Case report
A 52-year-old man was referred for exertional dyspnoea. He had a history of untreated restrictive perimembranous ventricular septal defect lost to follow-up and controlled systemic hypertension. There was no reported familial history of sudden cardiac arrest or pacemaker. Repeated transthoracic heart ultrasound scans showed an isolated left-to-right perimembranous ventricular septal defect with evidence of left chamber overload. The Doppler velocity on the left-to-right ventricular septal defect shunt was more than 5 m/s suggesting normal pulmonary pressures.
Percutaneous ventricular septal closure was decided after informed consent of the patient. Under transoesophageal echography guidance (Figure 1a, 1b) and using a retrograde approach from the right femoral artery, the ventricular septal defect was closed, via a 6-French delivery sheath, by a 10/8 KONAR-MF™ ventricular septal defect Multifunctional Occluder (MFO) device (Lifetech Scientific, Shenzhen). The MFO device measured 4 mm long and was composed of a truncated cone-shaped plug measuring 10 mm on its left side and 8 mm on its right side, an inflexible 14-mm left retention disk, and a flexible 14-mm right retention disk (Supplementary Figure S1). After deployment, almost the entire device was inside the defect, the left disk being slightly tilted into the left ventricular entry (Figure 1c). There was no valvular injury and only a small residual shunt with a 2-mm-colour-doppler jet width, so the device was released (Figure 1d). The fluoroscopy time was 10 minutes and 39 s, and 25 mL of contrast medium was used for the unique initial angiography. There was no acute complication. Twenty-four hours after the procedure, transthoracic ultrasound showed no residual shunt. The patient was discharged 48 hours post-procedure. The repeated electrocardiograms before discharge, at 2 months, and 1 year post-procedure, were similar to the pre-procedural electrocardiogram (Figure 2a).
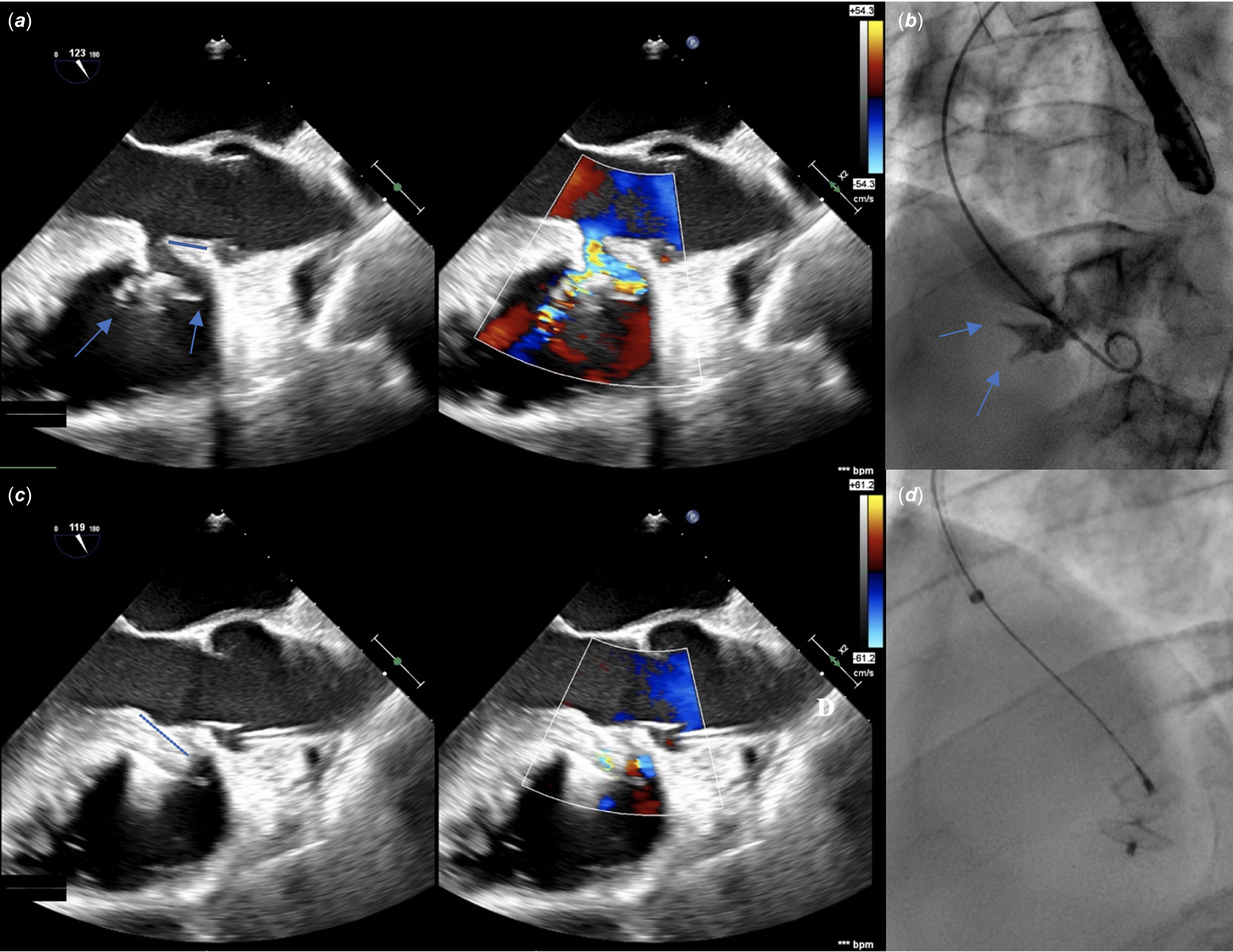
