Postoperative chylothorax occurs in about 3.8% of paediatric patients following cardiac surgery and is associated with increased morbidity and mortality. Reference Buckley, Graham and Gaies1 Chyle is composed of predominantly fat, protein, lymphocytes, and electrolytes. This typically accounts for 200 kcal/L in a healthy patient. The clinical consequences of continued chylous drainage include electrolyte disturbances, risk of infection, and nutrient losses compounding the existing increased energy demand secondary to surgery, critical illness, and postoperative recovery. Chylothorax amplifies the underlying burden of growth failure inherent to many cardiac lesions. Reference Buckley, Graham and Gaies1,Reference DiLauro, Russell, McCrindle, Tomlinson, Unger and O'Connor2
Materials and methods
The Chylothorax Work Group was formed in October 2020. Members represent 22 centres and consist of more than 60 multi-disciplinary providers: physicians, surgeons, advanced practice providers, and dietitians.
Using the Chylothorax Work Group infrastructure, a group of experts were identified to develop contemporary and clinically relevant guidance related to nutritional management of paediatric postoperative chylothorax. The experts included four dietitians (KF, MH, AT, and PV), one Nurse Practitioner (MW), and two cardiac intensive care physicians (EG and DB). The dietitian-specific expertise accumulates to 38 years of experience caring for children with CHD.
Literature was reviewed using the PubMed search terms chylothorax, paediatric, postoperative, CHD, chylothorax management, growth failure, and malnutrition between the years 2001 and 2021.
Results
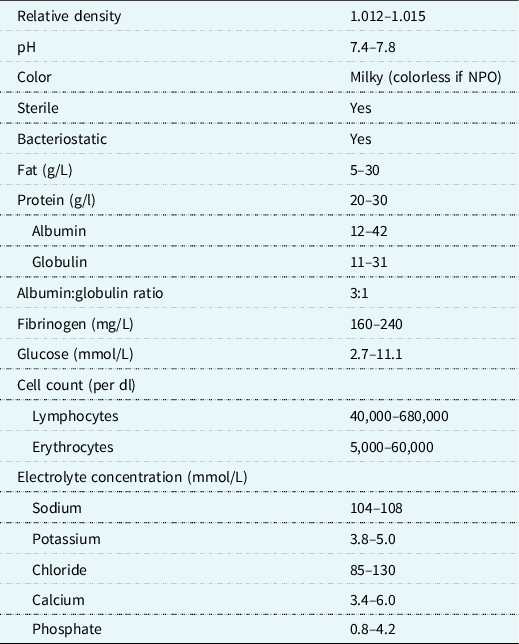
In the management of chylothorax, the overarching nutritional goals are to decrease chylous drainage, maintain adequate volume and electrolyte status, and prevent further malnutrition. The thoracic duct and its lymphatic tributaries transport about 4 L of chyle per day. Reference Hermon, Tenner, Burda, Strohmaier, Schlager and Golej3 Chyle is composed of lipids, proteins, fat-soluble vitamins, lymphocytes, and electrolytes (Table 1). The continuous loss of lymphatic fluid may result in the loss of nutrients, proteins, immunoglobulins, coagulation factors, and vitamins leading to respiratory compromise, nutritional deficiency, infections, haematologic complications, and metabolic derangements Reference Christofe, Pessotti, Paiva and Jatene4 in an already vulnerable population. The prevalence of chylothorax is increased in the most nutritionally at-risk populations such as neonates, infants, single-ventricle anatomy/physiology, with or without arch reconstruction and those with genetic syndromes. Reference Buckley, Graham and Gaies1,Reference Mery, Moffett and Khan5 With the significant risk for malnutrition in this population, a standardised approach to management in conjunction with close monitoring from a registered dietitian in collaboration with the multidisciplinary team is paramount.
Table 1. Composition of chyle
Fat-modified diet therapies
In growing infants and children, dietary fat delivers a major source of energy. The predominant fat source in breastmilk, infant formulas, and or the unrestricted child’s diet is from long-chain triglycerides. In addition to serving as a concentrated source of calories, fatty acids play a role in cell signalling and gene expression, are a structural component of cell membranes, and participate in nervous tissue myelin production, all of which are important components of growth and development. Reference Medicine6 Additionally, both surgery and critical illness potentiate metabolic alterations by inducing the stress response, which results in the catabolism of endogenous stores of protein, carbohydrate, and fat to provide energy. Reference Mehta and Compher7
The most common initial management strategy for chylothorax is to restrict long-chain triglycerides intake, altering the type of dietary fat, and thus promoting a diet enriched with medium-chain triglycerides. Fat absorption starts within the intestinal lumen. Fat globules known as micelles form after emulsification of dietary fats by bile acids. Long-chain triglycerides are converted to chylomicrons within the enterocytes, stimulating chyle production. Chyle is absorbed through lymphatic capillaries and in normal flow patterns travel through the lymphatic system via the thoracic duct to the bloodstream. Medium-chain triglycerides, consisting of triglycerides with saturated fatty acids of 8 to 12 carbon length, are absorbed directly into the portal venous circulation without micelle formation thus bypassing the lymphatic system and not contributing to chyle flow (Fig 1). Medium-chain triglycerides are suitable as an alternative fat source for the provision of additional calories in the setting of chylothorax. Reference Bengtsson8 The association between chylothorax resolution and amount and exposure to long-chain triglycerides has not been established. Additionally, when total fat is reduced or altered, other caloric sources will need to be optimised to ensure nutritional adequacy.
