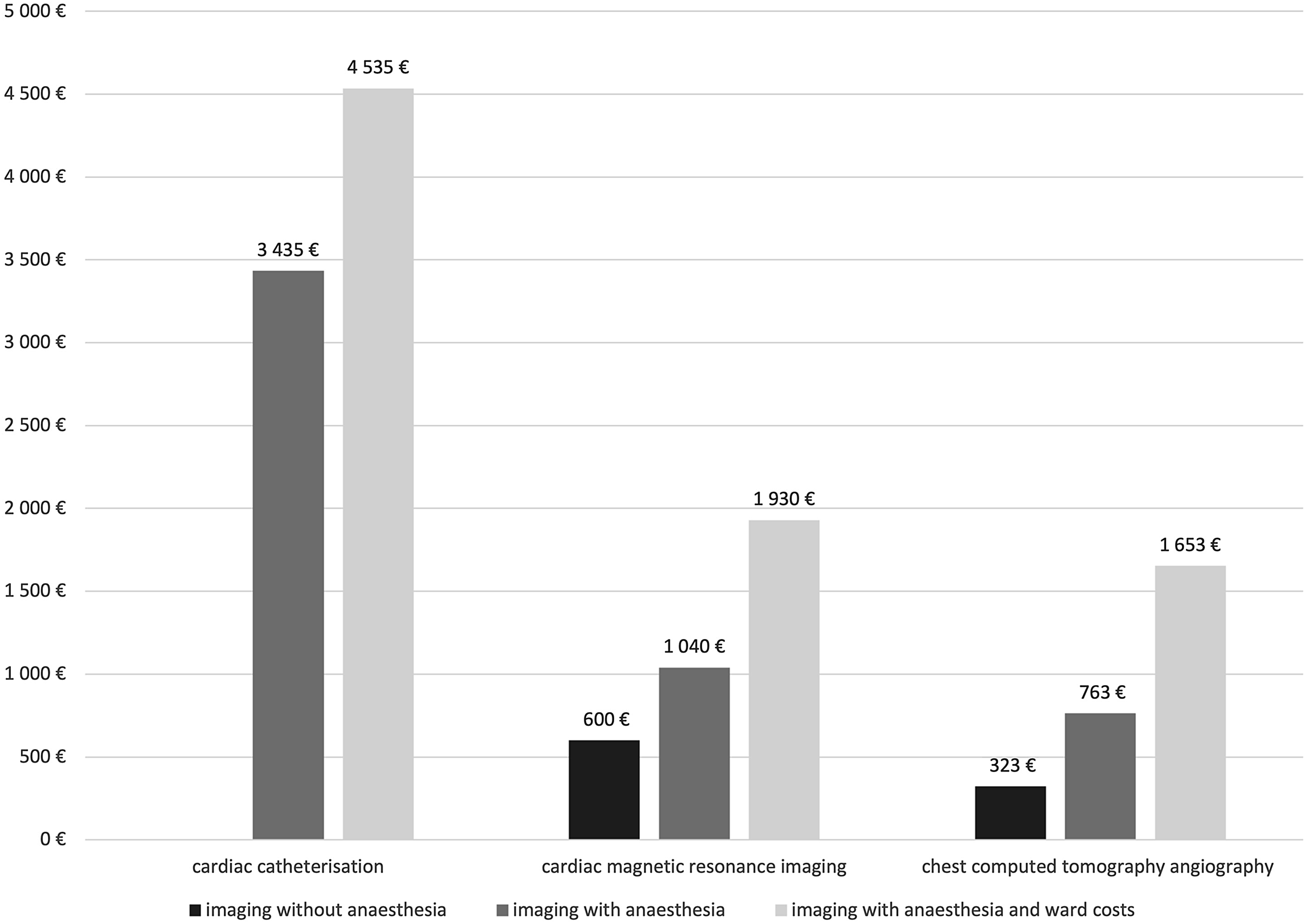
Fig. 1. Average institutional costs for individual studies of modalities (2017–2020).
