About 150,000 to 200,000 children with CHD are born every year in our country, Reference Qu, Liu and Zhuang1 and transposition of the great arteries accounts for 5 to 7%, which is the most common subtype of complex CHD. Reference Khairy, Clair and Fernandes2 The anatomical relationship of its aorta is reversed. Unlike its normal location, the aorta originates from the right ventricle and lies anterior to the pulmonary artery, which originates behind the aorta and originates from the left ventricle. Common cardiac anomalies associated with transposition of the great arteries include patent patent ductus arteriosus, atrial septal defect, ventricular septal defect, pulmonary stenosis, coarctation of the aorta, and aortic interruption. From the initial balloon atrial septotomy and pulmonary artery ligation for palliative treatment, to Mustard and Senning intra-atrial barrier surgery for physiological correction, and then to arterial switch operation, it has become the first choice for major children’s heart centres. According to The Society of Thoracic Surgeons Congenital Heart Surgery Database, from 2013 to 2016, the cumulative operative mortality rates for infant arterial switch operation and arterial switch operation with ventricular septal defect repair in developed regions were as low as 2.2 and 5.1%, respectively. The 20-year survival rate after arterial switch operation was reported in most developed regions is higher than 90%. Surgical results with low perioperative mortality over the last decades have now led to focus on the long-term outcomes. Theoretical long-term complications after the arterial switch include suprapulmonary stenosis, aortic root dilation, new aortic valve regurgitation, coronary artery malformation, and arrhythmias. Since 2011, neoaortic sinotubular junction reconstruction technique has been routinely performed in children with aortopulmonary artery diameter less than 1:2. By folding the new aortic wall at the level of the sinus tubular junction, the diameter ratio of the sinus tubular junction to the aortic annulus of the new aorta was 0.8, which was closer to the normal aortic root anatomy. Although the management of transposition of the great arteries has been optimised in developed countries over the past 60 years, arterial switch operation operation has achieved good results. However, arterial switch operation has been carried out in developing countries for only 20 years, and there are few summaries of the long-term outcomes after arterial switch operation. The aim of this study was to evaluate the 16-year arterial switch operation experience at Beijing Children’s Hospital and to determine early and late mortality and late morbidity, as well as the need for reoperation and catheter intervention, and second, to investigate risk factors for late complications and reintervention. Finally, propensity matching was used to determine the role of the neoaortic sinotubular junction reconstruction technique in arterial switch operation. Patients treated with neoaortic sinotubular junction reconstruction n Fuwai Hospital were matched with patients treated with non-neoaortic sinotubular junction reconstruction Beijing Children’s Hospital using the closest propensity score matching to evaluate whether neoaortic sinotubular junction reconstruction reduces long-term complications of arterial switch operation.
Materials and methods
Study design
Institutional Committee on Clinical Investigation of Beijing Children’s Hospital affiliated with Capital Medical University and Fuwai Hospital approved this protocol with a waiver of informed consent. Patients who received arterial switch operation biventricular correction in Beijing Children’s Hospital from January 2006 to January 2022 and patients who (not) combined with other intracardiac corrections were included as research subjects. Included diagnoses included Transposition of the great arteries-Intact ventricular septum, Transposition of the great arteries-Ventricular septal defect, and Transposition of the great arteries-Taussig-Bing anomaly. A total of 185 patients were enrolled. Thirty patients who underwent modified arterial switch operation with neoaortic sinotubular junction reconstruction technique after 2011 in Fuwai Hospital were also included. Perioperative clinical data and postoperative follow-up data of all children were collected from the ward medical record system, outpatient medical record system, and telephone follow-up in our hospital. Patients underwent outpatient review at 1, 3, 6, and 12 months in the first postoperative year and annually thereafter. Echocardiography chest X-ray and electrocardiogram were routinely performed during follow-up.
Definition
Emergency surgery was defined as life-saving surgery requiring surgery within 24 hours of admission. Early mortality was defined as death that occurred during hospitalisation. Late mortality was defined as death that occurred after discharge from the hospital. Mild, moderate, and severe aortic valve regurgitation was defined as the proportion of the valve regurgitation area in the left ventricular outflow tract <25, 25 to 65%, and ≥65%. Right ventricular outflow tract obstruction was considered mild with an echocardiographic peak gradient between 20 and 40 mmHg, moderate between 40 and 80 mmHg, and severe ≥80 mmHg. To account for the range in body size for neo-aortic measurements during childhood (0–18 years), Z-scores were calculated for each patient by using paediatric reference values and body surface area (DuBois method). Reference Pettersen, Du, Skeens and Humes4 Aortic root dilation was defined as Z-score ≥3. Late reintervention includes late reoperation and catheter intervention. Aorto-pulmonary diameter mismatch was defined when the ratio of pulmonary artery root diameter to aortic root diameter was found to be >1.5 during the operation.
Surgery
The authors previously reported the surgical technique of arterial switch operation performed by the cardiac surgery team of Beijing Children’s Hospital. Reference Wang, Li and Ding5 All patients underwent moderate hypothermic cardiopulmonary bypass under general anaesthesia with endotracheal intubation. The median incision was used to establish cardiopulmonary bypass through the aorta and superior and inferior vena cava, histidine-tryptophan-ketoglutarate cardioplegia was perfused for myocardial protection, and the ascending aorta was clamped. Coronary artery was removed free from the anatomic aortic sinus in a “U” shape or button and replanted in the corresponding anatomic pulmonary sinus. The surgical procedure of Fuwai Hospital is basically the same as that of Beijing Children’s Hospital, but the technique has been improved since 2011. neoaortic sinotubular junction reconstruction technique was routinely performed in patients with aortopulmonary artery diameter less than 1:2. The reconstruction more closely approximated normal aortic root anatomy by folding the new aortic wall at the level of the sinotubular junction, resulting in a sinotubular junction to annulus diameter ratio of 0.8 in the new aorta (Supplementary Figure 1).
Statistical analyses
Mean ± SD or median (min, max) was used to describe continuous variables, and the Student’s t-test or the Wilcoxon–Mann–Whitney U-test was used to compare differences between groups. Descriptive statistics for categorical variables were reported as frequency/percentage and were compared using the Pearson’s χ2 or Fisher’s exact test. Freedom from time-dependent endpoints was studied using Kaplan-Meier and Log-rank calculations. Multivariate logistic regression was used to identify predictors of new aortic valve regurgitation, aortic root dilation, right ventricular outflow tract obstruction, and reintervention. Freedom from time-dependent endpoints was studied using Kaplan-Meier and Log-rank calculations. Propensity score matching was performed on the neoaortic sinotubular junction reconstruction group and the non-neoaortic sinotubular junction reconstruction group. Demographic characteristics and preoperative basic clinical data were used as synergistic variables, and the calliper value was set at 0.01. Propensity score matching was performed using the logistic regression formula with a matching ratio of 1:1, and the propensity score value and weight were calculated. The clinical data of the two groups after matching were analysed, and the late complications and morbidity of the two groups after matching were compared. All statistical analyses were performed by SPSS 22.0 software, and P ≤ 0.05 was considered statistically significant.