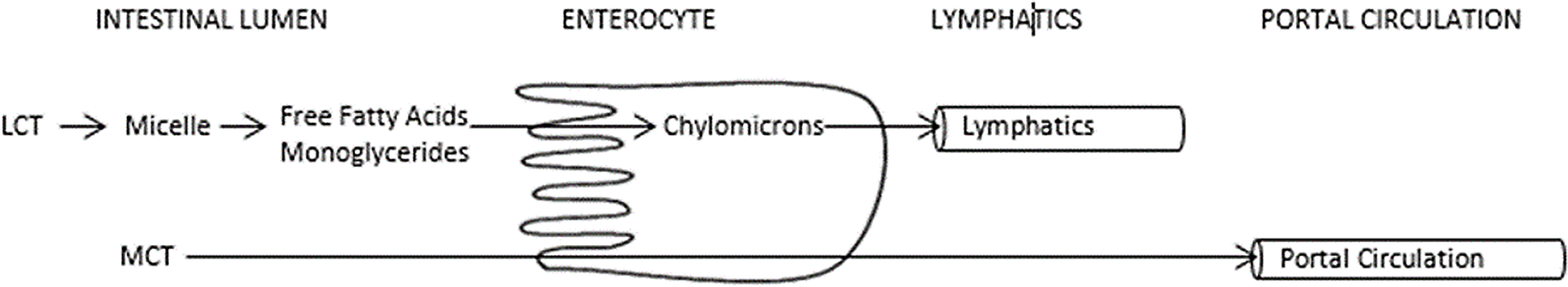
Figure 1. Medium Chain Triglyceride Absorption.
Neonates and infant diet management
Postoperative chylothorax is four times more likely to develop in neonates compared with older children Reference Buckley, Graham and Gaies1 and can have detrimental effects on growth and clinical outcomes. Complete reduction of fat is not recommended as fat is crucial in provision of calories for sufficient growth and development. Optimizing nutrition delivery while reducing chylous drainage can present a challenge as the fat sources in human milk and traditional infant formulas are predominately long-chain triglycerides. Providing defatted fortified human milk or medium-chain triglycerides predominant infant formulas is safe and effective with adjunctive medical therapies in the treatment of chylothorax. Common practice includes trial of an medium-chain triglyceride-based infant formula or defatted human milk for 2-7 days and monitoring total chest tube drainage before considering parenteral nutrition. Reference Marino, Bell, Woodgate and Doolan9 Formulas commercially available for infants with chylothorax, specifically those with feeding intolerance or documented milk protein allergies, are limited. Often, off label use of toddler and adolescent formulas are required but should be done with close nutritional guidance to avoid nutrient deficiency. Recommended formulas for use in children <1 year of age with chylothorax are shown in Table 2.
