Introduction
Congenital Heart Disease (CHD) is one of the most common birth defects. Reference Cassidy, Ilardi and Bowen1 Mortality from CHD has significantly declined in the last 2–3 decades, with >90% of individuals with CHD living into adulthood. Reference Cassidy, Ilardi and Bowen1,Reference Lopez, Morris, Sexson Tejtel, Espaillat and Salemi2 However, children with CHD are at risk of significant neurologic, cognitive, and psychosocial morbidity. Reference Cassidy, Ilardi and Bowen1,Reference Newburger, Sleeper and Bellinger3–Reference Pande, Noll and Afonso6 The aetiology of these morbidities is multifactorial and incompletely understood, but are thought to include abnormalities of brain development with increased vulnerability of the immature brain, as well as perinatal and perioperative factors, and home and environmental stressors. Reference Cassidy, Ilardi and Bowen1,Reference Lopez, Morris, Sexson Tejtel, Espaillat and Salemi2,Reference Marelli, Miller, Marino, Jefferson and Newburger4 While some discrete risk factors have been delineated, many are unmodifiable, and much remains unknown.
Acute kidney injury (AKI) after surgery for CHD is common and is independently associated with longer hospital length of stay, longer mechanical ventilation days, and mortality. Reference Akbulut B.B., Albayrak and Güner7–Reference Alten, Cooper and Blinder12 In hospitalised children, AKI has been found to be associated with worse neurocognitive outcomes. Reference Pande, Noll and Afonso6,Reference Pande, Smith and Soranno13,Reference Bone, Feinglass and Goodman14 We previously showed that infants who developed cardiac surgery associated acute kidney injury (CS-AKI) in the first year of life have a higher frequency of developmental delay (compared to patients with 0 or 1 episodes of CS-AKI in the first year of life) and had worse scores in cognitive, language, and motor domains on neurodevelopmental testing. Reference Pande, Noll and Afonso6 Ours was the first study to examine the association between CS-AKI and neurodevelopmental delays.
Although most existing literature that uses the Bayley Scale for Infant Development has assessed the relationships between risk factors and delay within the comprehensive domains of language, motor, and cognition, few have focused on language and motor subscales, including receptive and expressive language and gross and fine motor. These subscales are critically important to understand how children with these delays can be best supported to flourish. Deficits in these subscales can significantly affect reasoning, executive function, learning, attention, and social-emotional skills, which can translate into a need for extra school services, requirement of physical and occupational therapy, remediation in school, and even affect employment. Reference Newburger, Sleeper and Bellinger3–Reference Verrall, Blue and Loughran-Fowlds5
To better characterise the relationship between recurrent CS-AKI and neurodevelopmental delay, we performed a secondary analysis of our original cohort, analysing the receptive and expressive subscales of language development, as well as the gross and fine motor subscales of motor development.
Methods
Study population
As previously defined, Reference Pande, Noll and Afonso6 patients who underwent surgery for CHD during the first year of life and who underwent neurodevelopmental testing at 18–24 months of age using the Bayley Scales of Infant and Toddler Development Third Edition at the Texas Children’s Hospital Cardiac Development Outcomes Program Clinic were included. Patients with genetic diseases known to be associated with neurocognitive impairment were excluded. This study was approved with waiver of consent by the Baylor College of Medicine Institutional Review Board. As previously described, Reference Pande, Noll and Afonso6 information on innate patient factors as well as peri- and intra-operative variables, including surgical complexity, were gathered from the electronic medical record and the Cardiac Development Outcomes Program database.
