Necrotising enterocolitis is a serious life-threatening intestinal condition with high morbidity and mortality. Reference Neu and Walker1 Necrotising enterocolitis is characterised by mucosal inflammation and injury that can result in necrosis and perforation of varying lengths of small and larger intestines. Reference Neu and Walker1,Reference Bazacliu and Neu2 Term-born infants with CHD are at increased risk for development of necrotising enterocolitis, Reference McElhinney, Hedrick and Bush3–Reference Kelleher, McMahon and James5 but they have different demographics, mechanisms, and outcomes than those with classical necrotising enterocolitis commonly found in premature neonates. Reference Siano, Lauriti, Ceccanti and Zani6 Although the pathophysiology of necrotising enterocolitis in cardiac infants is not fully elucidated, mesenteric hypoperfusion and subsequent bowel ischaemia with lower diastolic pressures and decreased systemic oxygen delivery are likely key contributors to disease. Reference Natarajan, Anne and Aggarwal7–Reference Stapleton, Eble, Dickerson, Andropoulos and Chang9
The incidence of necrotising enterocolitis in term infants with CHD has been reported between 3 and 11% with mortality rates varying from 13 to 57%. Reference McElhinney, Hedrick and Bush3–Reference Siano, Lauriti, Ceccanti and Zani6,Reference Cheng, Leung and Tam10–Reference Spinner, Morris and Nandi13 Major determinants of necrotising enterocolitis have been retrospectively explored in CHD populations, but a paucity of data examines risk factors for poor outcomes in necrotising enterocolitis associated with CHD. Reference McElhinney, Hedrick and Bush3–Reference Kelleher, McMahon and James5,Reference Leung, Chau and Hui14–Reference Baxi, Josephson, Iannucci and Mahle16 Specifically, there is little evidence evaluating patient characteristics, severity of disease, and cardiac function at the time of diagnosis of definitive necrotising enterocolitis and the role of nutritional regimens in outcomes in infants with CHD and necrotising enterocolitis. For example, there still remains no clear consensus on the role that preoperative feeding has on the morbidity and mortality related to the development of necrotising enterocolitis, or clear descriptions of feeding patterns after diagnosis. Reference del Castillo, McCulley and Khemani12,Reference Natarajan, Reddy Anne and Aggarwal17 In order to inform risk triage and prognostic counseling for term infants with CHD who develop necrotising enterocolitis, it is imperative to understand which factors impact outcomes after diagnosis.
With the increased recognition that the number of term infants with CHD affected by necrotising enterocolitis is substantial and that risk factors are different than premature infants with necrotising enterocolitis, we sought to define the incidence of necrotising enterocolitis in term infants with CHD, describe outcomes of infants and identify risk factors for morbidity and mortality with definitive necrotising enterocolitis at a large quaternary high-volume cardiac surgical centre.
Materials and methods
Study population
We performed a 20-year (2000–2020) single-institution retrospective cohort study in consecutive term infants (37 weeks gestation at birth) with CHD and necrotising enterocolitis admitted to the Boston Children’s Hospital cardiac ICU. We used Bell’s staging criteria to define necrotising enterocolitis and only included CHD infants with Bell’s stage ≥ II, which we classified as definitive necrotising enterocolitis. (Table 1). The institutional review board of Boston Children’s Hospital approved this study with waiver of consent.
Table 1. Bells staging criteria for necrotising enterocolitis (NEC)

Data collection
The Boston Children’s Hospital Heart Center database was utilised to identify all eligible term-born infants with CHD and necrotising enterocolitis. Two independent physician investigators manually reviewed each patient’s diagnostic imaging (abdominal radiographs and ultrasounds) and clinical documentation by cardiac intensive care and general surgery clinicians to ensure patients met criteria for Bell’s stage ≥ II (definitive). We collected demographic data (e.g., gestational age at birth, birthweight, age, and weight at necrotising enterocolitis diagnosis, sex, and race), post-operative diagnosis of necrotising enterocolitis, type of cardiac lesion, and characteristics of cardiac intervention, feeding patterns, and severity of illness.