Table 2. Oral and enteral formulas for use in fat modified diets3
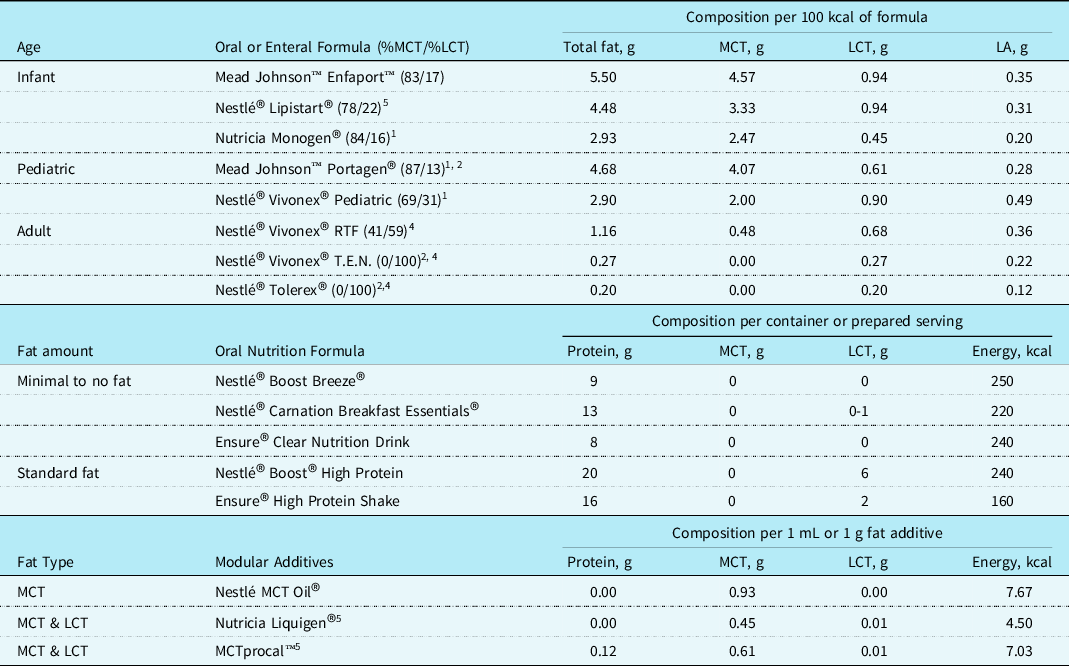
1 Nutritionally complete but off label use in patients under 1 year of age.
2 Nutritionally incomplete and requires trace mineral supplementation.
3 Typical use for oral intake and not for tube feed.
4 May be insufficient to meet essential fatty acid requirements.
5 Total includes other types of fat not listed.
Defatted human milk is preferred when available in an effort to avoid altering the gastrointestinal tolerance of breast milk. Fortunately, defatted human milk has been shown to be as safe and effective for the cessation of chylous drainage in comparison to traditional methods of stopping delivery of breastmilk and providing medium-chain triglycerides predominant infant formula. Reference Neumann, Springer, Nieschke, Kostelka and Dähnert10 Defatted human milk provides the known immunologic benefits of human milk to infants Reference Kocel, Russell and O'Connor11 and can promote sufficient growth with appropriate fortification. Reference Fogg, DellaValle, Buckley, Graham and Zyblewski12 Fat can be removed from human milk either through centrifugation, commercial cream separation, or natural separation techniques. Reference Chan and Lechtenberg13,Reference Lessen14 The most effective method for consistent fat removal is refrigerated centrifuge. Reference Drewniak, Lyon and Fenton15 This process includes samples being placed in a refrigerated centrifuge at 2°C for 15 minutes at 3000 rpm. Once centrifuged, a transfer lid and syringe can be used to remove the defatted portion of breast milk. Secondary options are often considered given the financial implications with the regular use of a refrigerated centrifuge. Commercial cream separators have become a safe method for hospital and home use. Reference Barbas, O'Brien and Forbes16 Natural separation is less reliable for consistent fat removal with the previously mentioned techniques being preferred. Reference Drewniak, Lyon and Fenton15 Once fat is removed from human milk, fortification is required from medium-chain triglyceride-based formulas and or medium-chain triglyceride-rich calorie modular to optimise caloric density and delivery found in Table 2. Defatted human milk is 10-12 kcal/oz on average and is insufficient as a sole source of nutrition for infants.
Children and adolescent diet management
There is no consensus on what constitutes a fat-restricted diet in terms of total fat grams/day or caloric delivery derived from fats. The 2020 Dietary Guidelines for Macronutrients recommends for children ages 1-3 to consume 30-40% calories from fat, ages 4-18 to consume 25-35% calories from fat. 17 Fat modification for the purpose of chylothorax has been reported to be from <10 g total fat/day Reference Winder, Vijayarajah and Reeder18 to <30% of calories from fat/day. Reference Sunstrom, Muralidaran and Gerrah19,Reference Pike, Okuhara, Toyama, Gross, Wells and Starnes20 Duration of effective therapy also varies among centres. The recommended duration of fat modification ranges between 10 days and 6 weeks for fat-modified diets. Reference Biewer, Zürn and Arnold21–Reference Cormack, Wilson, Finucane and West24 Prolonged oral diets providing <10% calories from long-chain triglycerides for greater than 1-3 weeks put children at risk for essential fatty acid deficiency. Reference Barr, Dunn and Brennan25 The amount of oral fat tolerated also depends on the age of the child, their caloric requirements, and the severity of disease. Nutrition optimisation during dietary fat restriction may include more meals, snacks, and oral supplements throughout the day to help reach nutrition goals. When transitioning to a fat-modified oral diet, a registered dietitian should be utilised to guide families on appropriate food choices. Suggested dietary modifications to supplement nutritional delivery during fat restriction are shown in Table 3.