Bayley Scales of Infant and Toddler Development Third Edition developmental assessment
The Bayley Scales of Infant and Toddler Development Third Edition neurodevelopmental testing tool has three main domains: cognitive, language, and motor, with subscales in language (receptive and expressive) and motor (fine and gross) domains. Each subscale yields a raw score, which is converted to a scaled score based on normative data. The mean scaled score is 10 with a standard deviation of three. Scaled scores are then converted to standard scores in the language (expressive and reception communication) and motor (fine and gross motor) domains. Across the normative population, the mean composite score is 100 with a standard deviation of 15. Greater than one standard deviation below the mean is categorised as mild impairment and greater than two standard deviation below the mean is considered severe impairment. Reference Bayley15
Cardiac surgery associated acute kidney injury
Acute kidney injury was defined based on the Kidney Disease: Improving Global Outcomes creatinine criteria. Reference Fitzgerald, Basu and Akcan-Arikan16 Baseline pre-operative creatinine and maximum creatinine in the first 7 days after surgery for CHD were collected. At our institution, low volume peritoneal dialysis is often pre-emptively performed in infants who undergo surgery for CHD to control fluid balance and avoid use of diuretics in the immediate post-operative period. The patients who received dialysis for 72 hours or more were classified as stage three AKI. Patients who developed repeat episodes of AKI after additional, distinct surgeries for CHD in the first year of life were classified as recurrent CS-AKI.
Statistics
Baseline characteristics are summarised using mean with standard deviation, median with 25th and 75th percentiles, or frequency with percentage as appropriate. We compared continuous variables using t-test, one-way analysis of variance, Wilcoxon rank sum test, or Kruskal–Wallis test, and categorical variables using Fisher’s or chi-square as appropriate. Unadjusted and adjusted logistic regression was used to assess the association of risk factors with the odds of subscale specific delay. We determined, a priori, the variables that would be included in the multivariable analysis based on prior literature Reference Newburger, Sleeper and Bellinger3–Reference Verrall, Blue and Loughran-Fowlds5,Reference Ryan, Jones and Allen17 as well as variables that were found to be significant with a p value<0.05 on univariate analysis. Then, backward elimination was used to reduce the model down until only CS-AKI, birthweight, age at first surgery, days in the hospital in the first year of life, and any factors that remained statistically significant were included in the multivariable model.
Results
General characteristics – Supplementary Table 1
The demographic characteristics of this cohort have been described previously Reference Pande, Noll and Afonso6 and are displayed in supplementary tables. Briefly, our cohort of 203 patients was 58% (n = 118) male, 36% (n = 73) underwent more than one cardiac surgery during the first year of life, and 69% (139) had an initial surgical STAT category of 3–5. Thirty-one percent (n = 62) of patients had CS-AKI, of those 16% had recurrent CS-AKI, or 5% (10) of the total cohort. Of note, 21% (n = 43) required a Spanish language interpreter at the neurodevelopmental assessment.
Neurodevelopmental outcomes – Figure 1
Median (interquartile range) age at Bayley Scales of Infant and Toddler Development Third Edition assessment was 20 months (18.7–22.5). Forty-four percent (n = 89) had receptive language delay, 15% (n = 31) had severe receptive language delay, 48% (n = 96) had expressive language delay, 20% (n = 41) had severe expressive language delay. Twenty-five percent (n = 49) had fine motor delay, 10% (n = 19) had severe fine motor delay, 27% (n = 50) had gross motor delay, and 11% (n = 20) had severe gross motor delay.

Figure 1. CHD acute kidney injury subscales.
Recurrent cardiac surgery associated acute kidney injury and delay – Figures 2 and 3, Supplementary Tables 2 and 3
All patients with recurrent CS-AKI had significantly lower scores in each subscale (p < 0.02 for all). On unadjusted logistic regression, recurrent CS-AKI was associated with mild receptive language, expressive language, fine motor, and gross motor delays. Cardiac surgery associated acute kidney injury was also associated with severe delays in all subscales except gross motor on unadjusted analysis, regardless of primary language. In a separate unadjusted analysis comparing patients who had one surgical intervention, there was no difference in neurodevelopmental outcomes in patients who had one episode of CS-AKI compared to those who had zero episodes of CS-AKI. In patients who had more than one surgical intervention, comparing those who had zero, one, or more than one episode of CS-AKI, recurrent CS-AKI was significantly associated with mild fine motor delay (p = 0.032) and severe receptive language delay (0.005).