Primary outcome
The primary outcome was a composite of in-hospital mortality and post-necrotising enterocolitis morbidity defined as the need for extracorporeal membrane oxygenation, multisystem organ failure, and/or need for acute gastrointestinal intervention. Multisystem organ failure was defined by a post-necrotising enterocolitis severity of illness, as measured with the paediatric sequential organ failure assessment score. The paediatric sequential organ failure assessment score has been utilised to predict in-hospital mortality in critically ill paediatric patients and assesses six organ systems: respiratory, haematological, hepatic, cardiovascular, neurological, and renal. Reference Matics and Sanchez-Pinto18 A recent study in children with sepsis found that a paediatric sequential organ failure assessment score > 8 was associated with mortality. Reference Matics and Sanchez-Pinto18 We used a more conservative post-necrotising enterocolitis (over the 24–48 hours post-necrotising enterocolitis) paediatric sequential organ failure assessment score greater than or equal to the 90% percentile from our cohort, which was 14, to define multisystem organ failure in the primary outcome. Acute gastrointestinal surgical intervention for necrotising enterocolitis was defined as placement of peritoneal drain, laparotomy, bowel resection, or ostomy placement. Mortality attributable to necrotising enterocolitis was determined by two independent physician investigators. We also identified infants with necrotising enterocolitis totalis and recurrent necrotising enterocolitis. Recurrent cases of necrotising enterocolitis were only counted one time in the cohort.
Cardiac lesions
Primary cardiac diagnosis was categorised as cyanotic mixing conditions with increased pulmonary blood flow, single ventricle, left-to-right shunting conditions with increased pulmonary blood flow, left and right ventricular outflow tract obstruction lesions. Ductal-dependent lesions were separated into biventricular versus single ventricle circulation and furthermore divided into ductal-dependent pulmonary blood flow versus ductal-dependent systemic blood flow. Echocardiographic measurements of ascending aorta or isthmus size (z ≥ -3) and systemic ventricle systolic function were included. We evaluated the most recent echocardiogram completed prior to the episode of necrotising enterocolitis. For systolic function, we used a combination of qualitative and quantitative reporting. In our echocardiogram laboratory, we use ejection fraction, but when the windows are poor, we comment on the qualitative assessment. In addition we also captured haemodynamic assessment of pulmonary (Qp) to systemic (Qs) ratios by cardiac catheterisation when applicable. Prior cardiac interventions with cardiac bypass, cross-clamp, and deep hypothermic circulatory arrest times were incorporated. We utilised the Society of Thoracic Surgeons-European Association for Cardiothoracic Surgery mortality category to analyse the risk for mortality associated with congenital heart surgery procedures. The categories rank from 1 to 5 with category 5 defined as highest complexity with greatest risk of mortality. We categorised the patients into the category that their current cardiology physiology aligned with at the time of diagnosis of necrotising enterocolitis.
Nutritional characteristics
Parenteral and enteral nutritional regimens were assessed and included type of enteral feeds (e.g., breast milk, donor breast milk, formula, or a combination), maximum caloric fortification, time to achieve full feeds > 100 millilitre/kilogram/day pre- and post- necrotising enterocolitis, presence of surgically placed gastrostomy or jejunostomy tube, and need for pre- and post- necrotising enterocolitis total parenteral nutrition.
Additional clinical variables
Predictors of the primary outcome included baseline admission patient characteristics, cardiac diagnosis, cardiac intervention, presence of umbilical vein or artery catheter, vasoactive-inotropic score, central line bloodstream infection prior to the diagnosis of necrotising enterocolitis, need for invasive mechanical ventilation, presence of lactate > 4, new-onset seizures, transaminases > 500, product transfusion (plasma, platelets, and/or red blood cells), and feeding regimen. Prenecrotising enterocolitis severity of illness was assessed with a pre-necrotising enterocolitis paediatric sequential organ failure assessment score. The paediatric sequential organ failure assessment score was assessed “pre” (24–48 hours prior to the diagnosis of necrotising enterocolitis) and measured as a continuous score between 0 and 24. Additional post-necrotising enterocolitis severity measures included lactate, seizures, blood product administration and liver function tests (aspartate transaminase), vasoactive inotropic score, and need for invasive mechanical ventilation.
Statistical analysis
Categorical variables are summarised with frequencies and percentages and compared for patients who did and did not experience the primary outcome using Fisher’s exact test. Continuous variables are summarised with medians and ranges, or interquartile ranges as indicated and compared using the Wilcoxon rank sum test. Logistic regression analysis was also used to explore the relationships between patient characteristics and the primary outcome. Factors significant at the 0.2 level in univariate analysis were considered for inclusion in a multivariable model; p < 0.05 by the likelihood ratio test was required for retention in the final model. Odds ratios are presented with 95% confidence intervals. Data analysis was performed with SAS version 9.4 (SAS Institute, Cary, NC).
