Patients with CHD are at an increased risk of thromboembolic complications and frequently require anticoagulation to prevent thrombosis. Reference Giglia, Massicotte and Tweddell1 Current guideline recommendations for anticoagulation in paediatric CHD are based on low-quality evidence and have not been updated in almost a decade leading to significant variability in clinical practice regarding when to use anticoagulation and the choice of agent. Reference Giglia, Massicotte and Tweddell1,Reference Monagle, Chan and Goldenberg2 Although evidence for the use of direct-acting oral anticoagulants in paediatrics in general and in CHD specifically is increasing, lack of experience, long-term data, and the recommendation against use in patients with mechanical heart valves limits their use in many patients. Reference Otto, Nishimura and Bonow3 Likewise, low-molecular weight heparin requires twice daily injections making it challenging for patients and families. Therefore, vitamin K antagonists remain a frequently prescribed agent in patients with CHD and the only evidence-based oral anticoagulant in patients with mechanical valve prosthesis. Reference Otto, Nishimura and Bonow3
Anticoagulant therapy with the vitamin K antagonist, warfarin, requires intensive monitoring of a patient’s international normalised ratio (INR) to balance therapeutic effectiveness and potential adverse effects due to its narrow therapeutic index. Several studies and guidelines highlight the importance of maintaining the international normalised ratio within the target or a percentage of time in therapeutic range of greater than 65% as it is associated with improved safety and efficacy. Reference Kaatz4–Reference Faircloth, Miner and Alsaied6 Recent reports of patients with CHD have revealed a time in therapeutic range of only 42–45% emphasising the need for improvement in anticoagulation management in this patient population. Reference Basmaji, Samuel and Shohoudi7,Reference Portman, Jacobs and Newburger8
The benefits of a pharmacist-managed anticoagulation clinic in adults have been well documented in the literature and have demonstrated a higher time in therapeutic range with lower rates of bleeding, thromboembolic events, and hospitalisations. Reference Noor, Khan, Warsi, Aseeri and Ismail9–Reference Alghadeeer, Alzahrani, Alalayet, Alkharashi and Alarifi13 In addition to better clinical outcomes, pharmacist-managed anticoagulation clinics have shown improved patient and physician satisfaction. Reference Lodwick and Sajbel14,Reference Bishop, Young, Twells, Dillon and Hawboldt15 However, there is limited literature describing use of this model in paediatric institutions or in patients with CHD.
The Anticoagulation Program within our Heart Institute shifted from a nurse-physician-managed model to a pharmacist-managed model in 2013 with the hypothesis that this transition would result in improvement in the quality and consistency of anticoagulation. This model is a pharmacist-led, nursing-supported, collaborative model that provides warfarin and enoxaparin management through a consistent, centralised team. This model allows the pharmacist to prescribe and adjust anticoagulation as well as order and evaluate laboratory results. Responsibilities of this programme include all outpatient warfarin and enoxaparin prescriptions, monitoring including proactive reminders, counselling, procedural planning, and a dedicated nursing phone line. In addition, the quality metric, percent time in therapeutic range, is assessed monthly to ensure that it is appropriately maintained above a standard benchmark of 65% that is considered good quality anticoagulation control. Reference Kaatz4–Reference Faircloth, Miner and Alsaied6,Reference Connolly, Pogue and Eikelboom16–Reference Lip, Banerjee and Boriani18
Materials and method
This was a single-centre retrospective cohort study to evaluate the impact of a practice change to improve anticoagulation management at a paediatric heart centre. The evidence-based quality metric, time in therapeutic range, was used to assess the outcome of transitioning from a nurse-physician-managed model to a pharmacist-managed model. This was a quality improvement initiative and therefore considered exempt by the CCHMC institutional review board. Time in therapeutic range is calculated at our institution using the Rosendal method through Dawn AC® Anticoagulation Software based on patient international normalised ratio values that are manually entered into this system by the nursing team. Reference Rosendaal, Cannegieter, van der Meer and Briet19 The therapeutic values utilised by this software include the international normalised ratio target range established by the primary cardiologist±0.2 except for patients with a lower end goal of 1.5, which is the lower limit set in the software. International normalised ratios are excluded during periods of time when patients are instructed to hold warfarin due to procedures and international normalised ratios obtained during hospital admissions.
Models
Nurse-physician-managed model: Prior to November 2013, our institution utilised a nurse-physician-managed model for anticoagulation management. This was a non-protocol driven model without a centralised provider for warfarin management. There was one registered nurse who was involved in management of most warfarin patients. The registered nurse worked with each individual primary cardiologist to develop an anticoagulation plan and answer questions. However, there was no structured follow up or referral process, dedicated anticoagulation phone, organised method to track patients, centralised team, or proactive reminders.
