Since William Norwood’s initial report on the surgical management of hypoplastic left heart syndrome in the early 1980s, Reference Norwood, Lang and Hansen1 tremendous efforts have gone into improving outcomes for this once uniformly lethal disease. Advances in surgical technique and ICU care have contributed to increased survival. Despite advancements in treatment strategies, the stage 1 Norwood procedure remains one of the highest risk procedures in the field of congenital cardiac surgery. Reference Urencio, Greenleaf, Salazar and Dodge-Khatami2 Experienced congenital heart centres routinely report hospital survival rates above 90% for the Norwood procedure in the modern era. Reference Ohye, Schranz and D’Udekem3 Centres across the world have utilised different methods, protocols, and expert opinions for pre-and post-operative management; however, there is a lack of consensus mainly due to the lack of concrete evidence on the impact of specific management strategies for these neonates. Reference Johnson, Mussatto, Uhing, Zimmerman, Tweddell and Ghanayem4,Reference Natarajan, Stagg and Taylor5
Prostaglandins, diuretics, respiratory support, and inotropes remain the mainstay therapy to maintain neonates in optimal condition prior to surgery. Managing the delicate balance between pulmonary and systemic blood flow remains one of the key challenges before and after the Norwood operation. The gradual decline in pulmonary vascular resistance in the first few days of life often results in pulmonary over-circulation and systemic hypoperfusion in this patient population. Metabolic acidosis that occurs with these physiologic changes contributes to end-organ damage, potential right ventricular dysfunction, and poor post-operative outcomes after the Norwood procedure. Reference Hebra, Brown and Hirschl6–Reference Wernovsky, Ghanayem and Ohye11
Milrinone is a phosphodiesterase inhibitor that decreases the systemic and pulmonary vascular resistances and increases inotropy and lusitropy. Bianchi and colleagues demonstrated that pre-operative milrinone infusion increases cardiac output, improving both splanchnic and cerebral blood flow. Reference Bianchi, Cheung, Phillipos, Aranha-Netto and Joynt12 Krushansky demonstrated that pre-operative use of milrinone infusion in conjunction with inhaled nitrogen was able to obtain an adequate balance of systemic and pulmonary blood flow in neonates with single-ventricle physiology. Reference Krushansky, Burbano and Morell13 There is significant controversy about whether milrinone impacts pre-and post-operative survival in current literature with conflicting evidence. Reference Burkhardt, Rücker and Stiller14,Reference Kanazawa, Shimizu and Iwasaki15
At our institution, it has been our regular practice to initiate a milrinone infusion for neonates with hypoplastic left heart syndrome in the pre-operative period when evidence of metabolic acidosis and pulmonary over-circulation occurs, as evidenced by rising serum lactate and elevated systemic saturation. Based on our clinical experience, we hypothesised that patients with hypoplastic left heart syndrome who receive milrinone prior to the Norwood operation in the setting of over-circulation and elevated lactate levels would have an improvement in systemic perfusion and decreased lactate levels following initiation of milrinone. In this study, we aimed to investigate the impact of pre-operative milrinone infusion on pre-operative hemodynamics, oxygenation, metabolic markers (lactate levels), and clinical outcomes in neonates with hypoplastic left heart syndrome undergoing the Norwood operation. As a secondary aim, we sought to compare patients who received high dose of milrinone versus low dose of milrinone prior to the Norwood operation.
Materials and methods
Cohort selection
This is a single-centre retrospective cohort study that included all patients with hypoplastic left heart syndrome who underwent a Norwood operation between January 1st, 2000, and December 31st, 2019, at Children’s Healthcare of Atlanta, a free-standing, university-affiliated quaternary children’s hospital. An internal surgical database was queried, and eligible surgical encounters were identified. The study was approved by the Children’s Healthcare of Atlanta Institutional Review Board (IRB# 00000286). Informed consent was waived.
Demographics (age, weight, gender, and race), clinical characteristics (cardiac-ICU length of stay, post-operative length of stay, hospital length of stay, duration of mechanical ventilation, chromosomal abnormalities) and operative variables (cardiopulmonary bypass time and aortic cross-clamp time) were collected. Patients were stratified into two main groups based on the presence or absence of milrinone infusion prior to the Norwood operation. Indication for milrinone infusion was the development of elevation of lactate levels (normal serum lactate levels 0.5–1.6 mmol/l) in the setting of pulmonary over-circulation with elevated systemic saturation and was at the discretion of the attending physician. Patients who were in the cases group (i.e., received milrinone) were further stratified into two groups for a sub-analysis based on the dose of milrinone: low-dose milrinone (≤0.3 mcg/kg/min) and high-dose milrinone (≥0.5 mcg/kg/min).