Results
Patient characteristics
From January 2006 to January 2022, a total of 185 patients underwent arterial switch operation in Beijing Children’s Hospital, including 100 (54.05%) Transposition of the great arteries-Intact ventricular septum patients, 64 (34.59%) Transposition of the great arteries-Ventricular septal defect patients, and 21 (11.35%) Transposition of the great arteries-Taussig-Bing anomaly patients. Among the patients, there were 131 males (70.81%) and 54 females (29.19%). The proportion of prenatal diagnosis was 19.46%, and the proportion of premature infants was 13.51%. There were 109 (58.92%) cases and 76 (41.08%) cases in the two periods before and after January 2014. The median age at surgery was 24 (1,240) days, and the median surgical weight was 3.54 (2.2, 7.3) kg. Before arterial switch operation, 15 (8.11%) patients received pulmonary artery banding. Eleven (5.95%) with coarctation of the aorta, 20 (10.81%) with coronary malformation, 67 (36.22%) with aorto-pulmonary diameter mismatch, 35 (18.92%) with bicuspid native pulmonary valve. Nineteen (10.27%) of the patients underwent emergency surgery. The average CPB time and cross-clamp time in arterial switch operation patients were 182.66 min and 100.16 min, respectively. After the operation, the median duration of mechanical ventilation was 160 hours, and 6 (3.24%) patients underwent extracorporeal membrane oxygenation. To ensure the stability of pulmonary artery pressure and the normal work of the right ventricle, 97 (52.43%) patients delayed chest closure. The median ICU stay and hospital stay were 7 days and 21 days, respectively. The general information about the patients is shown in Table 1.
Table 1. Clinical baseline data of 185 patients

AR = aortic valve regurgitation; ARD = aortic root dilation; ASD = atrial septal defect; BPV = bicuspid native pulmonary valve; CICU = cardiac intensive care unit; COA = coarctation of the aorta; ECMO = extracorporeal membrane oxygenation; IVS = intact ventricular septum; LVOTO = left ventricular outflow tract obstruction; NAR = new aortic valve regurgitation; PAB = pulmonary artery banding; PDA = patent ductus arteriosus; PFO = patent foramen ovale; PR = pulmonary regurgitation; RVOTO = right ventricular outflow tract obstruction; TBA = Taussig–Bing anomaly; TGA = transposition of the great arteries; TR = tricuspid regurgitation; VSD = ventricular septal defect.
Early mortality
The early postoperative mortality was 7.03%. Among the 13 patients who died, nine patients died of circulatory failure caused by left heart failure, two patients died of being unable to withdraw from cardiopulmonary bypass after the operation and parents gave up ECOM assistance, and one patient died of pulmonary arterial hypertensive crisis on the first day after the operation. The remaining one patient died of cardiac arrest caused by malignant arrhythmia. Univariate analysis showed that prematurity (P = 0.000), preoperative emergency operation (P = 0.000), preoperative mechanical ventilation (P = 0.003), Transposition of the great arteries-Intact ventricular septum and Transposition of the great arteries-Taussig-Bing anomaly (P = 0.000), concomitant arch repair (P = 0.032) and left ventricular outflow tract obstruction resection (P = 0.000), previous pulmonary artery banding (P = 0.010), coronary artery malformation (P = 0.000), preoperative moderate or severe TR (P = 0.000), preoperative moderate or severe PR (P = 0.001), preoperative cardiac dysfunction (P = 0.000), prolonged CPB time (P = 0.005), and prolonged cross-clamp time (P = 0.003) were risk factors for early postoperative mortality in arterial switch operation patients, while delayed chest closure (P = 0.028) was a protective factor (Table 2). We found that most of the early deaths occurred within 3 days after arterial switch operation. Multivariate analysis found no independent risk factors.
Table 2. Univariable analysis of early mortality [Number (%)/Median (Min, Max)/Mean ± SD]
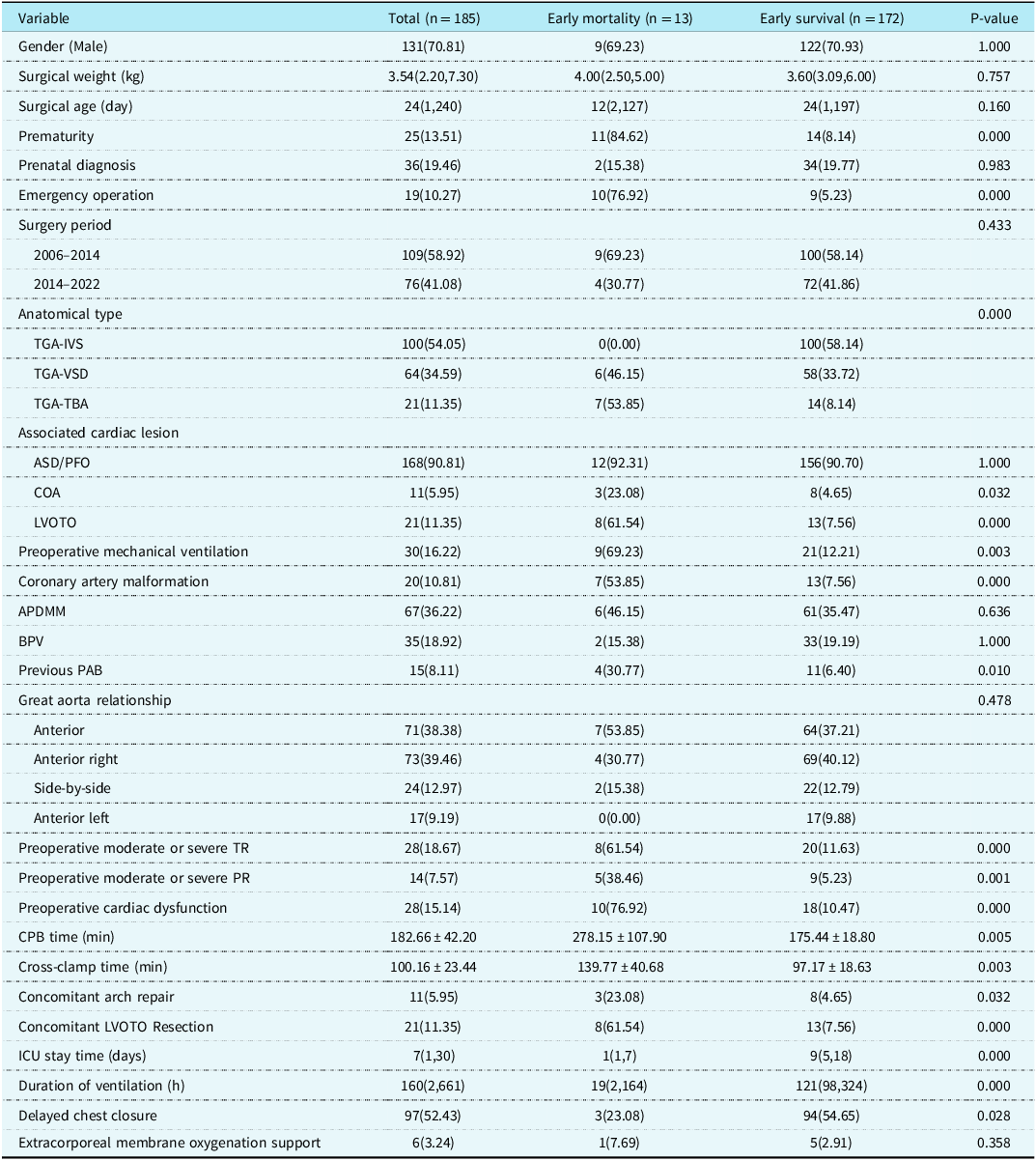
APDMM = aorto-pulmonary diameter mismatch; ASD = atrial septal defect; BPV = bicuspid native pulmonary valve; CICU = cardiac intensive care unit; COA = coarctation of the aorta; ECMO = extracorporeal membrane oxygenation; IVS = intact ventricular septum; LVOTO = left ventricular outflow tract obstruction; PAB = pulmonary artery banding; PFO = patent foramen ovale; PR = pulmonary regurgitation; TBA = Taussig-Bing anomaly; TGA = transposition of the great arteries; TR = tricuspid regurgitation; VSD = ventricular septal defect.