Results
Characteristic of the cohort
Over a 20-year time period, we identified 3,933 term infants with CHD admitted to the Boston Children’s Hospital cardiac ICU. There were 200 term infants with CHD and all stages necrotising enterocolitis (Table 1). Eighty-two infants had Bells Stage ≥ II necrotising enterocolitis, resulting in a 2.1% prevalence of CHD and definitive necrotising enterocolitis in term infants at our institution. The 82 infants were all born ≥ 37 weeks gestational age with median (range) birth weight 3115 g (1756–4810g). Twenty-eight percent (n = 23/82) of the patient were either small for gestational age (below the 10 percentile) and/or had foetal growth restriction. The median age at the time of necrotising enterocolitis diagnosis was 31 days (1–194), and the median weight was 3260 grams (2055, 6300). Necrotising enterocolitis was diagnosed postoperatively in 67% (n = 55/82) of patients at a median onset of 25 days postoperatively. The median cardiac ICU length of stay was 46 days (2, 300) with post-necrotising enterocolitis cardiac ICU median length of stay of 39 days (1, 283). Twenty-four percent (n = 20/82) of the patients experienced recurrent episodes of necrotising enterocolitis (Table 2).
Table 2. Patient characteristics
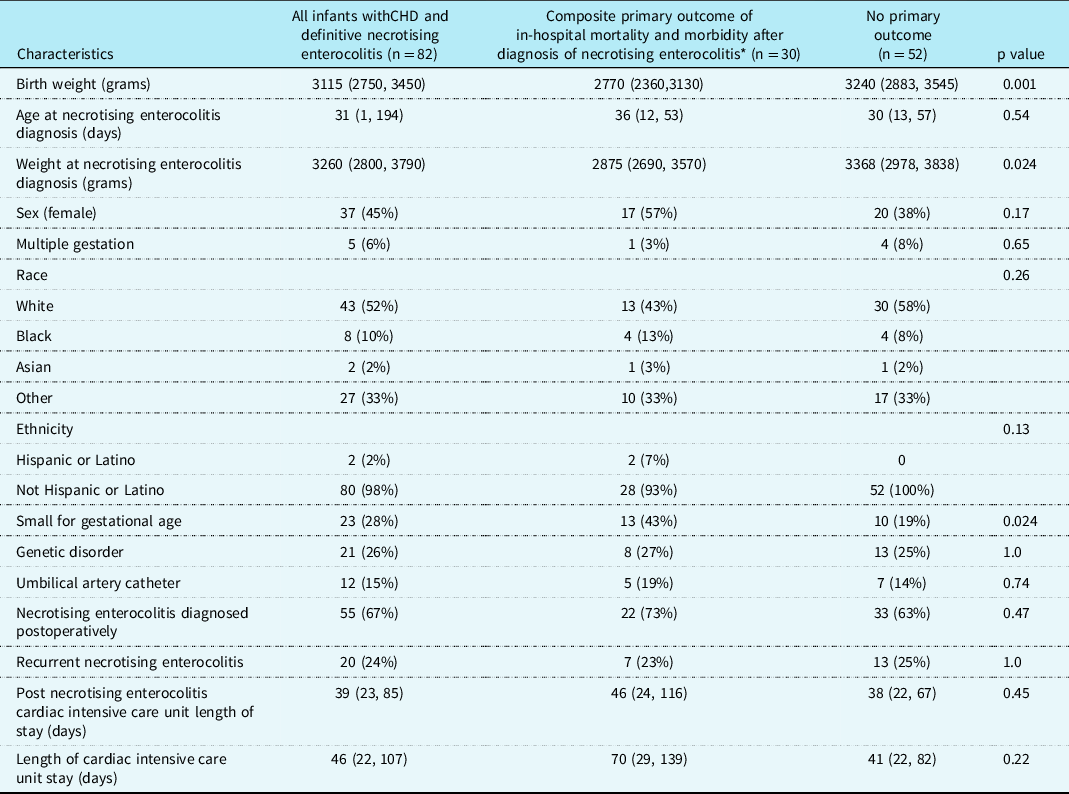
* The composite primary outcome was defined as in-hospital mortality and post-necrotizing enterocolitis morbidity (need for extracorporeal membrane oxygenation, multisystem organ failure based on the paediatric sequential organ failure assessment score, and/or need for acute gastrointestinal intervention). Small for gestational age (<10%).
Data are presented as median [interquartile range], or number (Percentage). Data are presented as column percentages.
Cardiac measures
Cardiac diagnoses are summarised in Table 3. Of 82 patients, six (7%), 18 (22%), 11 (13%), 17 (23%), and 30 (37%) neonates were Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery category 1, 2, 3, 4, 5, respectively. Sixty-eight percent (n = 56/82) of the patients had ductal dependent circulation with 11% (n = 9/82) having ductal dependent pulmonary blood flow and 39% (n = 32/82) having ductal dependent systemic blood flow.