Statistical analysis
We compared baseline characteristics and laboratory values of patients who received milrinone (cases) versus those who did not (controls). Continuous variables were expressed as median and interquartile ranges (IQR) and categorical variables as numbers and percentages. We used the Student’s t-test and Wilcoxon rank sum test to compare continuous variables. The Chi-squared and Fisher’s exact tests were used to compare categorical variables. Similar comparisons were made for the further sub-analysis of cases only, i.e., low-dose milrinone (≤0.3 mcg/kg/min) versus high-dose milrinone (≥0.5 mcg/kg/min). We performed multi-level mixed effects modelling to evaluate the longitudinal change of repeated measurements of lactate, PaO2, and mean arterial pressure. Serial lactate and PaO2 measurements were taken at milrinone initiation and the 2-, 4-, 6-, and 12-hour time points. For mean arterial pressure, serial measurements were taken hourly from 4 hours before milrinone initiation up to 24 hours post initiation. We conducted repeated measures analysis of variance to evaluate differences in serial measurements over time by milrinone dose and the interaction of time and dosage. A p-value of 0.05 was considered the cut-off for statistical significance. Statistical analyses were performed in SAS Enterprise Guide version 7.15 (SAS Institute, Inc., NC).
Results
Demographic data
There were 375 eligible patients during the study period, 79 (21.1%) received milrinone prior to the Norwood operation and were designated as cases, and 296 (78.9%) patients who did not receive pre-operative milrinone were designated as controls (Fig 1). Patient characteristics and a comparison between cases and controls are presented in Table 1. Patients in the cases group were slightly older at time of Norwood operation with a median age 6 days (IQR 5.0–9.0) vs 5 days (IQR 3.0–7.0) in the control group (p < 0.001). Cases were more likely to have chromosomal abnormalities 11.4% (n = 9) vs 5.1% (n = 15) (p = 0.041), be intubated 40.5% (n = 32) vs 32.1% (n = 77), sedated 30.4% (n = 24) vs 15.5% (n = 38), (p = 0.035), on neuromuscular paralysis 12.7% (n = 10) vs 2.9% (n = 7), (p = 0.0007), and had longer median cardiac ICU length of stay 20 days (IQR 13.0–33.0) vs 17 days (IQR 11.5–28.0), (p = 0.038) and longer median cross-clamp time 73 minutes (IQR 59–82.0) vs 67 minutes (IQR 53–79), (p = 0.008). There was no difference in weight at the day of surgery, gestational age, hypoplastic left heart syndrome variant, pre-operative respiratory support, and mortality (Table 1).
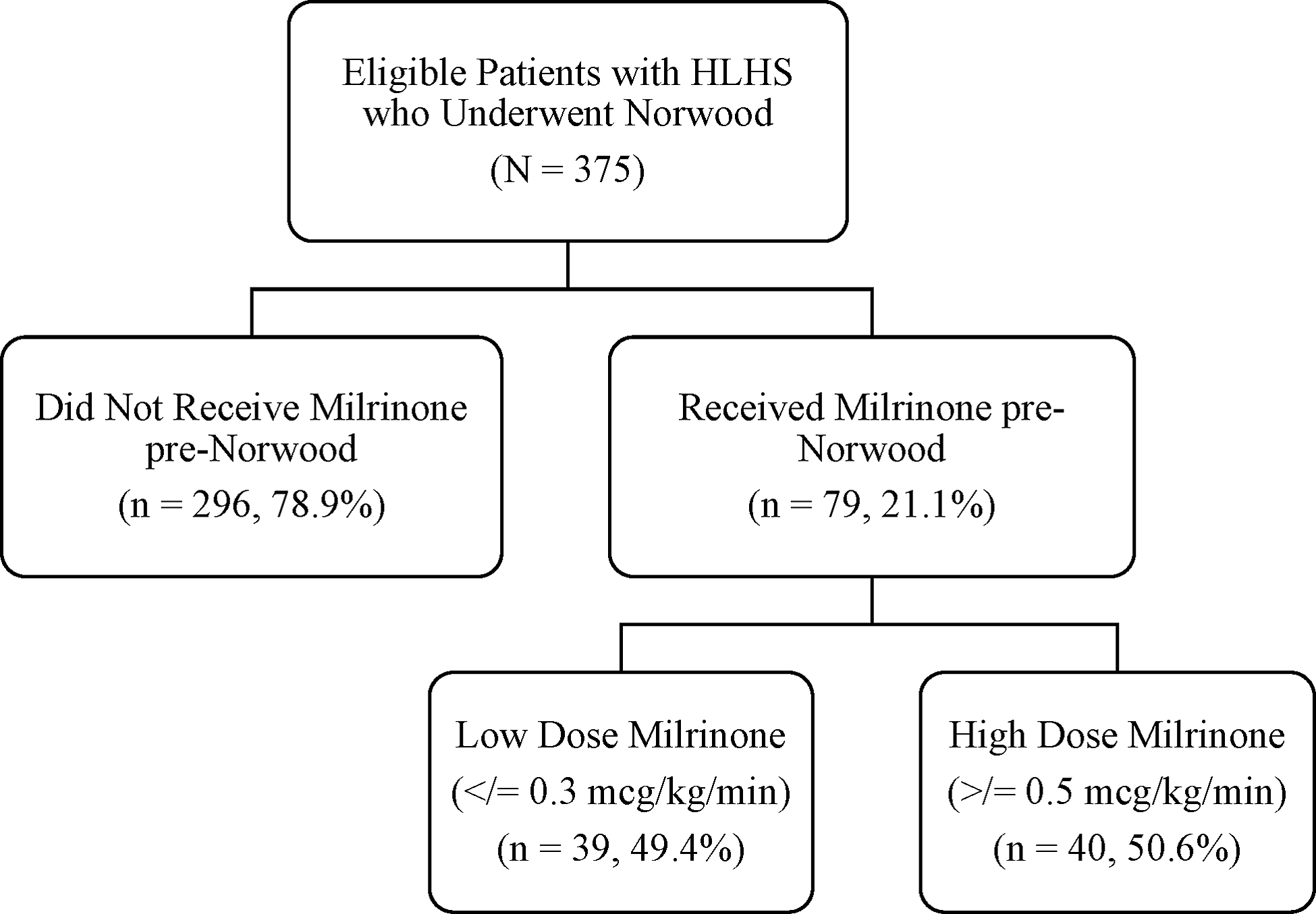
Figure 1. Study participants.