Late mortality
Of the 185 patients, 13 died early, six were lost to follow-up, and 166 completed long-term follow-up. The median follow-up time was 88.5 (2,190) months. There were five deaths (3,01%) during follow-up. One Transposition of the great arteries-Intact ventricular septum patient died of massive myocardial infarction caused by left coronary embolism 8 months after arterial switch operation. One Transposition of the great arteries-Taussig-Bing anomaly patient died of internal tunnel obstruction complicated with infective endocarditis 18 months after the operation. A Transposition of the great arteries-Ventricular septal defect patient underwent aortic valve replacement due to severe aortic regurgitation 13 months after arterial switch operation and died suddenly 5 months after the operation. The cause of death was unknown. One TGA-TAB patient with arterial switch operation underwent coarctation resection and anastomosis at the same time. The re-examination found that anastomotic stenosis underwent coarctation balloon dilatation 6 months after the operation, and died of chronic heart failure 6 months after the operation. The last Transposition of the great arteries-Ventricular septal defect patient developed aortic root dilation and new aortic valve regurgitation 18 months after arterial switch operation, underwent aortic valvuloplasty, and died of malignant arrhythmia 6 months after the operation. Among them, four (4.55%) died patients who underwent arterial switch operation before 2014, and one (1.28%) underwent arterial switch operation after 2014, however, the comparison between groups was not statistically significant (P = 0.360) (Fig. 1A). The overall survival rate of the arterial switch operation hospital survivors was 98.7% at 1 year, 97.4% at 2 years and 96.7% at 5 and 15 years(Fig. 1B); the survival curve was significantly different between transposition of the great arteries subgroups (P = 0.005) (Fig. 1C).
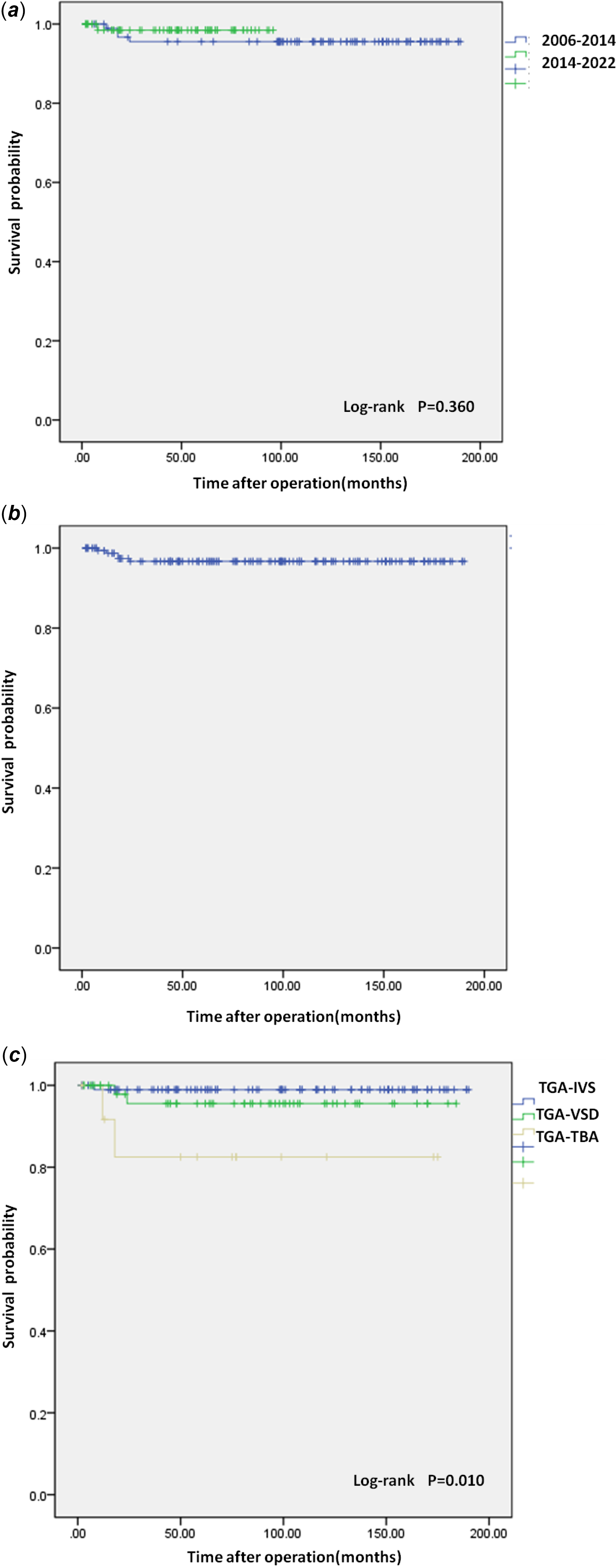
Figure 1. (a) Survival curve. Kaplan-Meier curves show the relationship between surgery period and mortality. (b) Overall Survival curve. (c) Survival curve. Kaplan-Meier curves show the relationship between the anatomical type and mortality. TGA-IVS = Transposition of the great arteries-Intact ventricular septum; TGA-TBA = Transposition of the great arteries-Taussig-Bing anomaly; TGA-VSD = Transposition of the great arteries-Ventricular septal defect.
New aortic valve regurgitation
Of the 166 follow-up patients, 19 developed late new aortic valve regurgitation. Univariate analysis found that Aorto-pulmonary diameter mismatch (P = 0.000), Bicuspid native pulmonary valve (P = 0.031), concomitant left ventricular outflow tract obstruction resection (P = 0.045), and new aortic valve regurgitation at discharge (P = 0.003) were risk factors for late new aortic valve regurgitation (Table 3). Multivariate analysis suggested that low surgical weight (OR = 0.336, P = 0.036), Aorto-pulmonary diameter mismatch (OR = 11.364, P = 0.000), preoperative Pulmonary artery banding (OR = 7.895, P = 0.042), and new aortic valve regurgitation at discharge (OR = 3.639, P = 0.036) were independent risk factors for late new aortic valve regurgitation (Table 4).