Table 3. Suggested dietary modifications to supplement nutritional delivery during fat restriction
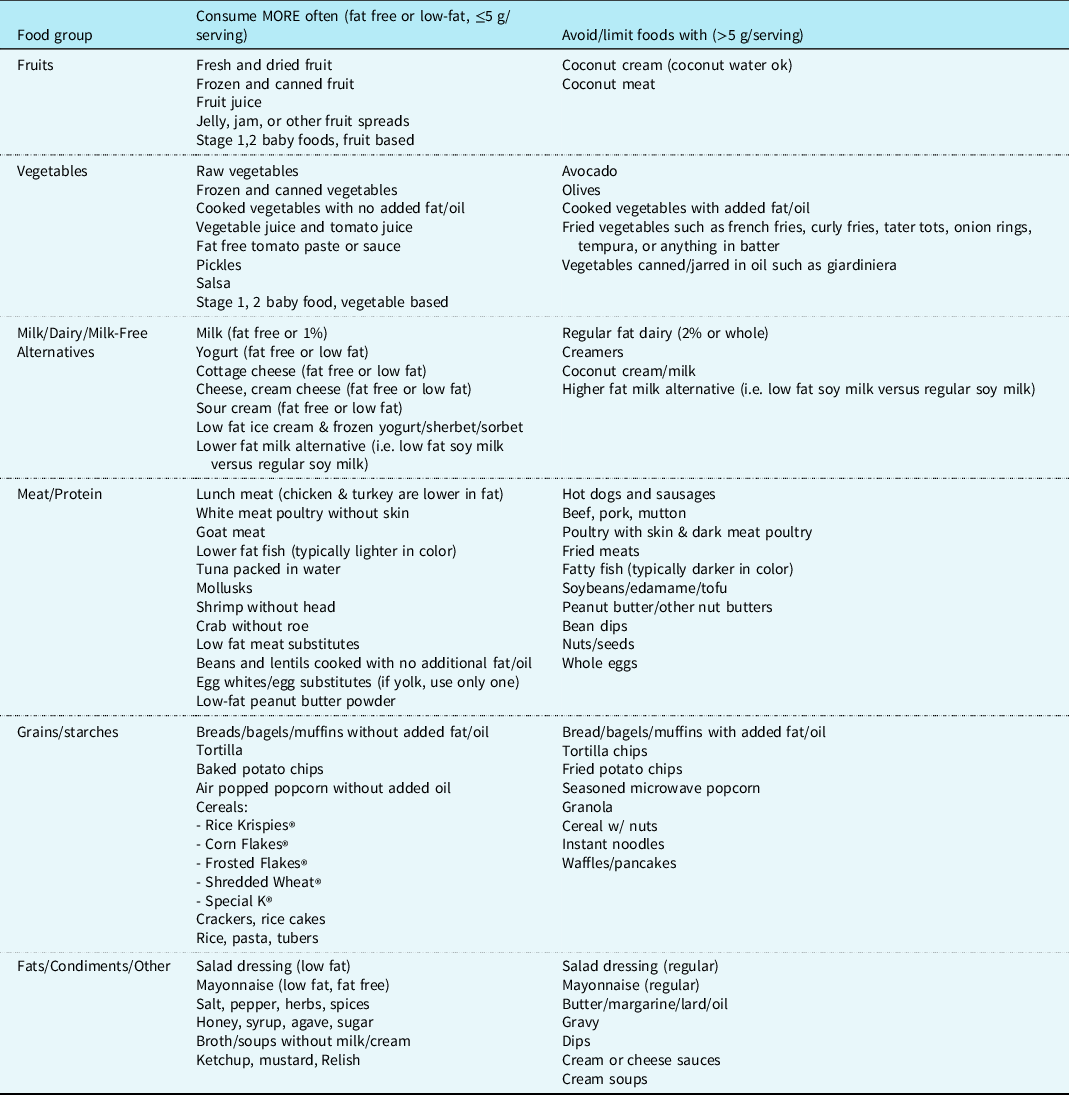
Daily total fat should be considered. Check labels for serving size and number of fat grams.
The Fontan procedure carries the highest incidence of chylothorax and is associated with increased risk of mortality. Reference Lo Rito, Al-Radi and Saedi26 Proposed mechanisms for chylothorax as a consequence of the Fontan circulation include elevated systemic venous and hydrostatic capillary pressures, increased pulmonary vascular resistance, and low systemic vascular resistance, which leads to congestion of the lymphatic system causing increased production of lymphatic drainage. Reference Mery, Moffett and Khan5,Reference Lo Rito, Al-Radi and Saedi26–Reference Chan, Lau, Wong, Cheng, Chau and Cheung30 To prevent chylothorax in this high-risk population, some have trialed preoperative prophylactic fat restriction. Sunstrom et al employed a diet with <30% calories from fat in conjunction with diuresis and fluid restriction in 14 patients in the immediate postoperative Fontan period until chest tube was removed at 6 days compared to previous average of 11 days prior to this intervention. Patients without chylous drainage resumed a normal diet post chest tube removal,while those with chylous drainage were discharged on fat restriction for 6 weeks from surgery. Pike and colleagues also provided a prophylactic fat restriction to 30% calories from fat in all Fontan patients for a full 6-week duration. Prophylactic fat restriction may provide the benefit of reducing lymphatic pressure and drainage, resulting in decreased chest tube days and hospital length of stay. Reference Sunstrom, Muralidaran and Gerrah19,Reference Pike, Okuhara, Toyama, Gross, Wells and Starnes20
The ability to reach goal caloric and protein requirements on a fat-modified diet may be challenging in the setting of a poor appetite. It is important to counsel patients and their families to be attentive to protein consumption given that low-fat foods are often low in protein. In some instances, oral or enteral supplementation may be required to obtain a low-fat, high-protein diet that is not otherwise achievable for those with significantly reduced oral intake. Medium-chain triglycerides predominant or fat-restricted oral nutrition supplement should be considered to optimise nutritional intake as needed (see Table 2).