Table 1. Patient characteristics of entire cohort (cases and controls)
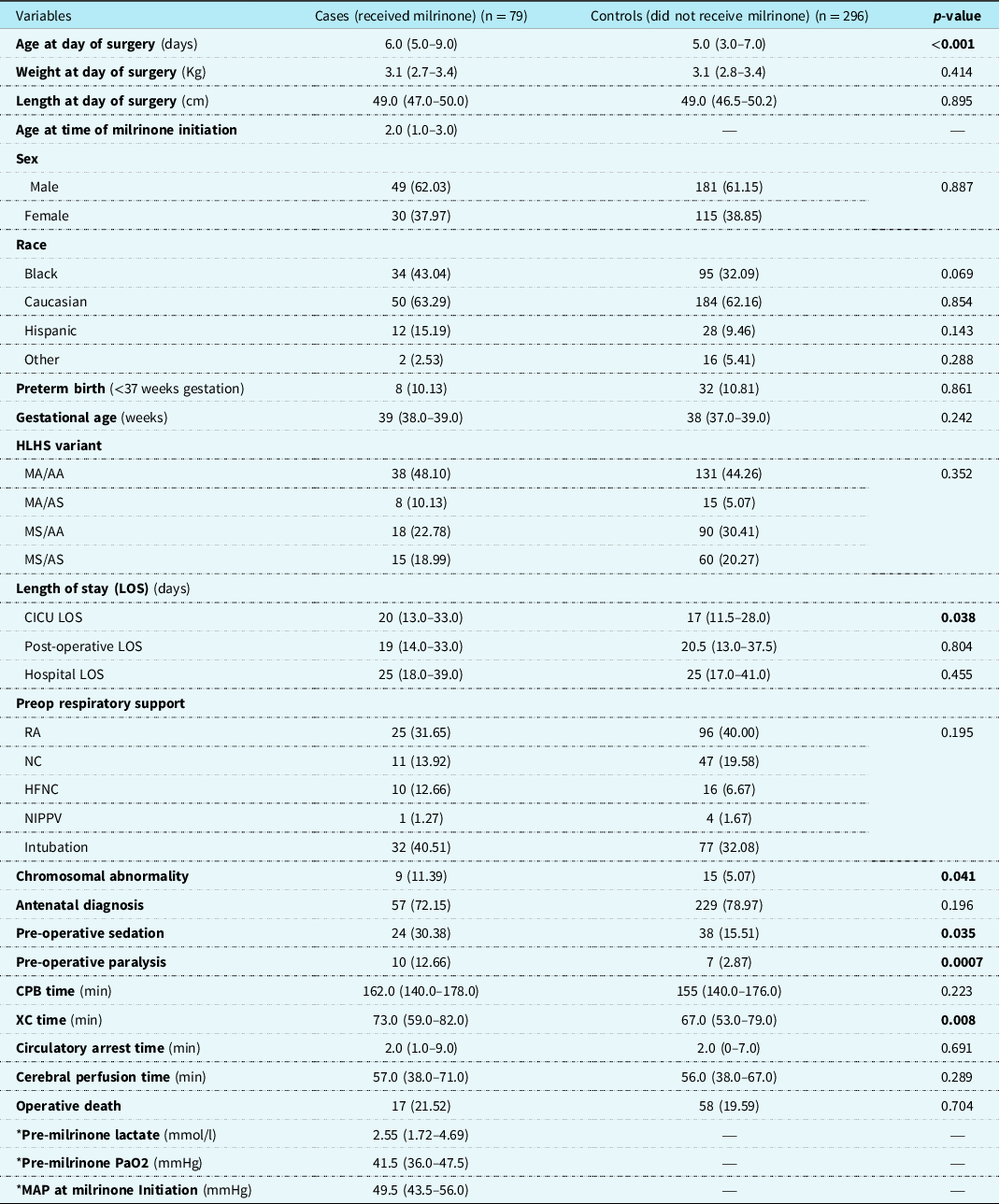
Results depicted in n (percent), median (interquartile range).