Table 3. Cardiac diagnosis, physiology, and interventions
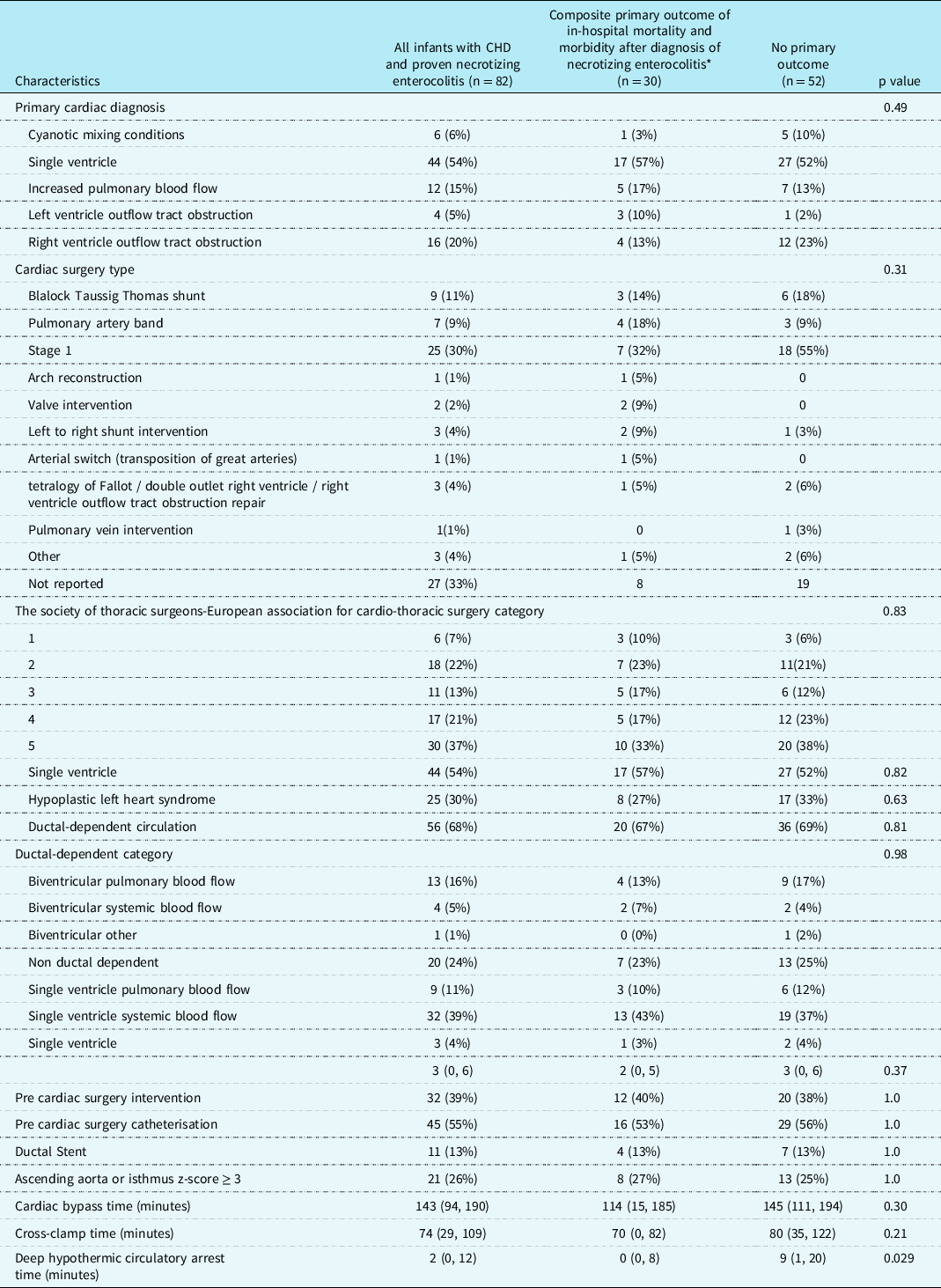
* The composite primary outcome was defined as in-hospital mortality and post-necrotizing enterocolitis morbidity (need for extracorporeal membrane oxygenation, multisystem organ failure based on the paediatric sequential organ failure assessment score, and/or need for acute gastrointestinal intervention).
Values are number (percent) or median [25th, 75th percentiles].
Nutritional factors
Seventy-two percent of infants (n = 59/82) were on full feeds ( ≥ 100 milliliter/kilogram/day) prior to development of necrotising enterocolitis with 64% (n = 38/59) of infants on breast milk, 32% (n = 19/59) feeding both formula and breastmilk, and 39% (23/59) of these infants had feeds fortified to ≥ 28 kilocalorie/ounce (Supplemental Table 1). Following necrotising enterocolitis, 83% (n = 63/82) patients were able to achieve full enteral feeds with mean time to full feeds of 21 days. Forty-eight percent (n = 39/82) of patients had gastrostomy or jejunostomy tubes placed following necrotising enterocolitis. Thirty-three percent (n = 10/30) of patients who experienced the primary outcome were unable to achieve full enteral feeds post necrotising enterocolitis.