Parental nutrition therapies in high-volume chylothorax
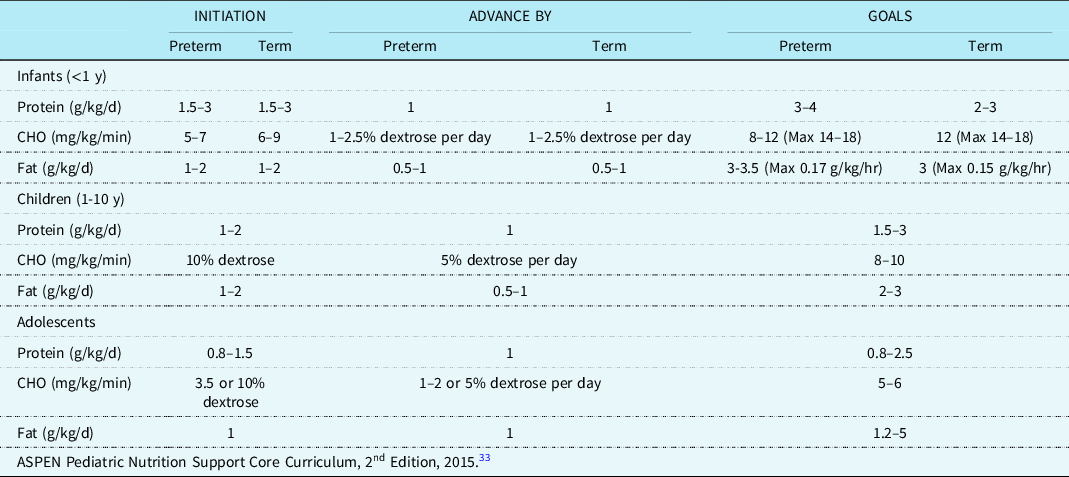
If enteral fat modifications fail to reduce persistent high-volume chylothorax (>20 mL/kg/day), a period of nil per os with parenteral nutrition should be considered. There is a lack of consensus regarding the duration of nil per os. Prior studies reference an nil per os duration ranging from five to twenty-one days. Reference Christofe, Pessotti, Paiva and Jatene4,Reference Winder, Vijayarajah and Reeder18,Reference Shin, Lee, Park and Park31 Fluid restriction when possible in conjunction with diuresis are common strategies for reducing central venous pressures, which may be exacerbating chylous drainage. Reference Panthongviriyakul and Bines32 Hence, the caloric delivery via parenteral nutrition is often restricted particularly in the early postoperative period and even more so in those with chylothorax. Of note, intravenous lipid emulsions can be given while nil per os and are delivered directly into the bloodstream therefore bypassing the lymphatic system providing a valuable source of calories and essential fats. Reference McCray33,34 The American Society for Parenteral and Enteral Nutrition provides nutrition care guidelines for critically ill children. Table 4 displays recommended parenteral nutrition initiation, advancement, and target macronutrient distribution for different age groups.
Table 4. ASPEN parenteral nutrition administration.