HLHS: hypoplastic left heart syndrome; MA: mitral atresia; MS: mitral stenosis; AA: aortic atresia; AS: aortic stenosis; LSO: length of stay; RA: room air; NC: nasal canula; HFNC: high flow nasal cannula; NIPPV: non-invasive positive pressure ventilation; CPB: cardiopulmonary bypass; XC: cross-clamp; PaO2: partial pressure of oxygen; MAP: mean arterial pressure.
* Data obtained only on patients who received milrinone preop.
Patients stratified to low-dose milrinone and high-dose milrinone
Patients receiving milrinone (cases) were stratified into two groups based on the milrinone dose. The two groups were low-dose milrinone (milrinone dose ≤ 0.3 mcg/kg/min) versus high-dose milrinone (milrinone dose ≥ 0.5 mcg/kg/min). There were 39 (49.4%) patients in the low-dose group and 40 (50.6%) patients in the high-dose group. There was no difference between the groups in regard to gender, race, gestational age, birth weight and length, chromosomal abnormality, hypoplastic left heart syndrome variant, different categories of length of stay, and lactate levels over time. Patients in the high-dose group were on milrinone for a longer duration prior to Norwood operation than the low-dose group median 4 days (IQR 3.0–5.9) vs 2.9 days (IQR 2.0–4.3), (p = 0.017). Patients in the low-dose group were more likely to have an antenatal diagnosis 87.2% (n = 37) vs 57.5% (n = 23), (p = 0.0005), more likely to be on neuromuscular paralysis 20.5% (n = 8), vs 5.0% (n = 2), (p = 0.048), and have longer median circulatory arrest times 3 minutes (IQR 2–10) vs 2 minutes (IQR 1–2), (p = 0.0057) (Table 2).
Table 2. Patient Characteristics Stratified by Milrinone Dose
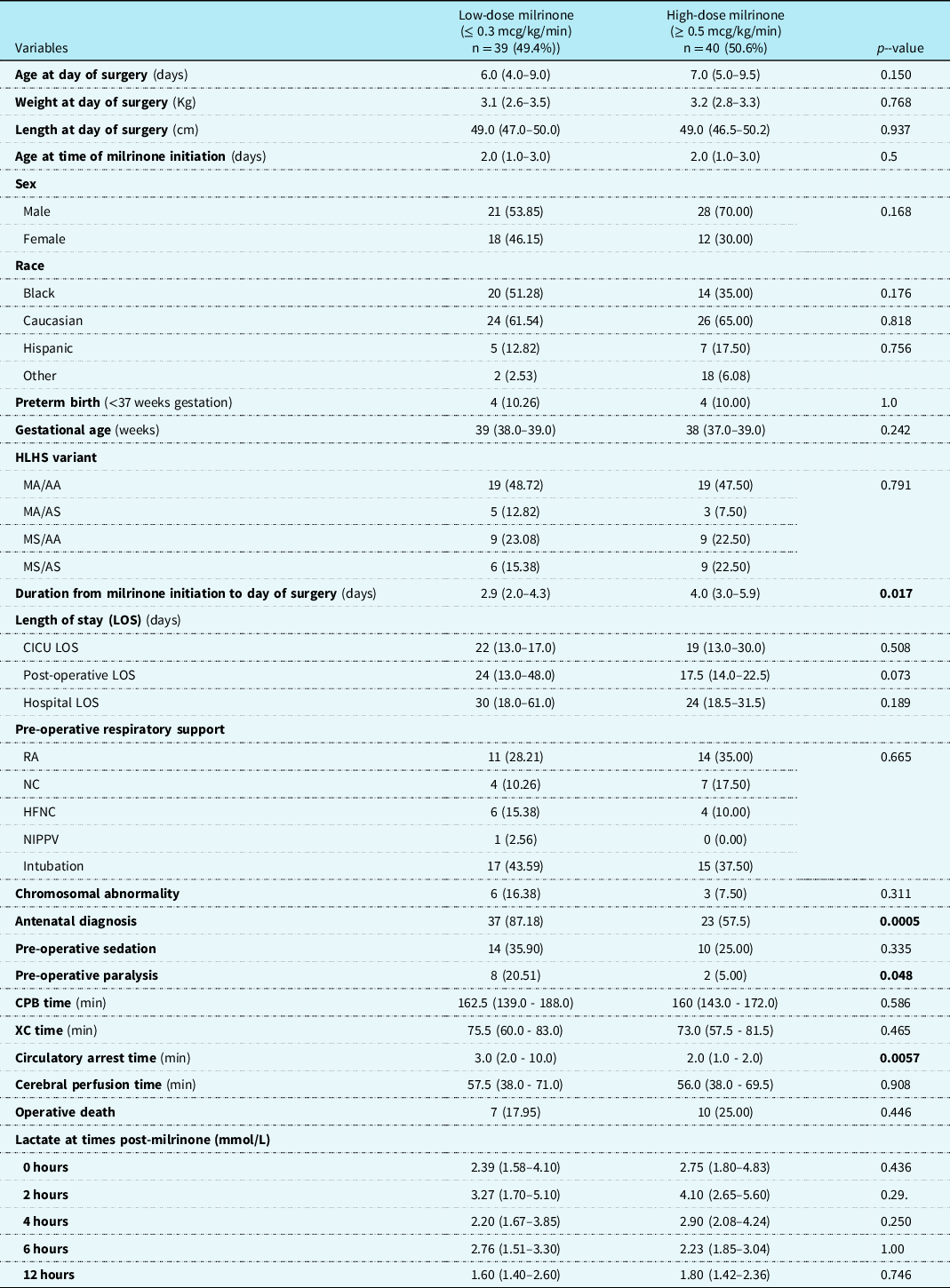
Results are depicted in n (percent) and median (interquartile range).