Table 3. Univariable analysis of NAR [Number (%)/Median (Min, Max)/Mean ± SD]
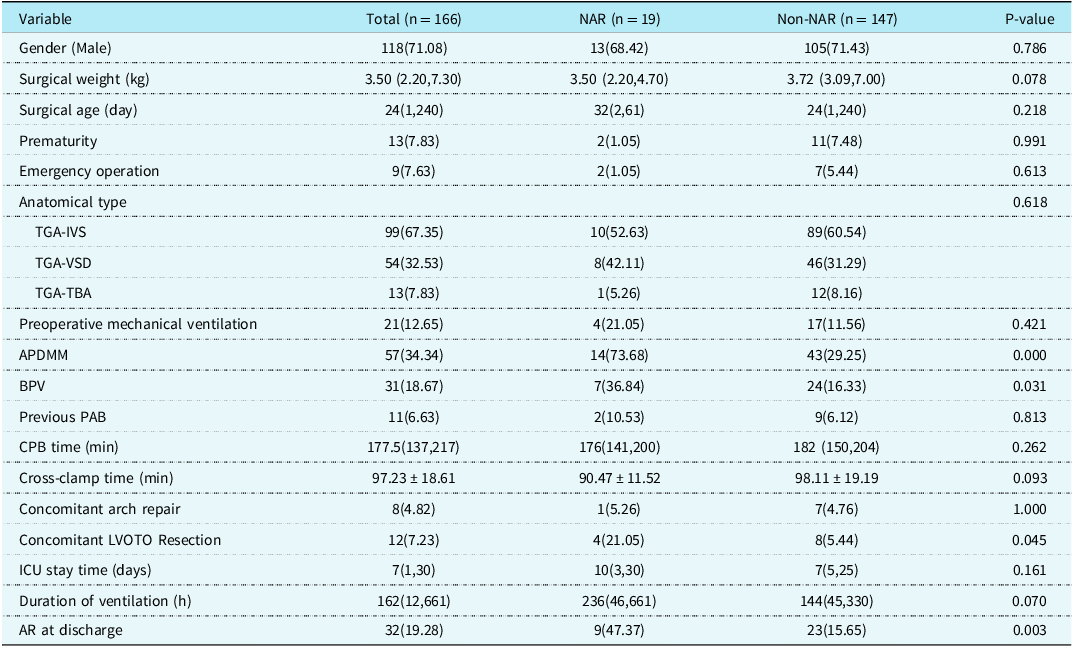
APDMM = aorto-pulmonary diameter mismatch; AR = aortic valve regurgitation; BPV = bicuspid native pulmonary valve; CICU = cardiac intensive care unit; IVS = intact ventricular septum; LVOTO = left ventricular outflow tract obstruction; NAR = new aortic valve regurgitation; PAB = pulmonary artery banding; TBA = Taussig-Bing anomaly; TGA = transposition of the great arteries; VSD = ventricular septal defect.
Table 4. Risk factors for NAR by multivariable analysis
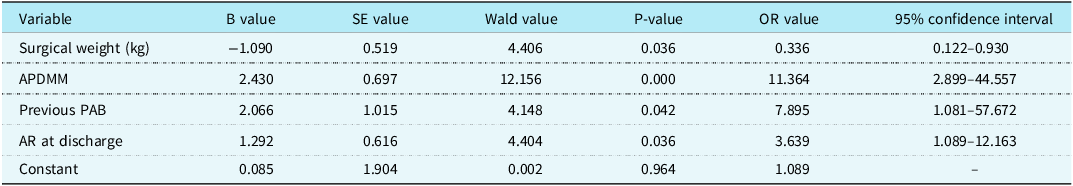
APDMM = Aorto-pulmonary diameter mismatch; AR = aortic valve regurgitation; NAR = new aortic valve regurgitation; PAB = pulmonary artery banding.
Aortic root dilation
Twenty-eight cases developed aortic root dilation during late follow-up, including 13, 10, and 5 cases of Transposition of the great arteries-Intact ventricular septum, Transposition of the great arteries-Ventricular septal defect, and Transposition of the great arteries-Taussig-Bing anomaly, respectively. Univariate analysis showed that low birth weight infant (P = 0.006), aorto-pulmonary diameter mismatch (P = 0.001), bicuspid native pulmonary valve (P = 0.000), prolonged duration of ventilation (P = 0.040) and new aortic valve regurgitation at discharge (P = 0.016) were risk factors for late aortic root dilation. Compared with non-aortic root dilation, patients with late aortic root dilation had a trend of more Transposition of the great arteries-Ventricular septal defect and Transposition of the great arteries-Taussig-Bing anomaly proportions in anatomical types, but the difference was not statistically significant (P = 0.067) (Table 5). Multivariate analysis found that aorto-pulmonary diameter mismatch (OR = 4.762, P = 0.002), bicuspid native pulmonary valve (OR = 6.906, P = 0.000), preoperative pulmonary artery banding (OR = 7.092, P = 0.019), and new aortic valve regurgitation at discharge (OR = 2.933, P = 0.040) were independent risk factors for late aortic root dilation (Table 6). Late aortic root dilation occurred in 28 hospital survivors, with a median time delay of 54 (7,133) months. Cumulative incidence rate of late aortic root dilation at 1, 5, and 10 years was 3.1, 10.8, and 24% respectively (Fig. 2A). Patients with Transposition of the great arteries-Taussig-Bing anomaly had a higher trend of occurring aortic root dilation than patients with Transposition of the great arteries-Intact ventricular septum and patients with Transposition of the great arteries-Ventricular septal defect (P = 0.080), although the statistical difference was not particularly significant (Fig. 2B). The cumulative incidence rate of late aortic root dilation was significantly different between concomitant arh repair group and non-arh repair group (P = 0.000) (Fig. 2C). The cumulative incidence rate of late aortic root dilation was significantly different between APDM group and aorto-pulmonary diameter mismatch group (P = 0.005) (Fig. 2D).
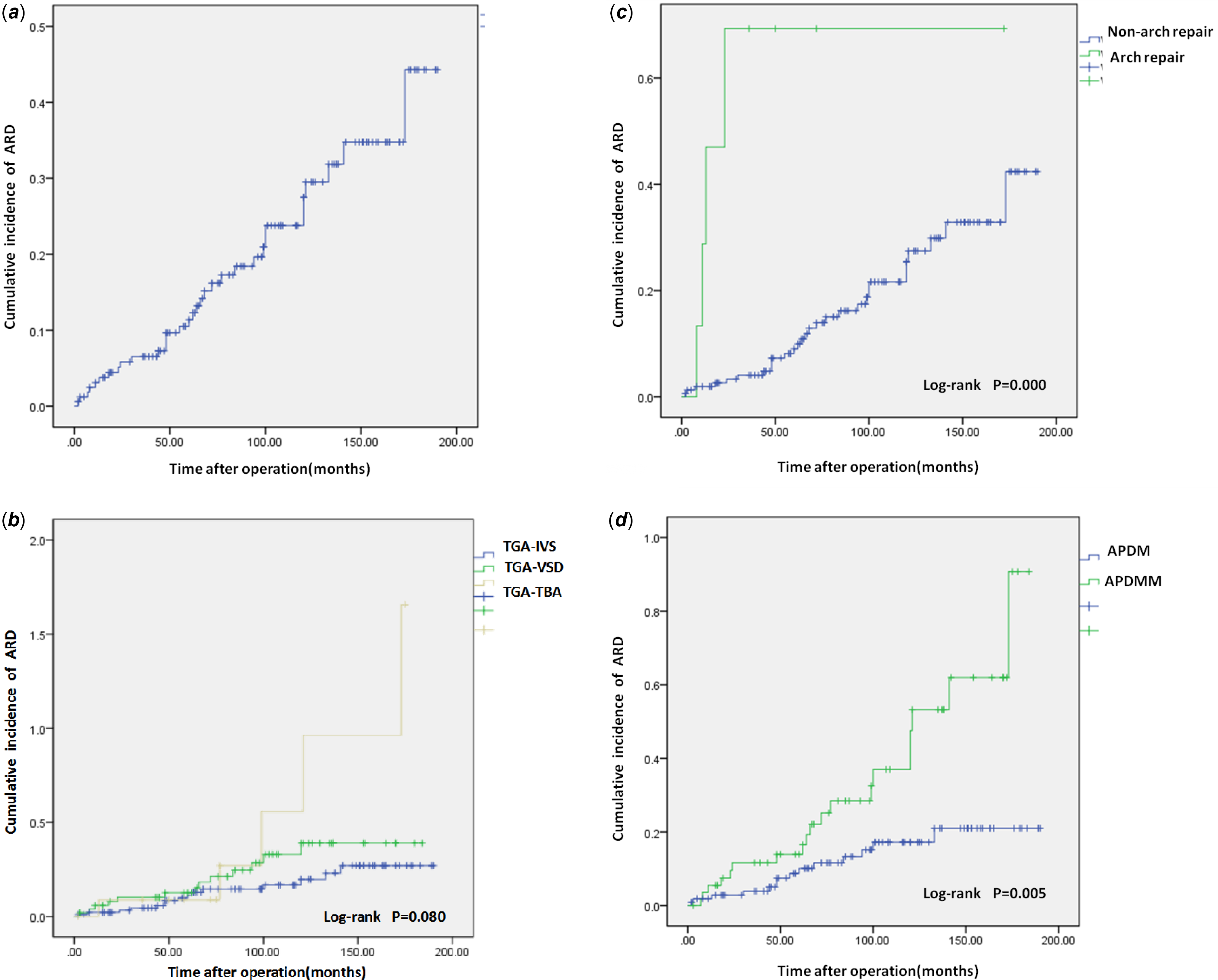
Figure 2. (a) Overall cumulative incidences curve about aortic root dilation. (b) Cumulative incidences curve. The curves show the relationship between the anatomical type and aortic root dilation. (c) Cumulative incidences curve. The curves show the relationship between the arch repair and aortic root dilation. (d) Cumulative incidences curve. The curves show the relationship between the aorto-pulmonary diameter mismatch and aortic root dilation. TGA-IVS = Transposition of the great arteries-Intact ventricular septum; TGA-TBA = Transposition of the great arteries-Taussig-Bing anomaly; TGA-VSD = Transposition of the great arteries-Ventricular septal defect.