Discussion
Our results from a national referral centre for paediatric cardiac surgery show non-invasive imaging to be safe for routine surveillance of low-risk patients with univentricular heart defects (risk stratification accordingly to Prakash et al. Reference Prakash, Khan, Hardy, Torres, Chen and Gersony6 ). In addition to non-invasiveness, this approach shows the advantages of a diminished need for general anaesthesia and sedation, reduction in the number of complications, and – for cardiac magnetic resonance – avoidance of ionizing radiation. A further advantage of non-invasive imaging arises from the cost savings, in that moving over to non-invasive imaging saved our institution at least €2500–4000 for each catheterisation replaced. The cost savings were higher when the non-invasive imaging did not require anaesthesia (in our data, 60% of cardiac magnetic resonance examinations succeeded without any general anaesthesia).
In the first phase, we observed an excellent correlation between CT angiography findings and intra-operative findings when we replaced routine pre-Glenn catheterisation with CT angiography. This result is consistent with earlier work showing CT angiography and catheterisation to be equally accurate for pre-operative evaluation for this patient group. Reference Han, Vezmar and Lesser13 Our work is consistent with a previous study, Reference Han, Vezmar and Lesser13 finding the complication rate to be higher during catheterisation, as no complications emerged during our CT angiography. One factor in this is the significantly shorter duration of CT angiography relative to catheterisation, which adds to CT angiography’s safety by reducing the need for anaesthesia. In our study, all catheterisations were performed under general anaesthesia with controlled ventilation, whereas light sedation without ventilation control sufficed for most CT angiography studies. Furthermore, today’s cardiac CT scanners permit high-quality sedation-free cardiac CT angiography with free-breathing, Reference Han, Overman and Grant14 which is a routine practice for pre-Glenn patients at our hospital.
While we found that CT angiography and catheterisation produced the same radiation dose, a previous report Reference Han, Vezmar and Lesser13 shows a sharp contrast to this. In a roughly similar study population (of 16 pre-Glenn patients), the radiation dose for CT angiography was similar to ours, but the dose for catheterisation exceeded this tenfold. The most likely reason is the difference in the dose-estimation methods employed. Han et al. used conversion factors to estimate the effective dose from dose area product and CT dose index values for fluoroscopy and CT, respectively. This is much less accurate and less specific than the simulation-based methods we used. For fluoroscopy, our true Monte Carlo simulation accounts for such factors as the projections unique to each child and the beam characteristics. For CT, our dose-estimation technique had higher accuracy. To some extent, the differences found in the fluoroscopy doses may also reflect local practices regarding the device’s dose parameters, the length of fluoroscopic loops, and device age.
The second phase, in which we replaced routine pre-and post-total cavopulmonary connection catheterisation with cardiac magnetic resonance, proved encouraging. It supports prior work finding cardiac magnetic resonance useful for the pre-operative evaluation prior to the total cavopulmonary connection Reference Harris, Cosulich and Gillespie15,Reference Yeong, Loughborough, Hamilton and Manghat16 : cardiac magnetic resonance provided sufficient anatomical data before the total cavopulmonary connection operation and was consistent with the operative findings. For pre-and-post-total cavopulmonary connection follow-up, cardiac magnetic resonance displays additional advantages such as functional imaging of the ventricles and assistance with flow assessments.
The significant reduction in the need for general anaesthesia yielded by the transition from catheterisation to cardiac magnetic resonance at our institution constitutes a major advantage, as cardiac magnetic resonance is considered generally safe for children and adolescents, with risks linked mainly to general anaesthesia. Reference Buechel, Grosse-Wortmann and Fratz17 Recall that no complications occurred during cardiac magnetic resonance in our study. Another significant safety advantage of cardiac magnetic resonance lies in the lack of ionising radiation – this is especially vital for such a challenging patient group, requiring repeated imaging.
Gadolinium-derived contrast agents are routinely used in cardiac magnetic resonance studies of patients with univentricular heart defects. Severe complications from using these agents, such as nephrogenic systemic fibrosis, are rare in children. Extravasation of contrast and allergic reaction occur more frequently. 18 No adverse effects related to gadolinium-derived contrast agents occurred in our patients. All patients were screened for renal dysfunction prior to the imaging study.