HLHS: hypoplastic left heart syndrome; MA: mitral atresia; MS: mitral stenosis; AA: aortic atresia; AS: aortic stenosis; LOS: length of stay; RA: room air; NC: nasal canula; HFNC: high flow nasal cannula; NIPPV: non-invasive positive pressure ventilation; CPB: cardiopulmonary bypass; XC: cross-clamp.
Serial lactate levels were reviewed from the time of initiation of milrinone to the 12-hour time point. Lactate levels continued to rise following initiation of milrinone as demonstrated by a higher median level at hour 2 of infusion (no milrinone bolus at initiation). A significant decrease in lactate level occurred by hour 4, with further decrease over time to the 12-hour mark p < 0.001) (Fig 2). Both the high-dose and low-dose milrinone groups demonstrated similar initial rise in lactate level by hour 2, and then continued decrease over time without significant difference between the two groups (p = 0.497). Mean arterial blood pressure did not significantly change from prior to initiation of milrinone through the 12-hour post initiation time point (p = 0.087). There was also no significant difference between blood pressure for the low- versus high-dose milrinone groups (p = 0.857) (Fig 3). There was no significant difference in PaO2 following initiation of milrinone over time (p = 0.166) and no difference in PaO2 between the high and low-dose milrinone groups (p = 0.624) (Fig 4).
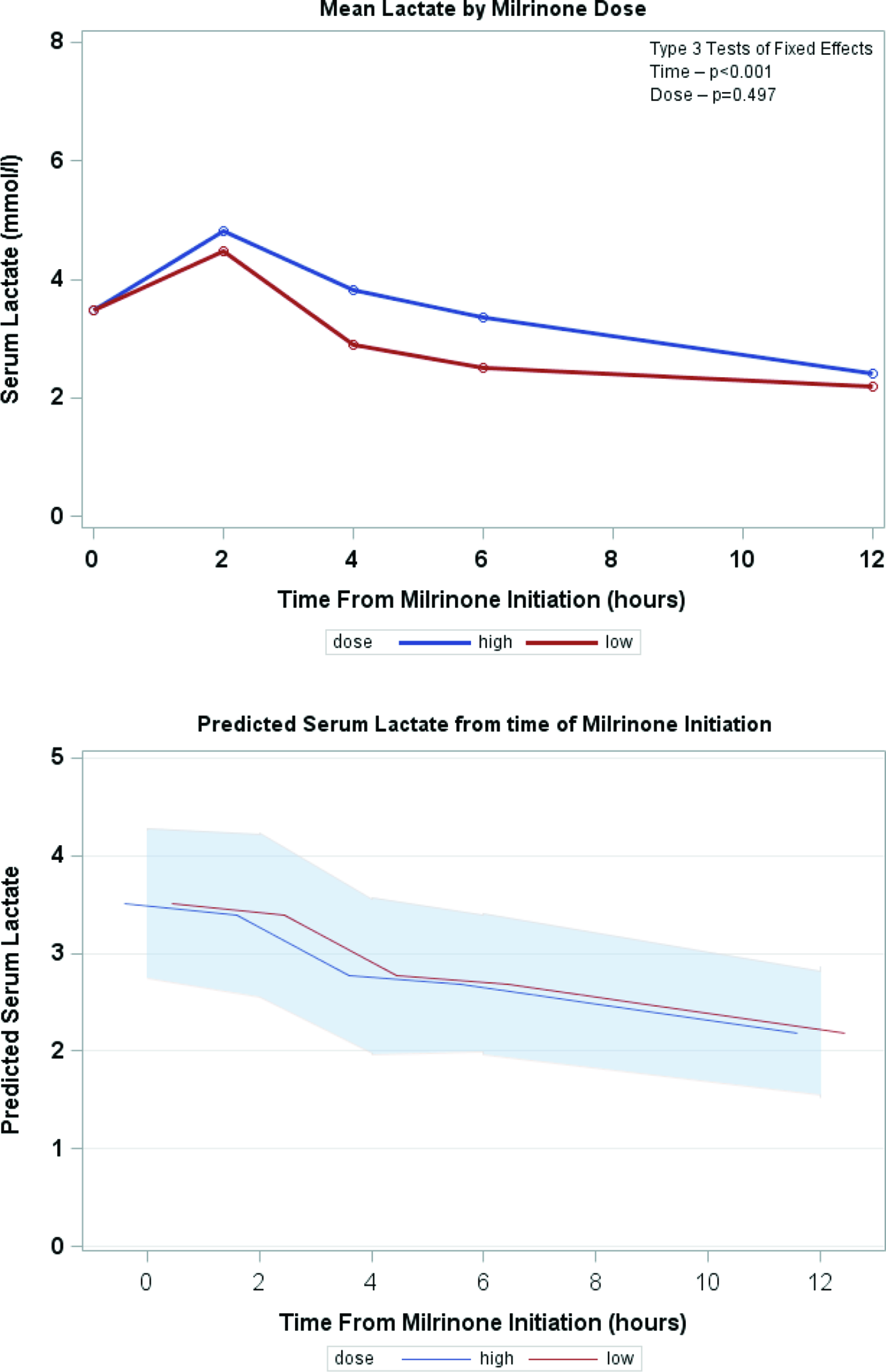
Figure 2. Mean serum lactate over time.