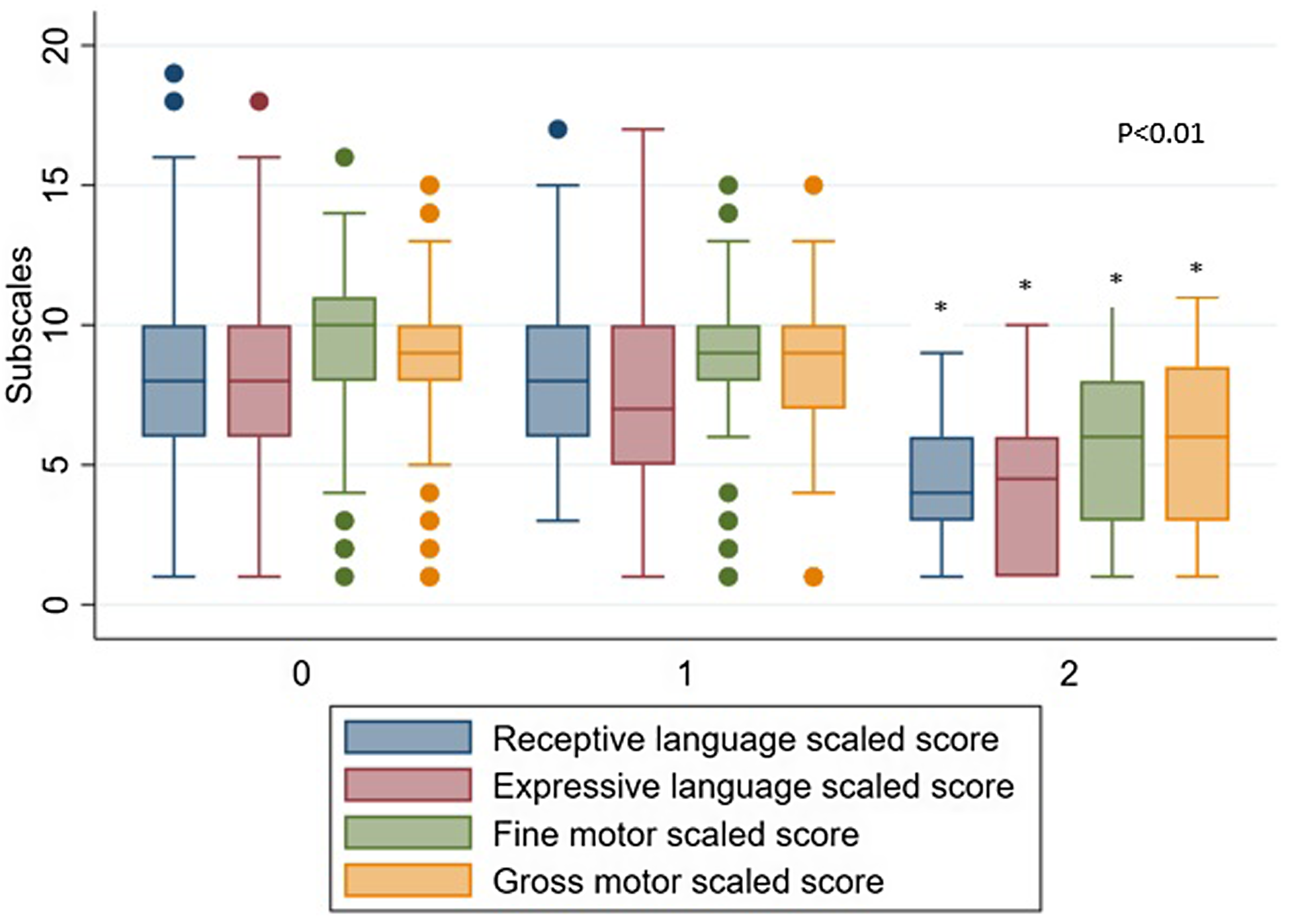
Figure 2. CHD AKI subscales.