We routinely measured peripheral venous pressure in conjunction with cardiac magnetic resonance. Prior work identified a strong correlation between peripheral and central venous pressure in children, Reference Anter and Bondok19 and recently, peripheral venous pressure was proved as a good predictor of central venous pressure in the adult population of Fontan patients. Reference Tan, Small, Gallotti, Moore and Aboulhosn20
All patients at our institution routinely undergo cardiac magnetic resonance examination and peripheral venous pressure measurements prior to the total cavopulmonary connection operation, 1 year after that, and 1–2 times after this throughout childhood and adolescence (see Fig 2). The optimal timing and frequency of post-total cavopulmonary connection cardiac magnetic resonance studies remain unclear, Reference Zaki, Kelleman, James Parks, Slesnick, McConnell and Oster21 though the American Heart Association’s scientific statement on evaluating a child or adult with a Fontan circulation suggests cardiac magnetic resonance once every 2–3 years. Reference Rychik, Atz and Celermajer10
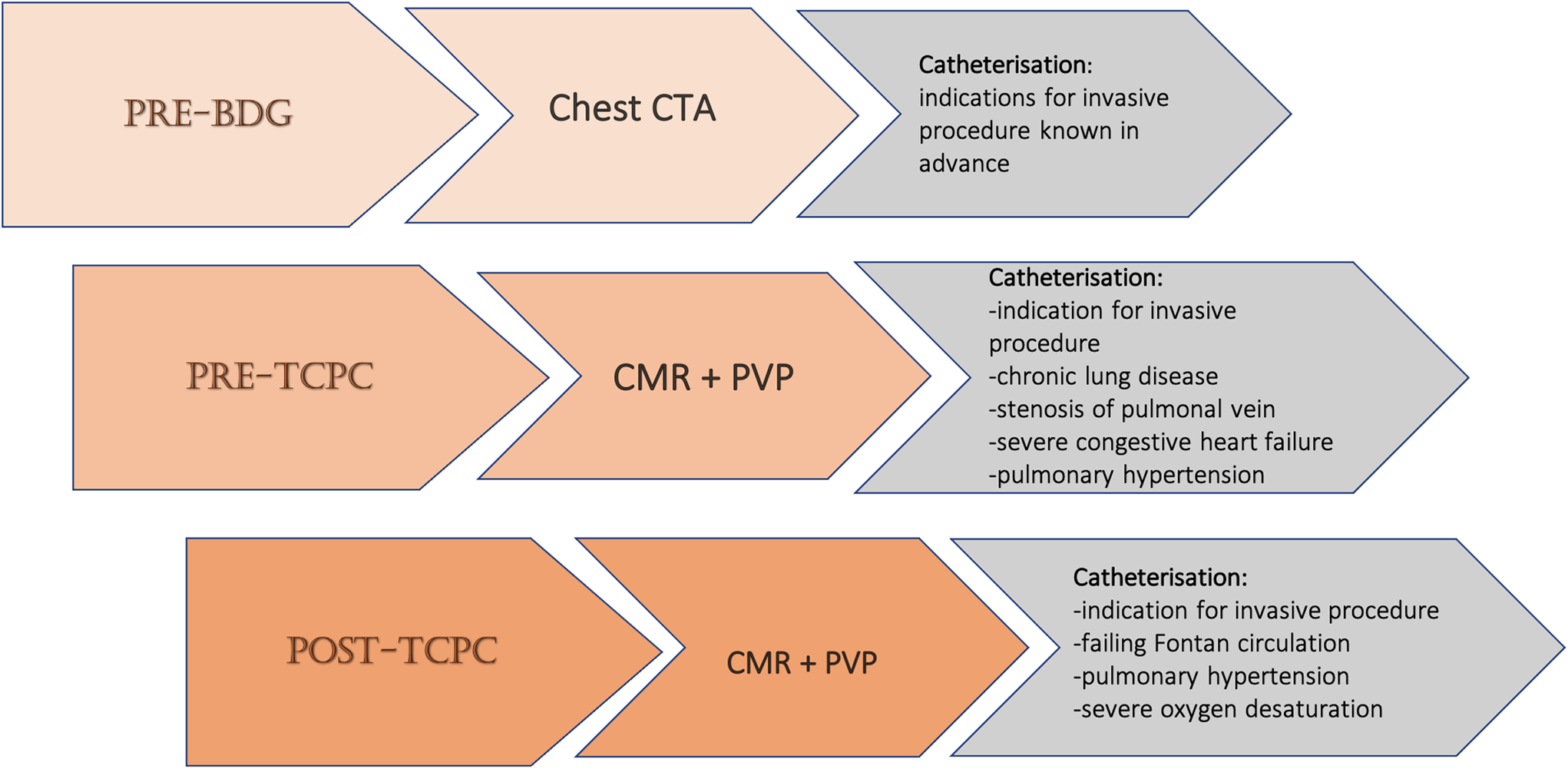
Fig. 2. Institutional imaging follow-up protocol for the stages of reconstruction for the univentricular heart defects. Abbreviations: BDG = bidirectional Glenn operation; PVP = peripheral venous pressure; TCPC = total cavopulmonary connection.