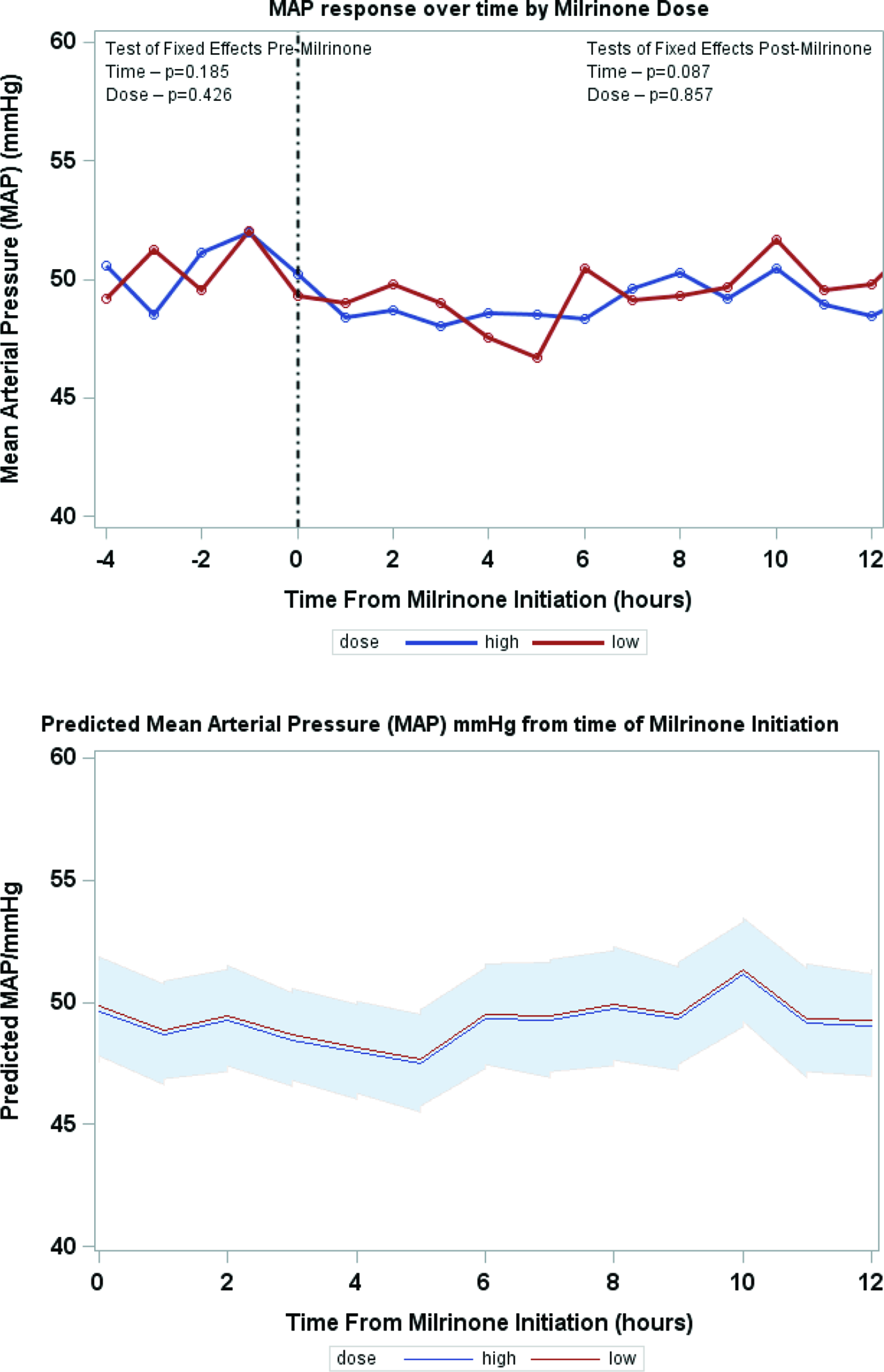
Figure 3. Mean MAP over time.