Figure 1. Initial long-axis transoesophageal ultrasound 2-dimension and colour Doppler views (a) and angiography (36°-left-anterior-oblique and 16°-cranial projection) (b), and the corresponding ultrasound (c) and radiological projection (d) after device deployment for the perimembranous ventricular septal defect closure. Before closure (a and b ), note the aneurysm with the two right ventricular exits (blue arrows), and the 10-mm-length aortic rim (blue line). The entry on the left side measured 10 mm, and two exits on the right side measured 6 and 4 mm, respectively, the larger being superior and closer to the aortic valve. The depth of the aneurysm of the septal leaflet of the tricuspid valve from the left ventricular entry was 22.5 mm. After closure (c and d), the left disk was tilted into the left ventricular entry (blue dotted line).
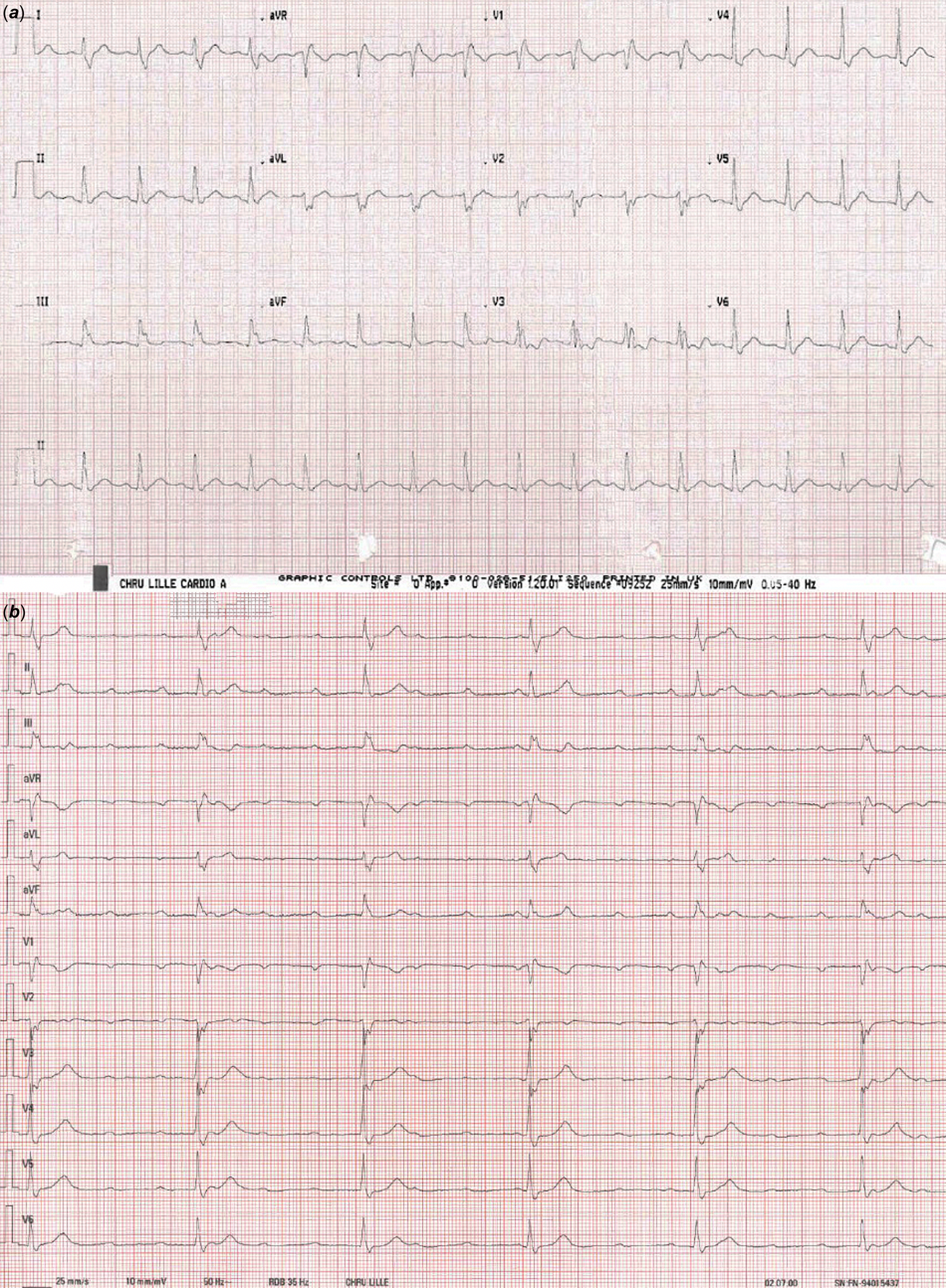
Figure 2. Twelve-lead electrocardiogram on the day before the procedure of ventricular septal defect closure depicting complete right bundle branch block (QRS duration 160 ms) ( a), and twelve-lead electrocardiogram 20 months after the procedure showing complete atrioventricular block (b).
Follow-up was uneventful until 18 months when the patient was admitted to the emergency for vasovagal symptoms: the electrocardiogram was unchanged and the patient was discharged home with no other investigation. The patient still complained of fatigue and was urgently hospitalised 2 months later for another episode of fainting. The electrocardiogram showed a complete atrioventricular block with severe bradycardia requiring isoprenaline (Figure 2b). An epicardial dual-chamber pacemaker DDD was successfully implanted.
Systematic review and discussion
A comprehensive literature review was performed. The aim was to assess the overall rate of complete atrioventricular blocks associated to the MFO device in perimembranous ventricular septal defect closure (Supplementary Figure S2). All included studies were observational with no control group, associated with a very high risk of bias (Supplementary Table S1). Reference Higgins, Morgan and Rooney1 Overall, 494 patients underwent the closure of a perimembranous ventricular septal defect with the