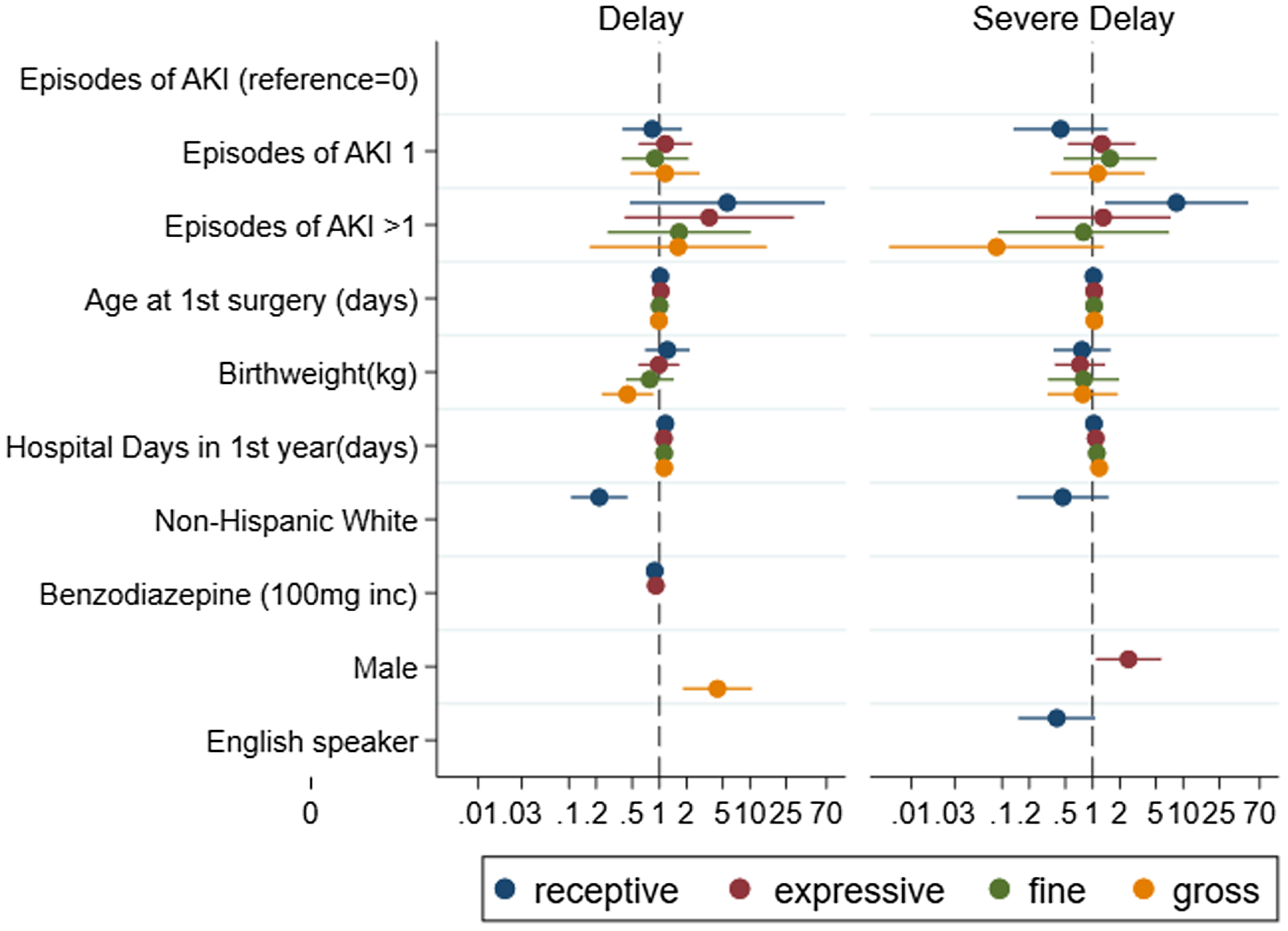
Figure 3. CHD AKI subscales.