Special considerations while on fat-modified diet
Essential fatty acid deficiency
Medium-chain triglyceride-rich diets where >85% of the fat source is medium-chain triglycerides used for the treatment of chylothorax may not provide adequate amounts essential fatty acids. Reference Marino, Bell, Woodgate and Doolan9 Essential fatty acid deficiency results when there is insufficient dietary intake of linoleic acid and alpha-linolenic acid from a limitation of dietary fat or altered absorption of fat. Reference Horsley and Susan Konek35 The human body is unable to synthesise linoleic acid (18:2 Δ9,12) and alpha-linolenic acid (18:3 Δ9,12, 15) due to the lack of enzymes that can introduce double bonds beyond the Δ9 site, therefore making them essential from the diet. Reference Gramlich, Meddings and Alberda36 Essential fats are necessary for healthy cell membrane formation, cholesterol metabolism, blood clotting, as well as proper brain and nervous system development. Reference McCray37 Prevention of essential fatty acid deficiency can be achieved, respectively, with intakes of 2-4% of total calories from linoleic acid and 0.2-1% of calories from alpha-linolenic acid. Reference Horsley and Susan Konek35,Reference Lapillonne38
In the presence of adequate enteral intake of essential fatty acids, tetraene products predominate in plasma. When the intakes of both linoleic acid and alpha-linolenic acid are low, triene formation is high and hence an elevated triene to tetraene (T:T) ratio. Mead acid (a triene) is produced at increased frequency in the absence of linoleic acid and alpha-linolenic acid due to enzymatic activity on non-essential fatty acids. Reference Gramlich, Meddings and Alberda36 An elevated T:T ratio >0.4 is considered diagnostic of essential fatty acids. Reference Holman39 Biochemical evidence of essential fatty acids occurs earlier than the clinical signs and symptoms, which may include dry scaly skin, skin lesions, dry dull hair, brittle nails, hair loss, poor wound healing, and poor growth. Reference McCray37,Reference Gramlich, Ireton‐Jones, Miles, Morrison and Pontes‐Arruda40 Screening for essential fatty acids via an essential fatty acid profile is recommended for patients receiving a fat-free diet for prolonged period (>3-4 wks), receive inadequate intravenous lipid emulsions/oil supplementation, and those who may lack adequate fat stores such as neonates and underweight children. Reference Gramlich, Meddings and Alberda36,Reference McCormick, Rosenberg, Tier and Balest41 If the infant or child is starting to show biochemical or physical signs of essential fatty acids, linoleic acid, and alpha-linolenic acid-rich fat sources such as sunflower, safflower, corn, soybean, flaxseed, canola, walnut, and fish oils can be added while still maintaining an overall low total long-chain triglycerides nutrition plan, see comparison of oils in Figure 2. Reference Anderson42
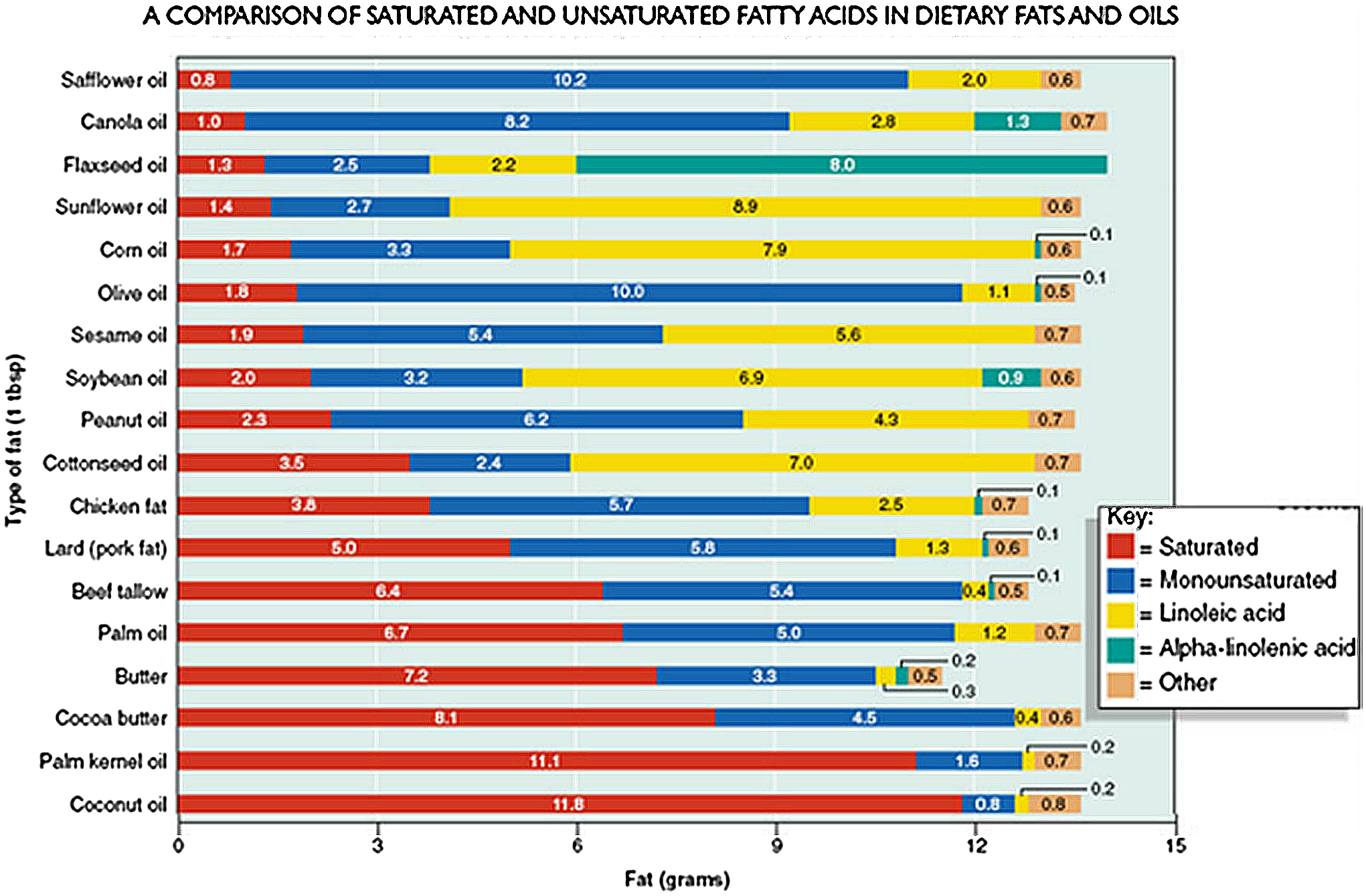
Figure 2. A Comparison of saturated and unsaturated fatty acids in dietary fats and oils.