MFO device. Three cases of persistent complete atrioventricular block were reported: 2 occurred early and resolved with the surgical ablation of the device and 1 late (the present case), totalling a complete atrioventricular block rate of 0.6%. Including transient cases, the overall rate of complete atrioventricular blocks was 1%. However, the rate of complete atrioventricular blocks requiring pacemaker implantation was only 0.2% (one patient, the present case). These figures were comparable to other transcatheter devices (1.1% and 0.5% for the overall complete block rate and pacemaker implantation-associated complete block rate respectively) but appeared lower than asymmetric membranous devices (4% and 1.1%, respectively), Reference Santhanam, Yang, Chen, Tai, Rajgor and Quek2 and lower than surgery (5% and 1%, respectively). Reference Schipper, Slieker, Schoof and Breur3 These differences in complete atrioventricular block rates were not significant in the literature but more recent devices such as the MFO had not been used. Reference Saurav, Kaushik and Mahesh Alla4
In the present systematic review, in 11 studies with available data totalling 320 patients, only 5 studies totalling 240 patients (75% of patients with available data, 48.6% of the whole cohort) had a follow-up of at least 12 months. As other cases of delayed complete atrioventricular block may not have been reported, a longer follow-up is necessary to confirm the long-term safety of the MFO device. Similarly, in the present review, the patients who had a persistent complete atrioventricular block were aged 5, 26, and 52 years. Nevertheless, most children undergoing this procedure were younger than 10. Indeed, in the literature, older age was associated with conduction disorders, Reference Bai, Xu and Li5 as was age less than 6 years with the Amplatzer ventricular septal defect occluders. Reference Carminati, Butera and Chessa6 This may have been explained by a higher diameter of the delivery sheath. Reference Wang, Zhou and Luo7 Further studies in younger patients with low-profile devices including the MFO are necessary.