Table 5. Univariable analysis of ARD [Number (%)/Median (Min, Max)/Mean ± SD]
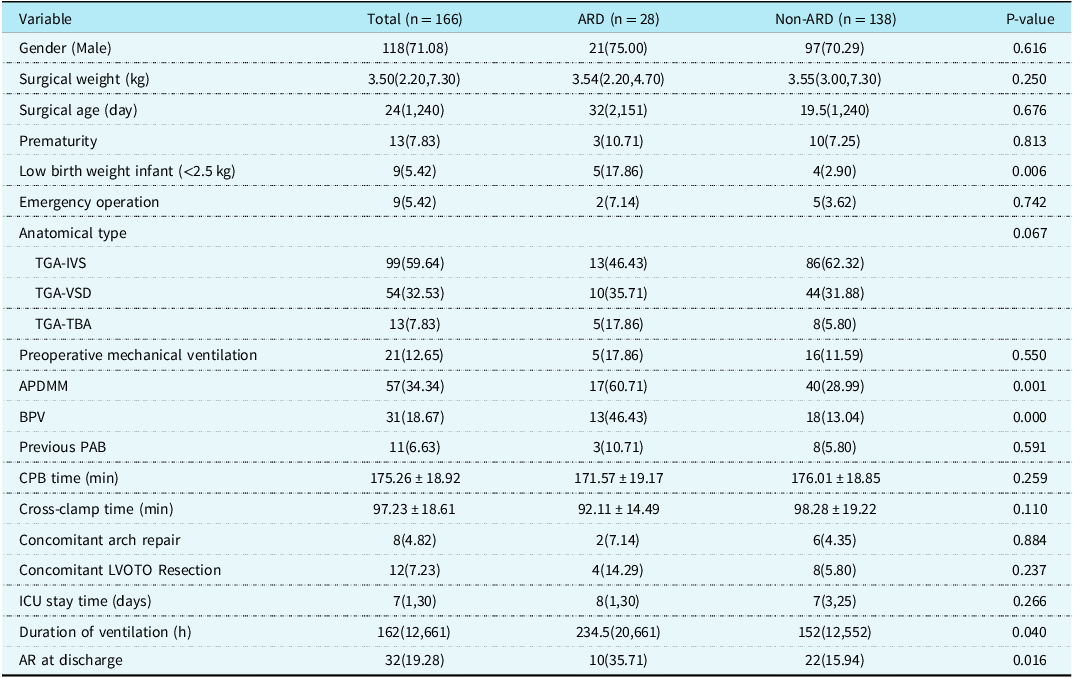
APDMM = aorto-pulmonary diameter mismatch; AR = aortic valve regurgitation; ARD = aortic root dilation; BPV = bicuspid native pulmonary valve; CICU = cardiac intensive care unit; IVS = intact ventricular septum; LVOTO = left ventricular outflow tract obstruction; PAB = pulmonary artery banding; TBA = Taussig-Bing anomaly; TGA = transposition of the great arteries; VSD = ventricular septal defect.
Table 6. Risk factors for ARD by multivariable analysis
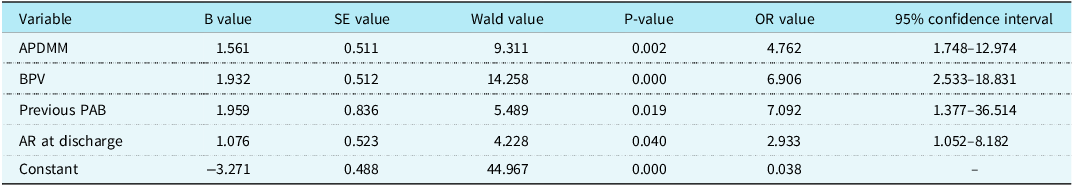
APDMM = aorto-pulmonary diameter mismatch; AR = aortic valve regurgitation; ARD = aortic root dilation; BPV = bicuspid native pulmonary valve; PAB = pulmonary artery banding.
Right ventricular outflow tract obstruction
Late right ventricular outflow tract obstruction occurred in 33 patients during follow-up. Among them, 15 were Transposition of the great arteries-Intact ventricular septum, 13 were Transposition of the great arteries-Ventricular septal defect, and five were Transposition of the great arteries-Taussig-Bing anomaly. Univariate analysis showed that older surgical age (P = 0.027), low birth weight infant (P = 0.020), aorto-pulmonary diameter mismatch (P = 0.002), and late aortic root dilation (P = 0.000) were risk factors for late right ventricular outflow tract obstruction. Patients with Transposition of the great arteries-Ventricular septal defect and Transposition of the great arteries-Taussig-Bing anomaly had a higher tendency to develop right ventricular outflow tract obstruction compared with patients with Transposition of the great arteries-Intact ventricular septum, although the difference was not statistically significant (P = 0.091). The intraoperative concomitant arch repair group appeared to have a higher proportion of patients with late right ventricular outflow tract obstruction than the non-arh repair group, although this was not statistically significant (P = 0.083). Multivariate analysis showed that older surgical age (OR = 1.012, P = 0.002) and late aortic root dilation (OR = 12.596, P = 0.000) were independent risk factors for late right ventricular outflow tract obstruction.