Limitations and strengths of the study
Our work is limited by its partly retrospective nature. We did not compare CT angiography and catheterisation in the same pre-Glenn patients since the patients could not be exposed to two radiation studies. However, our research gained strength from its setting: the national-wide referral centre for children with univentricular heart defects, with consecutive patients representing a national cohort. Moreover, detailed surgical verification was available for all pre-operative examinations. We want to point out that the required number of interventional procedures in these patients will affect the cost balance, and the institutional differences are to be expected.
Conclusion
Institutional transition from invasive to non-invasive imaging was safely performed in low-risk children with univentricular heart defects. A further advantage of non-invasive imaging arises from the cost savings. The cost savings were higher when the non-invasive imaging did not require anaesthesia.
Acknowledgements
The authors warmly thank the cardiac magnetic resonance team at the UK’s Evelina London Children’s Hospital for teaching and practical assistance during the project.
Financial support
This work was financially supported by New Children’s Hospital and the Pediatric Research Center. Funds were also received from the Finnish Ministry of Social Affairs and Health, Helsinki University Hospital, and the Finnish Pediatric Research Foundation.
Conflicts of interests
None.
Ethical standards
The study was designed to comply with the Helsinki Declaration and the conventions of the Council of Europe on human rights. The study fulfills all general ethics requirements for clinical studies. The Helsinki University Hospital Ethics Committee approved it (on 1 January 2017, HUS/108/2017).
Appendix
Table A1. Present study population consisted of low-risk patients with univentricular heart defects. Exclusion criteria for low-risk patients with univentricular heart defects classification according to the previously published data by Prakash et al. Reference Prakash, Khan, Hardy, Torres, Chen and Gersony6

Table A2. Summary of CT-scan parameters

AEC = automatic exposure control; NI = noise index.
Table A3. Summary of magnetic resonance sequence parameters
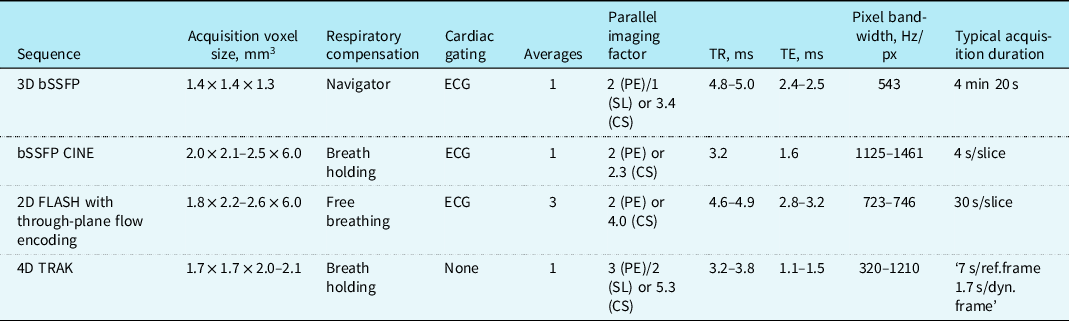
CS = compressed SENSE; PE = phase encoding direction; SL = slice encoding direction; ECG = electrocardiogram; TR = repetition time; TE = echo time.