The addition of intravenous lipid emulsions could be considered to help meet the essential fatty acids requirement. For infants, linoleic acid must be provided at 3% or greater of total kilocalories to meet the essential fatty acids requirement. An intravenous lipid emulsions infusion of 2.5-5 mL/kg/day (0.5-1 g/kg/day) should be adequate to prevent essential fatty acid deficiency. In children, essential fatty acid deficiency can be avoided by providing 2.5 mL/kg/day (0.5 g/kg/day) of intravenous lipid emulsions. Intravenous lipid emulsions may be given daily at a minimal dose or throughout the week (i.e. Monday, Wednesday, and Friday). 34
Biochemical monitoring
Biochemical monitoring, in addition to routine physical exams and tracking of somatic growth, is warranted for anyone receiving a fat-modified diet for >2 weeks. Large-volume output from chylothorax can cause high-protein losses, resulting in low albumin levels, and excessive losses of fat, electrolytes, immunoglobulins, and other minerals that may be protein bound, such as zinc, copper, and selenium. Reference Horsley and Susan Konek35 These minerals should be closely monitored in patients receiving parenteral nutrition, specifically when delivery is reduced secondary to supply shortage.
Chyle is rich in lymphocytes and immunoglobulins that play a critical role in immune response and infection prevention. Hoskote and colleagues demonstrated a significant correlation between duration of chyle loss and degree of lymphopenia including an association between prolonged chylous drainage with a nadir on day 5 (range 2-6 days) in neonates and worse secondary immunodeficiency. In children, loss of immunoglobins was seen more in large volume losses and less with duration of loss. Reference Hoskote, Ramaiah, Cale, Hartley and Brown43 A basic metabolic panel, phosphorus, albumin, and immunoglobulins level should be monitored. Supplementation and repletion of electrolytes, albumin, and/or immunoglobulins should be considered if abnormal, see Table 5 for recommended surveillance labs and timing.
Table 5. Suggested nutrition related biochemical monitoring for chylothorax.
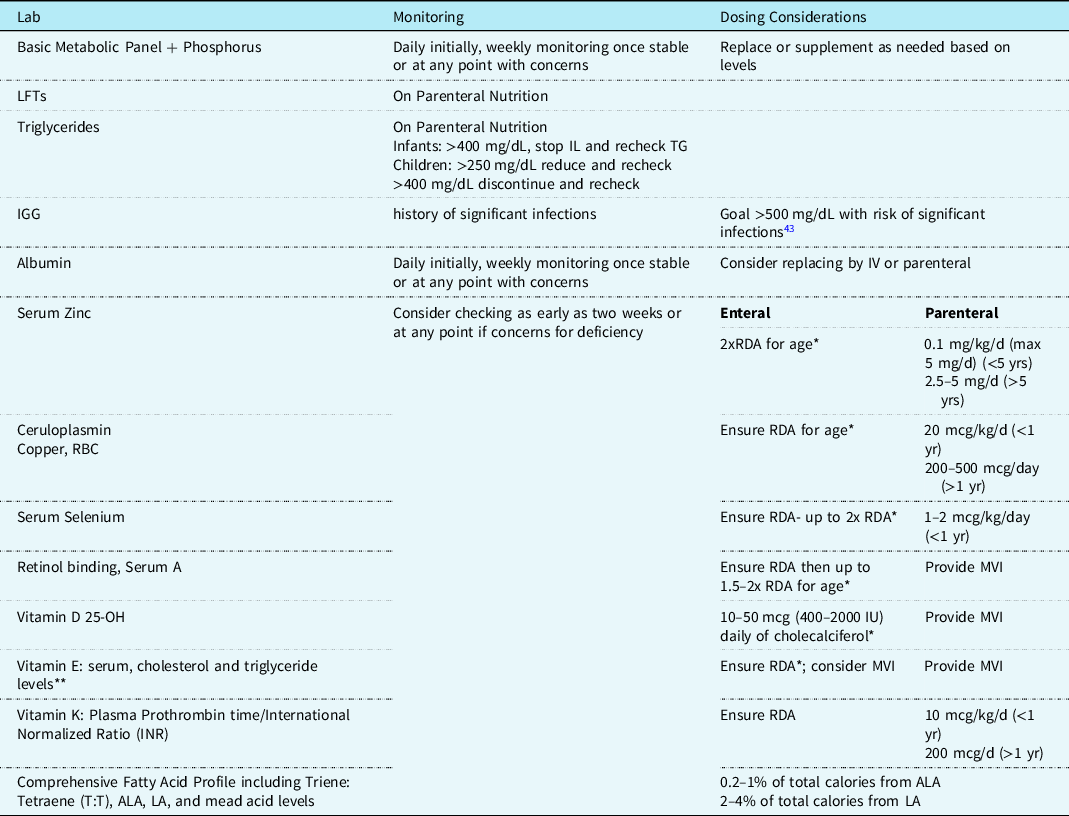
* Do not exceed upper limit (UL).