Primary outcome
Thirst-seven percent (n = 30/82) infants met the primary outcome. Of these patients, in-hospital mortality occurred in 47% (n = 14/30) (Fig 1). In nine of the 14 patients, death was attributed to complications from necrotising enterocolitis (Supplemental Table 2). Sixty-seven percent (n = 55/82) of patients developed necrotising enterocolitis postoperatively and 7% (n = 6/82) died from necrotising enterocolitis within four days of necrotising enterocolitis diagnosis. During the peri-necrotising enterocolitis period (within 24 hours surrounding diagnosis of necrotising enterocolitis), 7% (n = 5/82) of patients required extracorporeal membrane oxygenation, 11% (n = 9/82) had multisystem organ failure, 22% (n = 18/82) needed an acute gastrointestinal intervention, and 4% (n = 3/82) developed necrotising enterocolitis totalis. The median post-necrotising enterocolitis paediatric sequential organ failure assessment score total was 4 (75th and 90th percentiles were 7 and 14, respectively). Univariate and multivariable analysis for the primary outcome is presented in Table 4.

Figure 1. Study flow chart. The primary outcome was a composite of in-hospital mortality and post-necrotizing enterocolitis morbidity (extracorporeal membrane oxygenation, multisystem organ failure based on the pediatric sequential organ failure assessment score, and/or need for acute gastrointestinal intervention).
Table 4. Univariate and multivariable associations with primary outcome
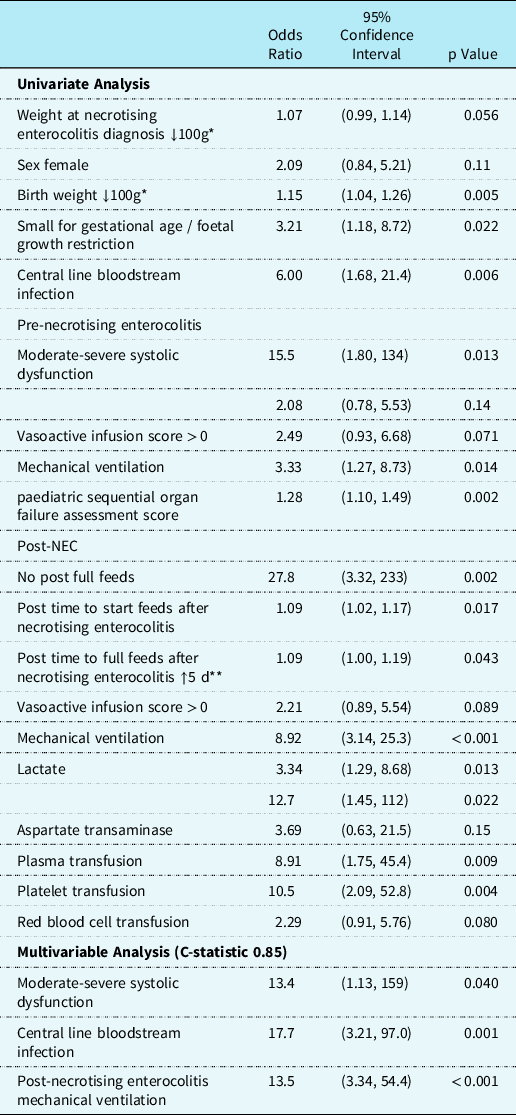
* ↓100g, For every 100 g decrease in weight at diagnosis of necrotising enterocolitis or birthweight, the odds ratio increases.
** ↑5 d, For every additional 5 days to reach full feeds after necrotising enterocolitis, the odds ratio increases.
Odds ratios estimated using logistic regression.
Univariate model
On univariate analysis (Table 4), infants with the primary outcome were more likely to have lower birth weight, small for gestational age/foetal growth restriction, central line bloodstream infection pre-necrotising enterocolitis diagnosis, require pre-necrotising enterocolitis mechanical ventilation, have higher pre-necrotising enterocolitis paediatric sequential organ failure assessment score and moderate to severe systolic dysfunction, elevated post-necrotising enterocolitis lactate, post-necrotising enterocolitis seizures, require post-necrotising enterocolitis mechanical ventilation, and receive plasma or platelet transfusions post-necrotising enterocolitis. Time to start and reach full feeds were also more delayed in the primary outcome group. There was no statistical association between the primary outcomes with presence of umbilical lines, prostaglandin administration, primary cardiac diagnosis, ductal circulation dependency, cardiac surgery type, Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery category, weight at necrotising enterocolitis diagnosis and pre-necrotising enterocolitis vasoactivescore.