It is unclear whether the complete atrioventricular block would have been the natural history of the current case. Among risk factors for conduction disturbances, pre-existing conduction disorders have been reported, including a pre-existing complete right bundle branch block and a longer QRS duration as in the present case. Reference Bai, Xu and Li5,Reference Jiang, Fan and Han8 Moreover, the occluder size, the diameter difference between the occluder and defect, Reference Wang, Zhou and Luo7 and the perimembranous ventricular septal defect localisation remote from the aortic valve as in the current case Reference Yang, Kong and Sheng9 were other suggested risk factors for complete left bundle branch block. In the present case, the left disk was tilted into the left ventricular entry: this may have increased pressure onto the inferior rim where conduction tissue is usually found. Yet no significant change in the device configuration was seen over time (Supplementary Figures S3 and S4). In the literature, a longer operation time, Reference Tang, Shao, Zhou, Hua, Luo and Wang10 per-procedural and early post-procedural transient complete atrioventricular blocks Reference Yang, Kong and Sheng9,Reference Leong and Alwi11 are other reported risk factors. The present procedure was yet straightforward using the retrograde approach. Unfortunately, no recovery of complete atrioventricular block occurring beyond 1-month post-implantation has been reported and a permanent pacemaker should be implanted. Reference Bai, Xu and Li5
Post-procedural follow-up, including regular systematic electrocardiograms, is necessary. The present patient complained of vasovagal symptoms 2 months before diagnosis, possibly related to high-grade atrioventricular block episodes. In case of unusual fatigue, fainting, or syncope, with a history of perimembranous ventricular septal defect percutaneous closure, electrocardiogram Holter monitoring should be realised and an implantable loop recorder should be discussed. Eventually, persistent asymptomatic post-procedural conduction disorders including right or left bundle branch block may be as high as 10%, Reference Wang, Zhou and Luo7 very rarely associated with symptoms of heart failure. The incidence and the impact of these anomalies have been poorly described with the MFO device in the literature.
Conclusion
We report for the first time a case of late complete atrioventricular block requiring pacemaker implantation after perimembranous ventricular septal defect percutaneous closure with the MFO device. Percutaneous closure should be avoided when a complete right bundle branch block exists. Post-procedural follow-up is mandatory with a low suspicion threshold for arrhythmia events in older patients. The rate of complete atrioventricular block requiring surgical ablation or pacemaker implantation is 0.6% with the MFO device with a median follow-up of 1 year. Long-term follow-up is warranted.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1047951124026891.
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
Acknowledgements
The authors acknowledge Dr Olivia Domanski, Dr Christelle Marquié, the patient, and the teams participating in his care.
Author contribution
All authors took part in the diagnosis. Saïd Bichali took part in study design, data collection, data analysis and interpretation, drafting of the article, and critical revision. François Godart took part in data collection and critical revision. Ali Houeijeh took part in critical revision. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Competing interests
Pr François Godart is a proctor for Occlutech. Dr Ali Houeijeh is a proctor for Abbott and Occlutech. The other author has no relevant financial or non-financial interests to disclose.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and the Helsinki Declaration of 1975, as revised in 2008. Informed written consent was obtained from the patient. The patient has consented to the case report submission to the journal.