In adjusted analyses, recurrent CS-AKI was significantly associated with severe receptive language delay (adjusted odds ratio 7.1, p = 0.03). The cumulative total number of hospital days in the first year of life was associated with receptive and expressive language, as well as gross and fine motor skills (all p < 0.01). Lower birthweight was significantly associated with mild gross motor delay (adjusted odds ratio = 0.44, p = 0.015). Minority race/ethnicity was associated with mild receptive delay (non-Hispanic White adjusted odds ratio = 0.2, p < 0.001) and severe receptive delay (non-Hispanic White adjusted odds ratio = 0.3, p = 0.03). Use of Spanish language interpreter was not significantly associated with language delay on adjusted analysis. Male sex was associated with delay in mild gross motor (adjusted odds ratio= 4.4, p < 0.001) and severe expressive language (adjusted odds ratio = 2.5, p = 0.03) subscales.
Discussion
The current study provides further important insights into the potential relationship between CS-AKI and neurodevelopmental outcomes in children with CHD. In patients with recurrent CS-AKI, nearly half the cohort had receptive and expressive language delay and a quarter had fine and gross motor delay. On adjusted analyses, recurrent CS-AKI was significantly associated with severe receptive language delay. This is a novel finding in children that may help direct preventative care.
It is well described that children with CHD have worse neurodevelopmental outcomes compared to their healthy peers, a finding that begins in infancy and often continues into adolescence and adulthood. Reference Cassidy, Ilardi and Bowen1,Reference Newburger, Sleeper and Bellinger3,Reference Marelli, Miller, Marino, Jefferson and Newburger4 They often have difficulty with speech and language with delayed acquisition, difficulty with phonation, and grammatical processing requiring speech or language therapy. Reference Cassidy, Ilardi and Bowen1,Reference Mussatto, Hoffmann and Hoffman18,Reference Rollins, Asaro and Akhondi-Asl19 These difficulties persist into school age, with lower standardised language assessment scores and frequent difficulties with behaviour and academic achievement. Reference Cassidy, Ilardi and Bowen1 Many unmodifiable risk factors exist in utero, such as smaller brain volumes, abnormal cortical folding, and lower markers of brain maturity. Reference Rollins, Asaro and Akhondi-Asl19 In the post-natal period, through often many surgeries, lengthy ICU and hospital stays, exposure to risk of complications, these infants are exposed to chronic hypoxaemia, enhanced inflammatory responses, and significant oxidative stress which may result in cerebral insult. Reference Cassidy, Ilardi and Bowen1,Reference Marelli, Miller, Marino, Jefferson and Newburger4 After multiple insults, infants are at risk of developing diminished memory, processing, motor, and language skills, which may necessitate supplemental services later in life, including physical and occupational therapies, school remediation requiring extra school services and even difficulty with gaining meaningful employment. Reference Rollins, Asaro and Akhondi-Asl19,Reference von Rhein, Buchmann and Hagmann20
Cardiac surgery associated AKI is common in children after surgery for CHD, with neonates being one of the highest risk populations due to a relatively low renal blood flow, glomerular filtration rates, and nephrogenesis. Furthermore, neonates in the cardiac ICU are exposed to low flow states, hypoxaemia, and significant inflammatory and oxidative stresses, as well as multiple common nephrotoxic drugs. Reference Alten, Cooper and Blinder12,Reference Selewski, Charlton and Jetton21 With the high risk of decreased renal clearance, ototoxicity with resultant hearing impairment is a well-described adverse event in neonates exposed with nephrotoxic drugs. Reference Wroblewska-Seniuk, Dabrowski, Szyfter and Mazela22 Hearing impairment is an important predictor of language delay, both of which are associated with Reference Wargo and Edwards23,Reference Zussino, Zupan and Preston24 worse health-related quality of life. Reference Le, Petersen, Mensah, Gold, Wake and Reilly25 Although it is difficult to determine the number of neonates with hearing impairment given lack of universal screening, it is estimated that neonates in the ICU have at least a 10-fold higher rate of hearing impairment compared to the general newborn population, and an even higher rate in preterm infants. Reference Nair, Janakiraman, Whittaker, Quail, Foster and Loganathan26
Children with CHD have lower total white matter and delayed temporal lobe growth, both of which are associated with worse verbal comprehension and receptive language delay. Reference Rollins, Asaro and Akhondi-Asl19,Reference von Rhein, Buchmann and Hagmann20 Furthermore, in animal models, cellular abnormalities and impaired blood flow are present after induced ischaemic AKI, Reference Liu, Liang and Chigurupati27,Reference Firouzjaei, Haghani and Shid Moosavi28 It is possible that injury to the hippocampus, which resides in the temporal lobe where structures whose growth predicts language development in children with CHD reside, may add to the explanation of our findings.