Multivariable model
Ten percent (n = 8/82) of the patients had moderate to severe systolic dysfunction at the time of necrotising enterocolitis diagnosis. Moderate to severe systolic dysfunction at the time of necrotising enterocolitis diagnosis was associated with primary outcome on the multivariable analysis (odds ratio 13.4, confidence intervals, 1.13–159). Presence of central line bloodstream infection prior to necrotising enterocolitis diagnosis was associated with poor outcomes (odds ratio 17.7, confidence intervals 3.21–97.0). The multivariable analysis revealed that post-necrotising enterocolitis mechanical ventilation (odds ratio 13.5, confidence intervals, 3.34–54.4) remained associated with the primary outcome (Table 4). When pre-necrotising enterocolitis paediatric sequential organ failure assessment score was added to the multivariable model there was an association was the primary outcome (odds ratio 1.19, confidence intervals 1.00–1.43), (Supplemental Table 3).
Discussion
Necrotising enterocolitis remains one of the most concerning diseases that an infant with CHD can suffer from in the neonatal period. In this large 20-year retrospective study, we identified definitive necrotising enterocolitis (Bell’s stage ≥ II) in 2.1% of term infants with CHD. Importantly, morbidity and mortality occurred in more than one-third of these patients. To our knowledge, this is one of the first studies to explore independent risk factors for adverse outcomes in term CHD infants following diagnosis of definitive necrotising enterocolitis. The main findings identified an association between adverse outcomes and moderate to severe ventricular dysfunction and central line bloodstream infections prior to the development of necrotising enterocolitis, and the need for mechanical ventilation after diagnosis of necrotising enterocolitis. The observations that the type of cardiac lesions, presence of single ventricle physiology, ductal dependency, and feeding regimen factors were not associated with the primary outcome may be as equally important and highlight that these features may not be risk factors for poor outcomes in infants with definitive necrotising enterocolitis and CHD. The main findings of this study capturing 20 years of granular data from > 3000 term infants with CHD can thus serve to inform risk-triage and prognostic counselling for families and infants with CHD and necrotising enterocolitis.
The overall mortality in neonates diagnosed with CHD and necrotising enterocolitis was 17% in our study with 11% of the cases being attributable directly to necrotising enterocolitis. Lau et al. described a necrotising enterocolitis prevalence of 3.4%, with a mortality rate of 26%, but their study included infants with suspected (Bells Stage I) and confirmed necrotising enterocolitis (Bells Stage ≥ II). Reference Lau, Cruz and Ocampo4 In addition, they focused on comparisons between ductal-dependent lesions with few other risk factors. A recent meta-analysis identified a necrotising enterocolitis incidence of 2.7% in term infants with CHD. Reference Siano, Lauriti, Ceccanti and Zani6 The proportion of term infants with CHD and necrotising enterocolitis in our study is lower than that reported in the current literature likely due to our exclusion of suspected (Bell’s Stage I) necrotising enterocolitis from our patient population. Reference McElhinney, Hedrick and Bush3,Reference Lau, Cruz and Ocampo4,Reference Siano, Lauriti, Ceccanti and Zani6,Reference Cheng, Leung and Tam10–Reference del Castillo, McCulley and Khemani12 As such, our findings likely represent a more accurate rate of definitive necrotising enterocolitis in term infants with CHD as compared to much of the other existing literature that includes suspected necrotising enterocolitis in their reporting.