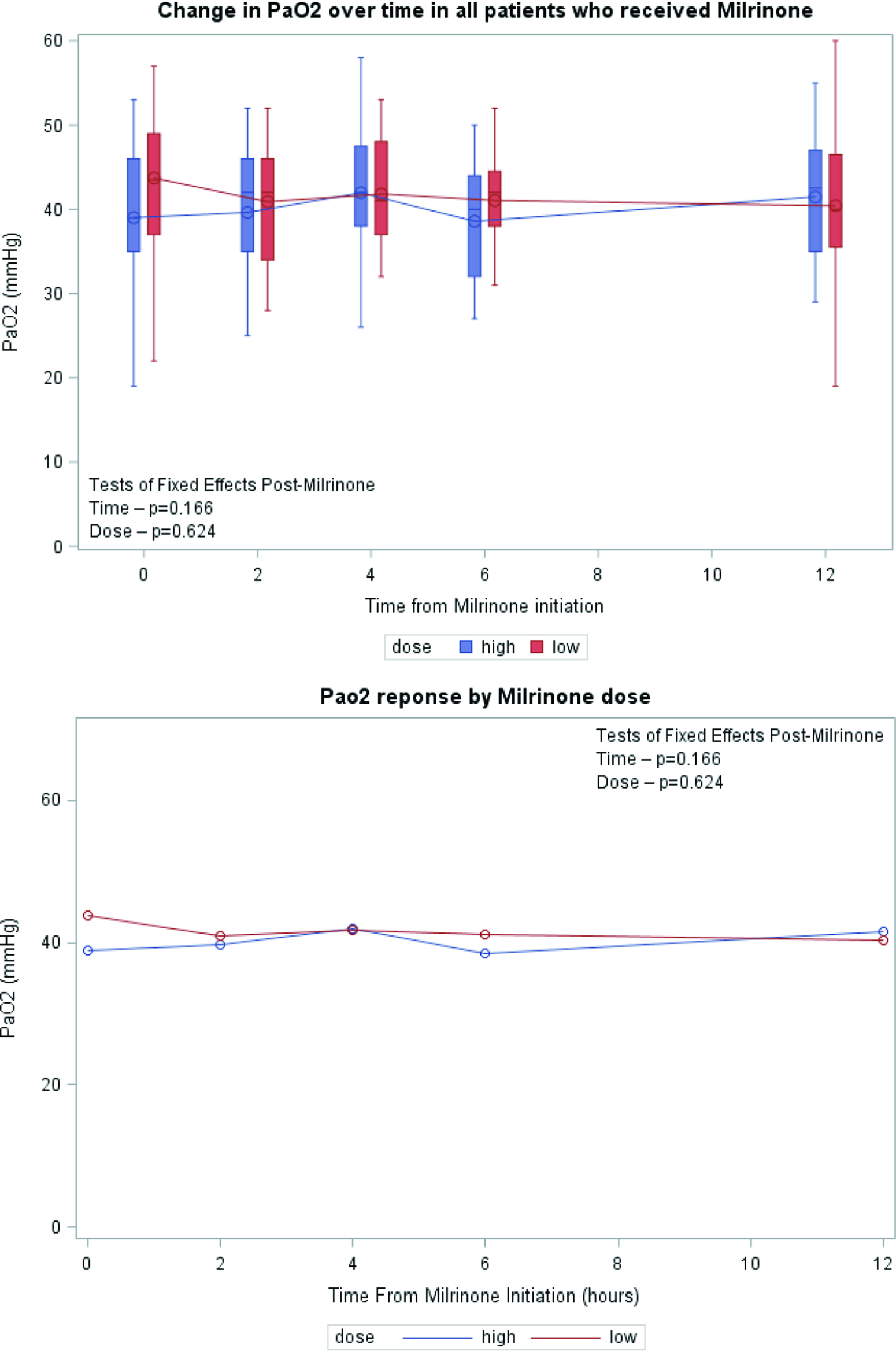
Figure 4. Mean PaO2 over time.
Discussion
Hypoplastic left heart syndrome patients are at risk of acquiring morbidities as they become over-circulated preoperatively, which in turn could affect post-operative outcomes. To our knowledge, this study is the largest to describe the milrinone effect on pre-operative hypoplastic left heart syndrome patients with elevated serum lactate levels. We demonstrate a statistically significant association between pre-operative milrinone administration and serum lactate reduction 4 hours after initiation, without a significant effect on mean arterial blood pressure.