Pharmacist-managed model: In November of 2013, a pharmacist-managed model was implemented by the Anticoagulation Program within our Heart Institute. Within this model, clinical pharmacists with specialised residency training are credentialed as part of the medical staff with a collaborative practice agreement allowing independent prescribing and monitoring of medication therapy. This new model also included proactive reminders for laboratory monitoring, standardised documentation of assessments and plans, and a dedicated anticoagulation telephone number monitored by a registered nurse within the Anticoagulation Program during daytime hours. The centralised team, consisting of a pharmacist and a nurse, answers anticoagulation-related questions, follows up on labs, initiates patient interviews, relays follow-up plans, and maintains a calendar with required laboratory monitoring listed for each patient enrolled in the programme. The pharmacists also proactively alter therapy for patient illness, dietary and/or formula changes, and new drug–drug interactions. For procedures, the pharmacist is responsible for communicating with proceduralists and primary cardiologists to develop patient-specific anticoagulation plans including enoxaparin bridging if appropriate and the team provides calendars to patients with medication instructions for all procedures. After hours, questions or concerns are fielded by Cardiology fellows on call with the pharmacists available for dosing and management questions. One consistent pharmacist is responsible for management of anticoagulation within the programme with a second pharmacist available for coverage when needed.
Cohorts
Model comparison cohort: Patients managed within our Heart Institute receiving warfarin for the full- time period between November 2011 and November 2014 were identified and included in the model comparison cohort. For the model comparison cohort, the time in therapeutic range was evaluated for the nurse-physician-managed model from November 2011 to November 2012 and for the pharmacist-managed model from November 2013 to November 2014. Patient characteristics reported are from the start of the time period evaluated. The time between December 2012 and October 2013 was not included as programme changes and interventions were being implemented and this time was considered a washout period. The programme was fully implemented by November 2013. The percent time in therapeutic range for each model during this time was compared using a paired t-test.
2022 cohort: Patients enrolled in our Heart Institute Anticoagulation Program receiving warfarin on 31st January, 2022 were identified and included in the 2022 cohort to describe this patient population and compare to the earlier model comparison cohort.
Patient characteristics for both cohorts including demographics, primary cardiologist, indication for anticoagulation, risk factors for thrombosis in patients with Fontan physiology (abnormal thrombophilia profile, history of thromboembolism, atrial arrhythmia, ventricular dysfunction, atrio-pulmonary Fontan, obesity, presence of a stent within the Fontan circuit, open fenestration, or pulmonary artery stump), mechanical valve data, international normalised ratio goal, and use of a home monitor were assessed. Home international normalised ratio testing was utilised when feasible based on insurance coverage and patient comfort. Patients report all results to the anticoagulation nurse and each international normalised ratio obtained via home monitors was assessed by the Anticoagulation Program.
Anticoagulation Program Quality Metrics: Data that were tracked by the Anticoagulation Program including monthly time in therapeutic range since November 2013, number of patient’s enrolled since 2015, number of international normalised ratios evaluated since November 2016, and new patient referrals since 2016 were included to describe the programme. This data was tracked by programme nurses and was reflective of the patients enrolled in the Anticoagulation Program at the end of each month.
Results
Model comparison cohort
Fifty-eight patients were managed for the full-time period between November 2011 to November 2014 by both the nurse-physician-managed model and the pharmacist-managed model and included in the model comparison cohort (Table 1). The median age for this cohort was 19 years (range: 13 months–58 years) with 57% being ≥18 years old. The most common primary indications for warfarin in this cohort were mechanical valve replacement (n = 29, 50%) and Fontan physiology (n = 22, 37.9%). For this cohort, the most common international normalised ratio goals were 2 to 3 (55%, n = 32) and 2.5 to 3.5 (37.9%, n = 22) (Table 1). This cohort consisted of patients with fourteen primary cardiologists. Each of these physicians individually managed their patients on warfarin in the nurse-managed model and one primary pharmacist managed all the patients on warfarin in the pharmacist-managed model.
Table 1. Study cohorts.

INR = international normalised ratio.
1 For patients with mechanical aortic and atrioventricular valve valves, the indication was categorised as mechanical atrioventricular valve.
2 Other: Biventricular repair with baffle, Kawasaki disease with dilated aneurysm, Stented Pott’s shunt, Coronary artery fistula closure, left ventricular dysfunction with history of stroke, bioprosthetic mitral valve replacement, atrial fibrillation.
3 Data not available.
In the model-comparison cohort, there was significant improvement in time in therapeutic range with the change from the physician-nurse-managed model to the pharmacist-managed model, 65.7% (12,897 of 19,636 days) versus 80.6% (18,650 of 23,131 days), p < 0.001 (Table 2)
Table 2. Comparison of time in therapeutic range (TTR) between the cohorts.