The presence of moderate to severe ventricular dysfunction prior to the diagnosis of necrotising enterocolitis in this study may also offer mechanistic insights into the pathophysiological drivers of necrotising enterocolitis in term infants with CHD. In contrast to the necrotising enterocolitis that appears following premature birth, infants with CHD and necrotising enterocolitis are thought to have gut hypoperfusion as a related risk factor for poor outcomes. It has long been suspected that features related to cardiac morphology and flow patterns were some of the main drivers for low flow states and ultimately adverse outcomes in infants with CHD and necrotising enterocolitis. Reference Carlo, Kimball, Michelfelder and Border15 Interestingly in this study, primary cardiac diagnosis (including single ventricle and hypoplastic left heart syndrome subtypes), cardiac surgery type, operative status (pre- versus post-operative), cardiac surgery type, Society of Thoracic Surgeons-European Association for Cardiothoracic Surgery category, and ductal dependence for pulmonary or systemic blood flow were not significantly associated with the primary outcome. Others have shown that patients who developed necrotising enterocolitis did not have diastolic flow reversal in the abdominal aorta, Reference Lau, Cruz and Ocampo4 suggesting that the “diastolic steal phenomenon” is not a significant identifiable risk factor for mortality. Collectively, these finding imply that there must be other factors contributing to low flow states (e.g. low cardiac output and shock). In our study, there was an increased risk of poor outcomes in infants with ventricular dysfunction prior to the diagnosis of necrotising enterocolitis, but there was no association with ductal-dependent systemic or pulmonary blood flow. Notably, there was no statistically significant difference in our study between patients with hypoplastic left heart syndrome with Sano (n = 13) and Modified Blalock Taussig Shunt (n = 9). Although this represents a relatively small sample size, our data may indicate that type of palliation strategy (Sano versus Blalock Taussig Shunt versus ductal stent) and prostaglandin duration will not increase the risk for adverse outcomes following development of necrotising enterocolitis, despite potential changes in mesenteric flow patterns. Reference Kelleher, McMahon and James5,Reference Harrison, Davis and Reid20,Reference Johnson, Ansong and Li21
There were several other elements on the univariate model associated with poor outcomes in our study. These factors included higher vasoactive-inotropic score, pre-necrotising enterocolitis paediatric sequential organ failure assessment scores, elevated lactate, seizures, plasma and platelet transfusions, and requirement of mechanical ventilation (pre- and post-necrotising enterocolitis). These factors reflect illness severity with presence of low cardiac output state and evolving shock. Reference Wright, Crowley, Charpie, Ohye, Bove and Kulik8,Reference ElHassan, Tang and Gossett22 Interestingly, pre-necrotising enterocolitis paediatric sequential organ failure assessment scores just misses statistical significance when it was included in the multivariable analysis and may have been associated with the primary outcome with larger numbers (Supplemental Table 3). The higher vasoactive-inotropic scores both prior to and following necrotising enterocolitis were not associated with increased risk of poor outcomes on the multivariable analysis likely due the fact that the timing of inotropes was not easily differentiated, which has also demonstrated in prior studies. Reference ElHassan, Tang and Gossett22
The major determinants of cellular metabolism/homeostasis include cardiac performance, markers of target organ function, and components that evaluate oxygen delivery and consumption. Blood transfusions are typically given to improve tissue oxygenation, and infants with CHD may require multiple blood transfusions. A temporal association between the administration of transfusions in premature infants and the subsequent development of necrotising enterocolitis has been noted in several retrospective studies. Reference Kelleher, McMahon and James5 There is only one retrospective case–control report that evaluated a link in term infants with CHD. Although there was a higher transfusion rate in those infants with CHD who developed necrotising enterocolitis, causality could not be demonstrated. Reference Baxi, Josephson, Iannucci and Mahle16 In our study, we found that infants with the primary outcome were more likely to have received plasma or platelet transfusions post-necrotising enterocolitis on univariate analysis, but there was no relationship between number of transfusions and the primary outcome in the multivariable analysis.
The association of the primary outcome with central line bloodstream infections prior to the diagnosis of necrotising enterocolitis may corroborate current theories on the role of infection in the pathophysiology of necrotising enterocolitis and CHD. Reference del Castillo, McCulley and Khemani12 Del Castillo et al. report increased number of positive blood cultures in patients with CHD who develop necrotising enterocolitis. Reference del Castillo, McCulley and Khemani12 There have been several proposed infectious aetiologies for increased risk of necrotising enterocolitis (e.g., gastrointestinal dysbiosis, feed types, and changes to mucosal barriers). Reference Scahill, Graham, Atz, Bradley, Kavarana and Zyblewski23 However, these factors are also commonly found with premature-related necrotising enterocolitis and may not be exclusive to term infants with CHD. Reference Neu and Walker1,Reference Bazacliu and Neu2
The role of preoperative initiation of enteral feeding in term infants with CHD remains a controversial topic with respects to its impact on the development of necrotising enterocolitis. Furthermore, there are no data on the relationship between feeding regimens and adverse outcomes in term infants with CHD who have already developed necrotising enterocolitis. In this study, we found that achievement of full enteral feeds prior to development of necrotising enterocolitis was not associated with the primary outcome and did not lead to an increased risk of mortality. These findings suggest that the benefit of enteral nutrition may outweigh prior concerns of increasing risk of poor outcomes following from necrotising enterocolitis. We also did not observe any association between the primary outcome and the type of milk (breast milk alone, formula alone, or a combination of both). We recognise that previous literature has shown a potential protective role of breast milk over formula in the development of necrotising enterocolitis, Reference Cognata, Kataria-Hale and Griffiths24 but when mothers own breast milk is not available, we would still advocate for enteral nutrition (donor breast milk or formula) over complete cessation of enteral feeds. Infants who survived following the primary outcome required significantly longer time to reach full feeds following diagnosis of necrotising enterocolitis on the univariate analysis. A significant number of patients with the primary outcome were also unable to achieve full enteral feeds following the diagnosis of necrotising enterocolitis. In our study, close to 50% of all infants with CHD and necrotising enterocolitis required a surgically placed feeding tube. This observation may further emphasise the importance of early feeding regimen initiation given the documented trend towards earlier postoperative feeding tolerance in patients who receive preoperative feeds. Reference Toms, Jackson, Dabal, Reebals and Alten25 Further studies are now needed to explore the mechanism of these nutritional findings.