** Lipid blood content suggested for accurate assessment of α-tocopherol, low levels during protein-energy malnutrition may falsely indicate deficiency. Reference Winbauer, Pingree and Nuttall44
Nutrition-focused physical exam monitoring
Nutrition-focused physical exam includes an examination of subcutaneous fat, muscle loss, oedema, and assessing for micronutrient-deficient specific clinical signs. A nutrition-focused physical exam should be performed on all patients at the time of chylothorax diagnosis and regularly throughout their treatment course to properly customise their nutrition interventions. As fat-soluble vitamin deficiencies, mineral deficiencies, and essential fatty acid deficiency are more common in this population, please see Table 6 for signs and symptoms of deficiency.
Table 6. Micronutrients commonly deficient in chylothorax and physical effects.
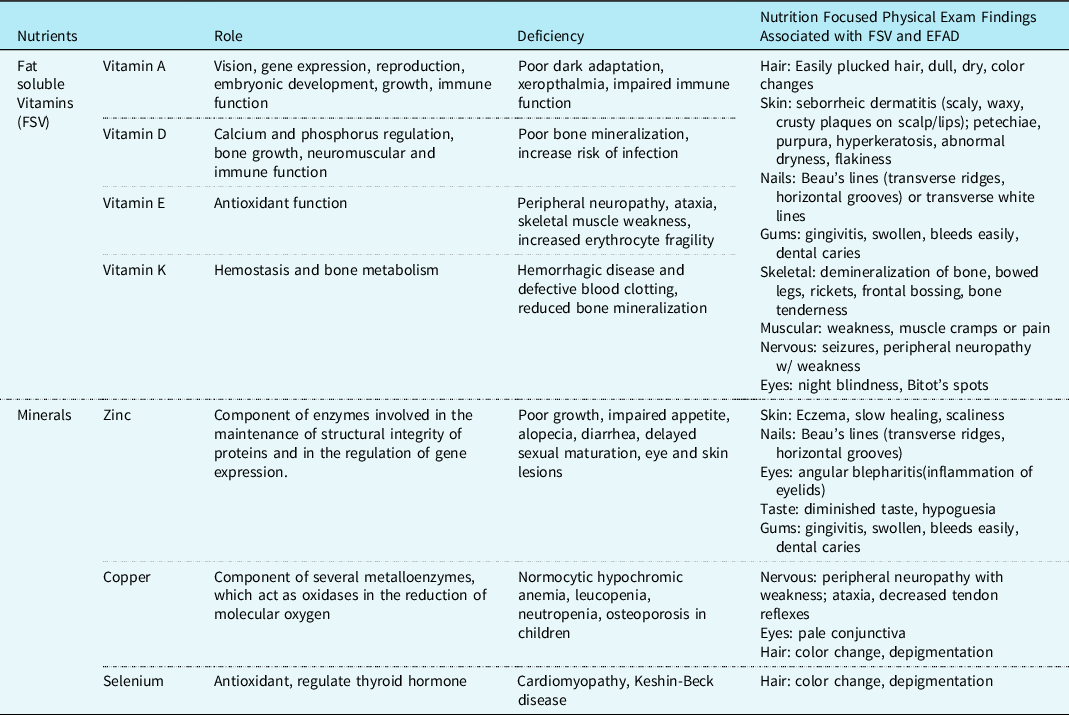
Note: many micronutrients are affected by metabolic stress and may not be an accurate reflection of body stores.
Discussion
To address the nutritional considerations unique to paediatric patients with chylothorax, we utilised the Chylothorax Work Group infrastructure to develop contemporary, clinically relevant, nutritional guidance based on expert experience, and literature review. The guidelines provide age-specific recommendations regarding delivery of fat-modified diets, management of parenteral nutrition, nutrition-focused physical exam, biochemical monitoring rationale, and recommendations. We suggest that optimal recovery from chylothorax will require clinicians to mitigate the myriad of nutritional insults associated with this complication. Within the Chylothorax Work Group, ongoing efforts are underway to better understand the nutritional influences of chylothorax and best practices when postoperative chylothorax occurs in children with children heart disease.
Acknowledgements
We wish to acknowledge the efforts of all participants of the Chylothorax Work Group for their time, energy, and enthusiasm to the creation of consensus management and ongoing chylothorax research.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.