At the outset of this study, we hypothesised that patients with hypoplastic left heart syndrome who receive milrinone pre-Norwood operation in the setting of over circulation and elevated lactate levels would improve systemic perfusion and have a decrease in lactate levels. It appears to require approximately 4 hours to significantly impact lactate following initiation of milrinone. This can be explained by the pharmacokinetics of milrinone and the lack of using a bolus dose prior to initiation of the milrinone infusion for our patients. Without a loading dose of milrinone, it takes at least 2 hours at 0.5 mcg/kg/min or more to reach therapeutic plasma concentration. Reference Vogt16 Krushansky and colleagues reviewed 49 single ventricle patients pre-Norwood who received milrinone and inhaled nitrogen to help balance their circulation and showed a statistically significant decrease in lactate levels over two-time points: admission and morning of surgery. While their study showed a positive effect, it was in combination with inhaled nitrogen which has been phased out of practice in most institutions, as it is of low impact. Reference Krushansky, Burbano and Morell13,Reference Thomas, Flores, Wong and Loomba17
Milrinone is recognised as a vasodilator, lowering both systemic and pulmonary vascular resistance with hemodynamic benefits to hypoplastic left heart syndrome patients. Reference Hoffman, Wernovsky and Atz18,Reference Zuppa, Nicolson and Adamson19 Due to the decrease in systemic vascular resistance, there is some hesitation in using milrinone in patients with borderline hemodynamics. This study demonstrates in the pre-operative hypoplastic left heart syndrome patient with borderline hemodynamics as evidenced by rising lactate levels, that milrinone administration is well tolerated, with no hypotension, and improvement in lactate levels. Moreover, when comparing low-dose milrinone versus high-dose milrinone, there was no effect on mean arterial pressure over time. Mills et al. showed that a milrinone-based vasodilator protocol in the hypoplastic left heart syndrome patients post-Norwood procedure had a decrease in systemic vascular resistance without serious hypotension and that the relationship between cardiac output and systemic vascular resistance was inverse. Reference Mills, Kaza and Walsh20 While Milrinone could lower pulmonary vascular resistance, its effect on pulmonary vascular resistance is too small to impact oxygen delivery compared to systemic vascular resistance. Our study showed that it did not have a statistically significant impact on PaO2 over time, or in relation to milrinone dose, indicating its minimal effect on pulmonary vascular resistance and the balance between systemic and pulmonary blood flow. When considering the recognised decrease in systemic and pulmonary resistance associated with milrinone use, the lack of significant rise in systemic saturation, and the improvement in lactate levels in our patient population, one may conclude that an improvement in cardiac output has occurred without significant alteration in the ratio of systemic to pulmonary blood flow, i.e., an increase in total cardiac output. This aligns with the work done by Loomba and colleagues, as they conducted a meta-analysis assessing milrinone hemodynamic effects in children, which showed that systolic blood pressure, mean arterial pressure, and Pao2 did not significantly change after initiation of milrinone. Reference Loomba, Dorsey, Villarreal and Flores21
The optimal milrinone dose has been a point of interest. Low-dose milrinone and high-dose milrinone were compared in our study; while the demographics and basic pre-operative findings were similar, low-dose milrinone patients received more neuromuscular blockade compared to high-dose milrinone and had a longer duration of milrinone therapy prior to the Norwood operation. This could be attributed to low-dose milrinone patients being sicker, and providers avoided higher doses of milrinone. There was no difference in mortality, time to clear lactate, and length of stay with all categories between low-dose and high-dose milrinone. Management of the pre-operative patient with hypoplastic left heart syndrome and elevated serum lactate varies between institutions. Some institutions perform a temporary bilateral pulmonary artery band to balance the circulations prior to the Norwood procedure, and others proceed more quickly to the Norwood operation when imbalance of the systemic and pulmonary circulations occurs. Another options would be to consider performing a hybrid procedure (ductal sent placement and bilateral pulmonary artery band) in a select population patients that are considered high risk. Our programme has managed patients who demonstrate elevated lactate and high systemic saturations preoperatively by initiation of milrinone and occasional initiation of mechanical ventilation. The mortality of hypoplastic left heart syndrome patients with pre-operative over-circulation and systemic under perfusion who received milrinone at our institution is similar to hypoplastic left heart syndrome patients who did not receive pre-operative milrinone and did not have over-circulation.
Our findings are subject to all the limitations inherent to a single-centre retrospective cohort study. As a single-centre study, the demographics, clinical characteristics, as well as surgical and medical management of these patients are specific to our institution and might not be generalisable to other healthcare systems. Also, the timing of milrinone initiation may be subject to selection bias as provider’s background training, clinical experience, and comfort level differ. Although we show that the use of milrinone in the setting of over-circulation improves clinical variables such as lactate levels, we cannot say for sure if this is due to the addition of milrinone or the combination of medical therapies that were added, such as respiratory support, sedation, and other measures that will decrease metabolic demand. Lastly, the selection of patients in this study was from the internal surgical registry that only included patients who underwent cardiac surgery. Hypoplastic left heart syndrome patients that were not operated on could include sicker patients who had milrinone initiated.
In conclusion, patients with hypoplastic left heart syndrome who received milrinone prior to Norwood operation in the setting of systemic under perfusion, and elevated serum lactate were slightly older at the time of Norwood procedure, more likely to have chromosomal abnormalities, require sedation, neuromuscular blockade, and have longer cardiac-ICU length of stay. A significant reduction in lactate levels occurs by 4 hours post initiation of milrinone which was maintained over time. There was no difference in response between milrinone doses (high-dose versus low-dose) with regard to lactate rate of clearance or blood pressure stability. The trend of mean arterial pressure and PaO2 measurements did not change over time and between milrinone dosing groups.
In summary, the use of milrinone in the pre-operative over-circulated hypoplastic left heart syndrome patient is well tolerated, is associated with improved lactate levels, and without significant hypotension or worsening of excess pulmonary blood flow.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and have been approved by the institutional committees (Institutional Review Board at the Children’s Healthcare of Atlanta).