Limitations
The strengths of this study need to be interpreted within the framework of its limitations. Causality cannot be proven from the retrospective study design, and we could only identify potential associations for hypothesis generation and testing in future prospective studies. The small sample size limits our ability to incorporate more factors in the multivariable model. While moderate to severe dysfunction and central line bloodstream infection prior to the diagnosis and the need for mechanical ventilation after the diagnosis of necrotising enterocolitis were all statistically significant, the high odds ratios and larger confidence interval may suggest that the model is not stable with this relatively small sample. We used predictive covariates prior to the development of necrotising enterocolitis in this risk-adjusted model to create a composite outcome to increase the number of adverse events. The association between small for gestational age/fetal growth restriction and adverse outcomes in infants with CHD and necrotising enterocolitis may have been significant on multivariable analysis within a larger sample size, but is likely confounded by other intrauterine environment factors, delayed surgical interventions prolonging unbalanced circulation periods, baseline feeding intolerance, and increased all-cause mortality as noted in prior studies. Reference McElhinney, Hedrick and Bush3,Reference Kelleher, McMahon and James5,Reference Natarajan, Reddy Anne and Aggarwal17,Reference ElHassan, Tang and Gossett22,Reference Hammer, McEnaney, Callahan, Baird, Hoganson and Jenkins26–Reference Leaf, Dorling, Kempley, McCormick, Mannix and Brocklehurst28 While necrotising enterocolitis can also be diagnosed by ultrasound, we defined pneumatosis and presence of portal venous gas by chest radiogram only. Future studies will need to continue to look at the role of ultrasound in the diagnosis of necrotising enterocolitis in term infants with CHD. We did not account for the impact of the timing of CHD diagnosis (before or after delivery) on the primary outcomes, and future work is needed to investigate these relationships. The nutritional findings in the study are important, but further evidence regarding the percentage of breast milk versus formula and the impact of donor milk will be valuable to examine, especially with the growing evidence of the dose-dependent effects of breast milk on cardiac performance in infants. Reference El-Khuffash, Lewandowski, Jain, Hamvas, Singh and Levy29 Our cardiac ICU’s approach to nutritional support has evolved over the 20 year study period, and we suspect this may have had additional impact on the primary outcome that could not be accounted for in the analysis due to sample size. For example, in 2009, we introduced a new enteral feeding algorithm, Reference Braudis, Curley and Beaupre30 and despite this new protocol, the prevalence of necrotising enterocolitis remained relatively the same between the epoch before (2000–2009, prevalence of 1.6%) and the epoch after (2010–2020, prevalence of 2.4%). The rate of the primary outcome was also the same between the two epochs (34 versus 36%), suggesting that those infants who had higher morbidity/mortality following necrotising enterocolitis cannot simply be explained by changes in feeding practice. More complex processes are likely at play in understanding the mechanistic link to the observed associations with poor outcome. The single-centre nature of the study may not be generalisable across all centres, Reference Lau, Cruz and Ocampo4 but our cohort reflects a large centre with high surgical volumes.
Conclusions
Definitive (Bell’s stage ≥ II) necrotising enterocolitis occurred in 2.1% of term infants with CHD, with death and adverse post-necrotising enterocolitis outcomes in more than one-third of patients. The presence of moderate to severe dysfunction and central line bloodstream infection prior to diagnosis and the need for mechanical ventilation after diagnosis of necrotising enterocolitis were all independent predictors of the primary adverse outcomes. The type of cardiac lesion, ductal dependency, and cardiac intervention were all not associated with the primary outcome. Importantly, the type of enteral nutrition and achievement of full enteral feeds prior to the diagnosis of necrotising enterocolitis were also not associated with the primary outcome in our study, suggesting the benefits of enteral nutrition in these patients may outweigh prior concerns of increased risk of poor outcomes. Further studies are needed to explore the mechanism of these findings in infants with CHD and necrotising enterocolitis to best inform risk triage and prognostic counselling for families.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S104795112300121X.
Acknowledgements
None.
Financial support
None of the authors have financial relationships relevant to this article to disclose.
Competing interest
The authors declare no conflicts of interest.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation of the Belmont Report and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committee of Boston Children’s Hospital.