Late intervention
A total of 18 patients (10.84%) needed a first reoperation or catheter intervention. Eight (44.44%) of these were open reoperations, ten were catheter interventions. The actuarial freedom from late reintervention was 96.3, 90.7, 88.8, 76.6% at 1, 5, 10, and 15 years, respectively (Fig. 3E). Late follow-up found that Transposition of the great arteries-Intact ventricular septum patients were more likely to be free from reintervention at a later stage than those of the other two groups (P = 0.003) (Fig. 3A). The Neonate group was more likely to be free from late reintervention than the Infant group (P = 0.000) (Fig. 3B). The non-aortic root dilation group was more likely to be free from reintervention at a later stage than the aortic root dilation group (P = 0.000) (Fig. 3C). The right ventricular outflow tract obstruction group had a higher probability of later reintervention than the Non-right ventricular outflow tract obstruction group (P = 0.000) (Fig. 3D). Cardiac reinterventions included percutaneous pulmonary artery (including its branches) stenosis stenting/percutaneous pulmonary artery stenosis balloon dilation/percutaneous pulmonary angioplasty (n = 5), aortic coarctation balloon dilation (n = 5), cardiac Pacemaker implantation (n = 3), aortic valvuloplasty (n = 2), aortic valve replacement (n = 1), pulmonary valve replacement (n = 1), and aortic root replacement (n = 1). Most reinterventions in this cohort occurred in the left and right ventricular systems, and coronary reinterventions were not found. Three patients died in the late stage of the reintervention, and they died 5 months, 6 months, and 6 months after the reintervention, respectively. Univariate analysis suggested that older surgical age (P = 0.002), Low birth weight infant (P = 0.026), complex anatomical type (P = 0.003), preoperative mechanical ventilation (P = 0.041), aorto-pulmonary diameter mismatch (P = 0.011), concomitant arch repair (P = 0.000), aortic root dilation (P = 0.000), 2006–2014 surgical period (P = 0.015) and right ventricular outflow tract obstruction (P = 0.000) were risk factors for late reintervention. Multivariate analysis found that older surgical age (OR = 1.015, P = 0.002), aortic root dilation (OR = 5.451, P = 0.017), right ventricular outflow tract obstruction (OR = 7.008, P = 0.003), and 2006–2014 surgical period (OR = 0.221, P = 0.041) were independent risk factors for late intervention.
Neoaortic sinotubular junction reconstruction technique improves aortic valve function and durability
In order to improve the surgical effect and reduce the incidence of new aortic valve regurgitation, 30 consecutive transposition of the great arteries patients with aorto-pulmonary diameter mismatch underwent arterial switch operation and neoaortic sinotubular junction reconstruction in 2011 in Fuwai Hospital, and they were classified as neoaortic sinotubular junction reconstruction group. Propensity score matching was used to select 30 patients who did not undergo neoaortic sinotubular junction reconstruction in Beijing Children’s Hospital during the same period as the non-neoaortic sinotubular junction reconstruction group. As shown in Table 7, the preoperative baseline characteristics of the two groups were similar and there were no significant differences. In the neoaortic sinotubular junction reconstruction group, the ratio of new sinus tube junction to aortic annulus diameter was 0.7 immediately after surgery. None of the 30 patients in neoaortic sinotubular junction reconstruction group developed new aortic valve regurgitation or aortic root dilation during follow-up. Aortic cross-clamp time and CPB time were longer in the neoaortic sinotubular junction reconstruction group than in the non-neoaortic sinotubular junction reconstruction group, but the difference was not statistically significant (P = 0.093; P = 0.262). In the non-neoaortic sinotubular junction reconstruction group, aortic root dilation and new aortic valve regurgitation occurred in seven and five cases, respectively, which was statistically significant compared with the neoaortic sinotubular junction reconstruction group (P = 0.003; P = 0.015).
Table 7. Baseline characteristics and clinical outcomes of patients in the neoaortic sinotubular junction reconstruction nd non-neoaortic sinotubular junction reconstruction roups after propensity score matching [Number (%)/Median (Min, Max)/Mean ± SD]
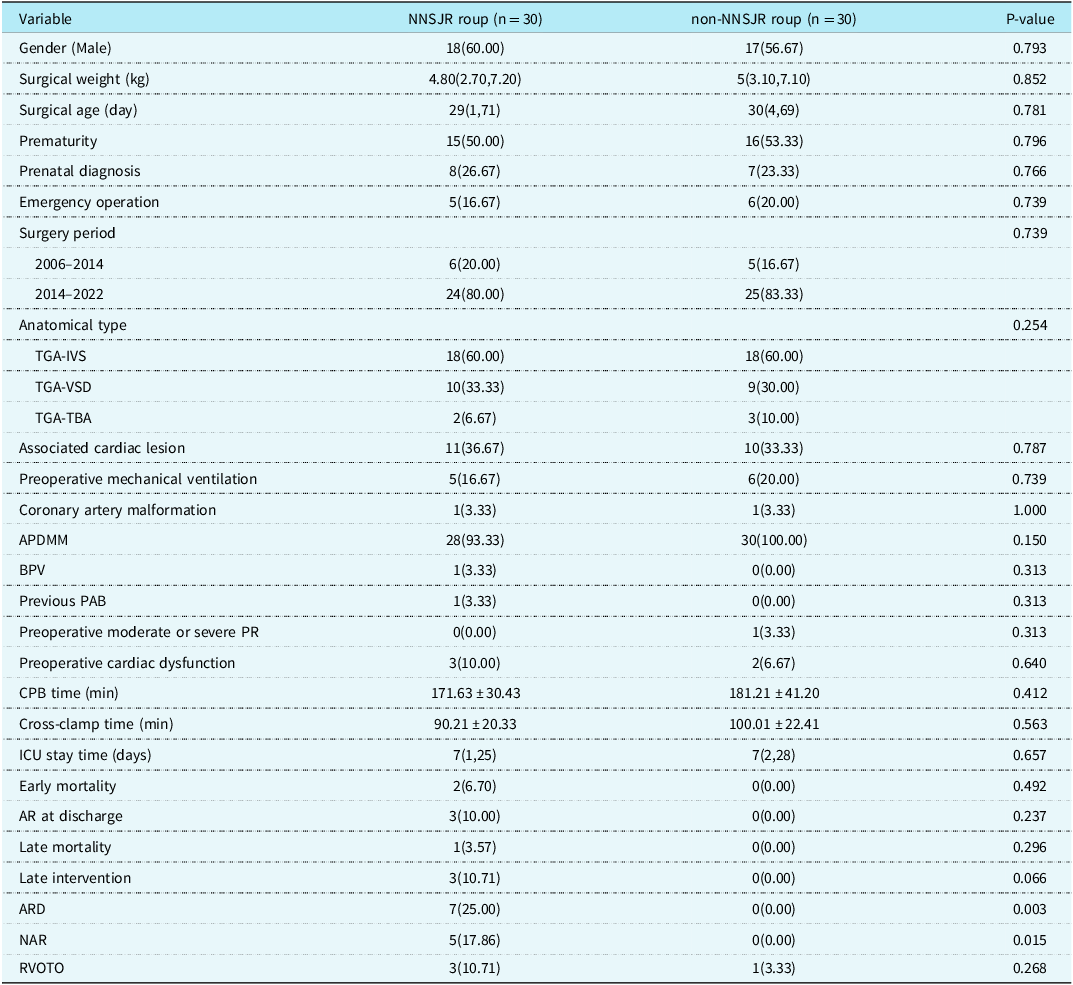
APDMM = aorto-pulmonary diameter mismatch; AR = aortic valve regurgitation; ARD = aortic root dilation; BPV = bicuspid native pulmonary valve; IVS = intact ventricular septum NAR new aortic valve regurgitation; NNSJR = neoaortic sinotubular junction reconstruction; PAB = pulmonary artery banding; RVOTO = right ventricular outflow tract obstruction; TBA = Taussig-Bing anomaly; TGA = transposition of the great arteries.