Patients in our cohort who had recurrent CS-AKI spent significantly more days in the hospital compared to those without recurrent CS-AKI. These patients are expectedly exposed to more hospital acquired morbidity just by virtue of being in hospital and do not receive the same routine neurodevelopmental care that infants engage in at home and in the outpatient world. Our findings show that patients with recurrent CS-AKI may represent a population that is at higher risk of language delay and may benefit from inpatient efforts to promote language development.
The American Heart Association guidelines recommend increased neurodevelopmental surveillance and evaluation for all children with CHD who are considered “high risk”, Reference Marino29 including those who had open heart surgery in the first year of life, those with cyanotic heart disease, and with additional risk factors. Reference Marino29 Many yet to be determined modifiable and unmodifiable factors must contribute to the worse neurodevelopmental outcomes in this patient population as current evidence is only able to explain a relatively small proportion of the variation in neurodevelopmental scores. Reference Ryan, Jones and Allen17,Reference Mussatto, Hoffmann and Hoffman18,Reference Marino29 Our findings suggest that repeat CS-AKI and the increased morbidity that affects this patient population may be a risk factor for worse neurodevelopmental outcomes in this population. Reference Hoste, Kellum and Selby9,Reference Alten, Cooper and Blinder12,Reference Pande, Smith and Soranno13
Furthermore, children with CS-AKI may benefit from interventions focused on language skills while in the hospital, as well as earlier and more frequent hearing and language assessments upon discharge and follow-up with speech language therapy services. Our data add further weight to the body of evidence supporting that child who develop repeated episodes of CS-AKI should be more closely followed in the outpatient setting to assess for resolution of kidney injury or progression to chronic kidney disease, as well as progression of hearing impairment.
Limitations
Our study has several limitations. As a single centre retrospective review of patients who voluntarily come to a follow-up clinic, a self-selection bias may exist. Furthermore, our results may not be generalisable to this patient population and require larger studies to validate our findings. Although the Bayley Scales of Infant and Toddler Development Third Edition is normed to English-speaking patients, we included use of a language interpreter in our analysis. Finally, we did not assess the aetiology of CS-AKI in this study; future studies should focus on this to help elucidate specific risk factors for CS-AKI.
Conclusion
Recurrent CS-AKI in the first year of life is associated with severe receptive language delay in children who undergo surgery for CHD in infancy. Our findings may offer further insight into the mechanism of difficulties with language skills known to exist in children with CHD. Children with recurrent CS-AKI require close outpatient follow-up and might benefit from earlier and frequent hearing screening to optimise timing of proactive therapies. Future multicentre studies should focus on better characterising and understanding this relationship to improve care and follow-up of high-risk children for neurodevelopmental delay.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1047951124025873
Financial support
None.
Competing interests
None of the study authors have a reported conflict of interest.