Discussion
Surgical treatment of transposition of the great arteries has historically been established and used many surgical methods until the advent of new treatments that limited their use. Physiological correction based on early atrial dam surgery is aimed at redirecting systemic and pulmonary venous return into the ventricle connected to the correct aorta. Atrial barrier surgery has excellent early clinical outcomes, but presents long-term problems of superior vena cava obstruction, barrier leakage, atrial and ventricular arrhythmias, tricuspid regurgitation, and right heart failure. Reference Horer, Karl and Theodoratou6,Reference Horer, Herrmann and Schreiber7 arterial switch operation has largely replaced atrial barrier surgery with excellent early and long-term outcomes. At present, arterial switch operation has become the consensus of domestic and foreign experts as the preferred surgical procedure for children with transposition of the great arteries. In four decades of arterial switch operation, a significant decrease in perioperative mortality to rates below 5% in high-volume heart centres has been achieved. Reference Cleuziou, Vitanova and Pabst8 In this study, the early mortality rate of arterial switch operation patients was 7.03%, and the late mortality rate was 3.0%. The early mortality rate was slightly higher than that of high-volume heart centres in some developed countries. Considering the basic medical conditions of our country, many patients are over-aged. The average age of operation is high, the optimal operation period is missed, and the perioperative management is more difficult.
New aortic valve regurgitation and Aortic root dilation
Neo-aortic regurgitation and root dilatation are common findings in patients with transposition after arterial switch operation. Histological studies have found that even in patients with transposition of the great arteries, the pulmonary artery differs significantly from the aorta in terms of the elastic layer of the arterial wall and smooth muscle actin. Although there are no obvious morphological and histological differences between the aortic and pulmonary arteries in neonates, the elastic fibrous tissue of the pulmonary valve shrinks with age in the low-pressure pulmonary circulation. Since the original pulmonary valve is used as the aortic valve after arterial switch operation, such patients are at risk of developing new aortic valve regurgitation in the long term. Reference Lu, Shoujun and Jun9 In this study, 19 (11.45%) patients developed new aortic valve regurgitation, and 28 (16.87%) patients developed aortic root dilation. The team at Leuven University Hospitals reported the results of a 35-year follow-up of 318 patients at their institution who experienced arterial switch operation from October 1981 to July 2018. They found that 12 percent (35) of survivors had moderate to severe new aortic valve regurgitation at the most recent follow-up. Reference Santens, Van De Bruaene and De Meester10 Roel L.F. van der Palen et al. Reference van der Palen, Blom and Kuipers11 retrospectively analysed the clinical data of 490 children treated with arterial switch operation at the Leiden University Medical Center, Leiden, between January 1977 and January 2020. They completed a 43-year long-term follow-up. After right ventricular outflow tract obstruction (50/83, 60.2%), aortic root dilation was the second most common indication for reoperation (15/83, 18.1%). In this study, the incidence of reoperation for new aortic valve regurgitation with root dilation was 22% (4/18), which was slightly higher than that of the former, considering that our centre is more active in the intervention of late complications. Yaqiong Xiao et al. Reference Xiao, Zhang and Su12 reported the mid-term follow-up results of 119 patients who received arterial switch operation in Union Hospital, Huazhong University of Science and Technology from 2007.1 to 2013.12. Ninety-three (85.3%) patients completed follow-up, and it was found that four patients (4.3%) developed moderate to severe new aortic valve regurgitation, which is better than this study. Because of their shorter follow-up time, we believe that with longer follow-up, more patients will develop new aortic valve regurgitation. Shinichiro Oda et al. Reference Oda, Nakano and Fujita13 reported that 145 patients underwent angiographic follow-up for at least 10 years, of which 21 (14.5%) patients developed neo-aortic valve insufficiency, which was similar to the results of this study. Tyson A. Fricke et al. Reference Fricke, Buratto and Weintraub14 retrospectively analysed the clinical data of 844 patients undergoing arterial switch operation at the Royal Melbourne Hospital from 1983 to 2015. Many patients with arterial switch operation have good long-term survival but develop significant new aortic valve regurgitation or require new aortic valve replacement after 25 years. 15% of patients had moderate neo-aortic valve regurgitation or required neo-aortic valve replacement after 25 years. Slightly higher than the results of this study, considering its longer follow-up time span.
Risk factors for new aortic valve regurgitation and aortic root dilation have been reported in recent years, but the results reported by different institutions are not the same. In this study, we found that aorto-pulmonary diameter mismatch, previous pulmonary artery banding, and minimal new aortic valve regurgitation at discharge were common independent risk factors for late new aortic valve regurgitation and aortic root dilation. However, low surgical weight was an independent risk factor specific to new aortic valve regurgitation, and bicuspid native pulmonary valve was an independent risk factor specific to aortic root dilation. Roel L F van der Palen et al. Reference van der Palen, van der Bom and Dekker15 reported findings from their centre in 2019. Risk factors for root dilatation were complex transposition of the great arteries anatomy (Transposition of the great arteries-Ventricular septal defect, double-outlet right ventricle with subpulmonary Ventricular septal defect) and male gender. Risk factors for aortic valve regurgitation ≥ moderate were: complex transposition of the great arteries anatomy and neo-aortic growth. Per millimetre increase in aortic root dimension, there was a 9% increase in the hazard of aortic valve regurgitation ≥ moderate. However, Bicuspid pulmonary valve did not relate to the presence of root dilatation or aortic valve regurgitation, which is different from our view. Emanuela Angeli et al. Reference Angeli, Gerelli and Beyler16 reported a long-term evaluation of the effects of the mitral pulmonary valve on new aortic valve regurgitation and aortic root dilation after arterial switch operation. In univariate analysis, weight at operation was the only independent variable that predicted increased aortic valve regurgitation (P = 0.02). Only the position of the great vessels (anteroposterior versus other position) was a higher risk of aortic root dilation for side-by-side or rightward position of the great arteries (univariate analysis: P = 0.03). Shinichiro Oda et al. Reference Oda, Nakano and Fujita13 believed that major risk factors for NRD were double-outlet right ventricle and presence of neo-aortic valve insufficiency. Rui Lu et al. Reference Lu, Shoujun and Jun9 summarised and analysed the results of new aortic valve regurgitation after arterial switch operation in 599 transposition of the great arteries children in the Pediatric Cardiac Surgery Center of Fuwai Hospital. They believe that previous pulmonary artery banding and aorto-pulmonary diameter mismatch are risk factors for severe aortic insufficiency, and no severe aortic regurgitation was found after neo-aortosinusoplasty in children with aorto-pulmonary diameter mismatch. Their conclusions are consistent with the conclusions of this study. We believe that aorto-pulmonary diameter mismatch and the previous pulmonary artery banding have changed the geometry of the aortic root and the sinotubular junction to varying degrees. The haemodynamics have changed, which is easy to cause turbulent flow, and the growth and development of the child itself are combined, with the growth over time, the probability of new aortic valve regurgitation and aortic root dilation occurrence is increasing. As for the relationship between low surgical weight and advanced new aortic valve regurgitation, it may be because the lower the surgical weight of the child, the more immature the pulmonary valve is, the texture is relatively brittle, and it is easy to be damaged during arterial switch operation. This requires us to perform more delicate surgical operations and pay attention to protecting the pulmonary valve when operating on low-weight children in the future.
In 2014, Arcieri et al. Reference Arcieri, Cantinotti, Pak, Bernabei, Assanta and Murzi17 proposed a V-shaped resection technique to reduce the mismatch between the new aortic root and the ascending aorta. Based on the same purpose, neoaortic sinotubular junction reconstruction technique was used in 30 consecutive patients with transposition of the great arteries who had mismatched two major arteries in Fuwai Hospital since 2011. All patients with neoaortic sinotubular junction reconstruction did not develop severe new aortic valve regurgitation during the postoperative follow-up period, and the results were significantly better than those without neoaortic sinotubular junction reconstruction. The experience of Fuwai Hospital is that neoaortic sinotubular junction reconstruction technique is easy to perform and does not significantly increase the aortic cross-clamp time and cardiopulmonary bypass time. neoaortic sinotubular junction reconstruction technique can significantly improve the incidence of long-term complications, and significantly reduce the incidence of late new aortic valve regurgitation and aortic root dilation after arterial switch operation.
Late intervention
Although survival has greatly improved, recurrent and new lesions remain and require reoperations or catheter interventions. According to previously published literature reports, the late reintervention rate ranges from 5 to 20%. Reference Fricke, D’Udekem and Richardson18–Reference Fricke, Donaldson and Schneider21 The late reintervention rate of this study was 10.84%, which was consistent with published reports. The long-term complications after arterial switch operation mainly include right heart system disease, left heart system disease, coronary artery dysfunction and arrhythmia. This cohort showed that right heart system intervention was the most common (6/18), arrhythmia intervention was the least common (3/18), and no coronary reintervention was seen, but one patient died of myocardial infarction due to left coronary embolism in late death patients without timely intervention.
Limitations
The most important limitation of this study is its retrospective nature. An additional limitation is the relatively small study size limiting the statistical robustness of any inference that may be drawn. In the future, longer follow-up and larger sample size multicentre clinical studies are needed to further evaluate our conclusion.
Conclusions
In conclusion, new aortic valve regurgitation, aortic root dilation, recurrent coarctation of the aorta, and right ventricular outflow tract obstruction are major indications for long-term reintervention. Aorto-pulmonary diameter mismatch, previous pulmonary artery banding, and trace new aortic regurgitation at discharge were common independent risk factors for late new aortic valve regurgitation and aortic root dilation. Low body weight was an independent risk factor specific to new aortic valve regurgitation and bicuspid native pulmonary valve was an independent risk factor specific to aortic root dilation. Older age at surgery and aortic root dilation are independent risk factors for late right ventricular outflow tract obstruction. Older age at surgery, surgery before 2014, late right ventricular outflow tract obstruction and late aortic root dilation are independent risk factors for late intervention. neoaortic sinotubular junction reconstruction technique may reduce the incidence of new aortic valve dysfunction after arterial switch operation, significantly reduce the incidence of new aortic valve regurgitation and aortic root dilation, and improve the incidence of late complications after arterial switch operation.
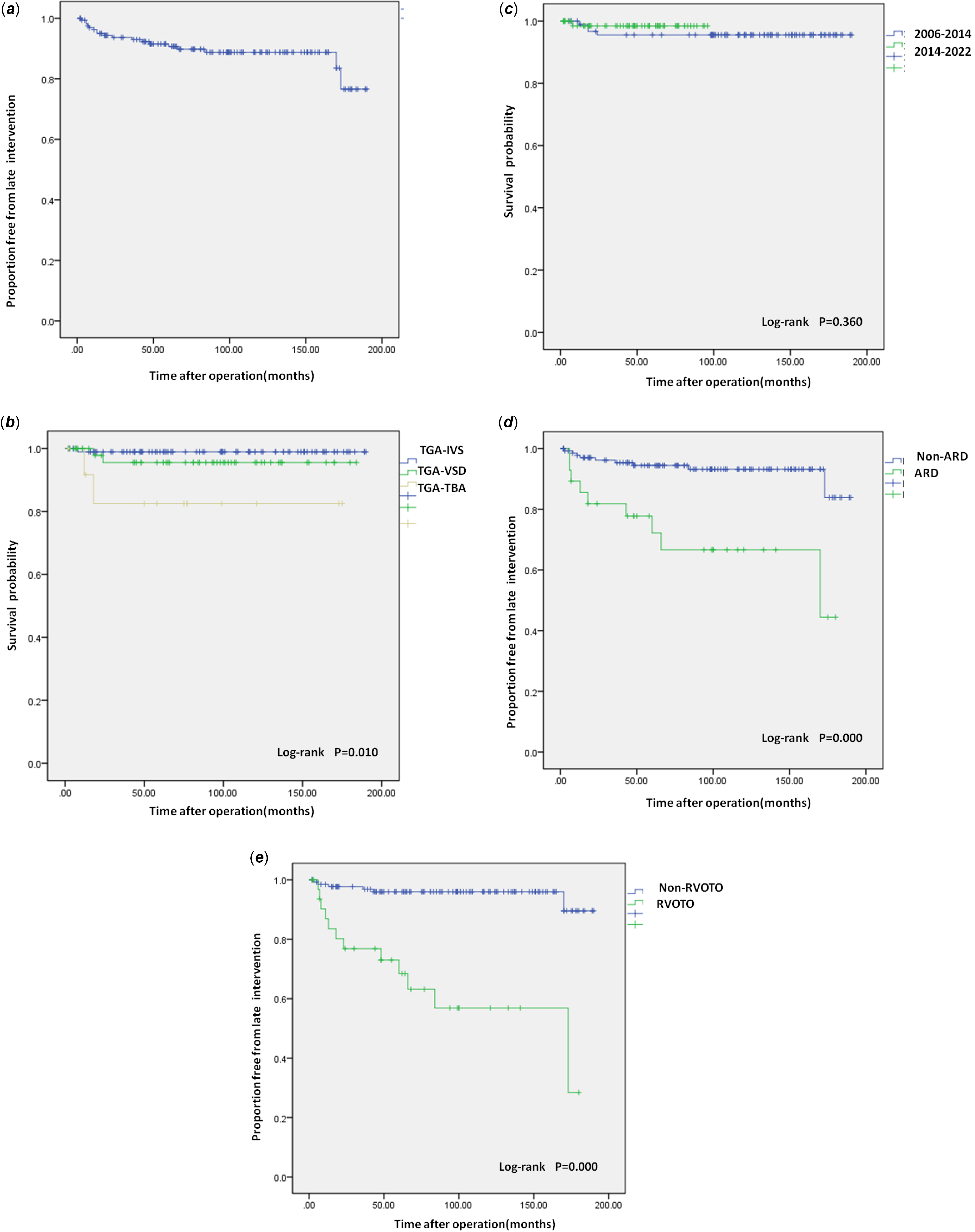
Figure 3. (a) Overall late intervention survival curve. (b) Late intervention survival curve. Kaplan-Meier curves show the relationship between the anatomical type and late intervention. (c) Late intervention survival curve. Kaplan-Meier curves show the relationship between the age group and late intervention. (d) Late intervention survival curve. Kaplan-Meier curves show the relationship between the aortic root dilation and late intervention. (e) Late intervention survival curve. Kaplan-Meier curves show the relationship between the right ventricular outflow tract obstruction and late intervention. RVOTO = right ventricular outflow tract obstruction; TGA-IVS = Transposition of the great arteries-Intact ventricular septum; TGA-TBA = Transposition of the great arteries-Taussig-Bing anomaly; TGA-VSD = Transposition of the great arteries-Ventricular septal defect.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1047951124000453.
Data availability statement
All data are incorporated into the article, and the data underlying this article are available in the article.
Acknowledgements
We acknowledge the roles of our colleagues, perfusionists, nurses, and others involved in the care of the study participants.
Author contribution
Zhangwei Wang: Conceptualisation; Data curation; Formal analysis; Investigation; Methodology; Software; Validation; Visualisation; Writing—original draft. Kai Ma: Writing – review & editing. Shoujun Li: Writing – review & editing.
Financial support
No funding was received for this work.
Competing interests
None declared.