1 P < 0.001 compared to pre-intervention time in therapeutic range.
2022 Cohort
One hundred nineteen patients were enrolled in the Anticoagulation Program in January of 2022 and were included in the 2022 cohort (Table 1). The median age was 24 years (range: 19 months to 69 years) with 70% ≥ 18 years old. The most common primary indication for warfarin was mechanical valve replacement (n = 81, 68%) and the most common type of mechanical valve used was On-X for aortic valve replacements and St Jude for mitral valve replacements (Table 3). Thirty-three (28%) patients in the 2022 cohort had Fontan physiology, 4/33 were anticoagulated for a mechanical valve replacement, 15/33 were anticoagulated for secondary prophylaxis due to a history of thrombosis, and 14/33 were anticoagulated for primary prophylaxis with no history of thrombosis.
Table 3. Anticoagulation Program 2022 cohort mechanical valve types.
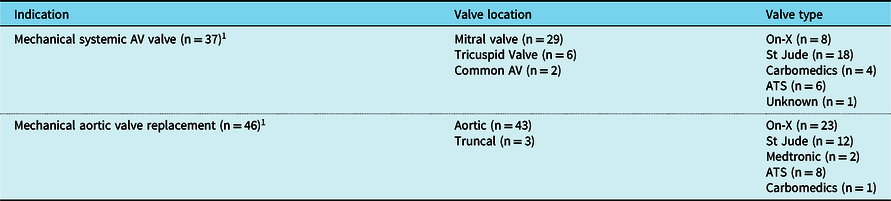
AV = atrioventricular.
1 Two patients had both aortic and mitral mechanical valve replacements.
The most common international normalised ratio goals for the 2022 cohort were 2 to 3 (44%, n = 53) and 2.5 to 3.5 (37%, n = 44) (Table 1). Fifty percent of patients utilised home monitors for point-of-care international normalised ratio testing. Patients of twenty-three different primary cardiologists were enrolled in the Anticoagulation Program. One primary pharmacist was the provider responsible for warfarin management of all patients enrolled in the Anticoagulation Program including all prescriptions and laboratory monitoring with a second pharmacist available for coverage when needed.
Anticoagulation Program data
Under the pharmacist-managed model, the Anticoagulation Program maintained a time in therapeutic range above the benchmark of 65% from November 2013 to December 2022 with a median monthly time in therapeutic range of 82% (range: 72–92%). The median number of international normalised ratios addressed per business day from 2016 through 2022 was 12 (Range: 3–27). Table 4 displays the available Anticoagulation Program data broken down by year.
Table 4. Heart institute Anticoagulation Program data 2014 through 2021.

* Data not included unless tracked for all months of calendar year.
Discussion
The transition to a pharmacist-managed model by the Anticoagulation Program at our paediatric heart institute demonstrated significant, meaningful improvement in time in therapeutic range from 66 to 81% that was consistently sustained by the programme from 2013 to 2022. This is above the reported time in therapeutic range ranging from 55 to 68% in large landmark clinical trials and the established threshold for good quality anticoagulation control that is associated with better clinical outcomes. Reference Connolly, Pogue and Eikelboom16,Reference Connolly, Ezekowitz and Yusuf20–Reference Patel, Mahaffey and Garg22 The time in therapeutic range maintained at our programme was also markedly above the time in therapeutic range of 41.9% reported in a recent multi-centre study of 567 patients with CHD receiving vitamin K antagonists and 45% reported in paediatric patients with cardiac disease in the setting of a randomised control trial. Reference Basmaji, Samuel and Shohoudi7,Reference Portman, Jacobs and Newburger8
Several studies and guidelines highlight the importance of maintaining a higher time in therapeutic range with vitamin K antagonists as lower time in therapeutic ranges are associated with increased thromboembolic and bleeding events. Reference Kaatz4–Reference Faircloth, Miner and Alsaied6,Reference Jones, McEwan, Morgan, Peters, Goodfellow and Currie23 In a previous study evaluating patients with CHD, thromboembolic events were significantly more likely in patients with a higher percentage of time below therapeutic range, and haemorrhagic events were significantly more likely in patients with a higher percentage of time above therapeutic range. Reference Basmaji, Samuel and Shohoudi7 The association of improved clinical outcomes with a higher time in therapeutic range and reported difficulty maintaining a high time in therapeutic range in this patient population emphasises the importance of implementing measures to improve quality of anticoagulation management. Several studies including systematic reviews and meta-analyses have demonstrated improvement in time in therapeutic range and outcomes with a pharmacist-managed model. Reference Noor, Khan, Warsi, Aseeri and Ismail9–Reference Alghadeeer, Alzahrani, Alalayet, Alkharashi and Alarifi13 Despite this, evidence for use in paediatrics or CHD is limited.
Similar to the recently published multi-centre study in patients with CHD, our patient population also primarily consisted of patients with CHD. Patients in that report were anticoagulated for atrial arrhythmias (63%), Fontan palliation (58%) or both, primarily with a goal international normalised ratio of 2 to 3 (90.7%), and patients with mechanical heart valves were not included. Reference Basmaji, Samuel and Shohoudi7 Compared to this report, the percentage of patients with Fontan physiology without mechanical valves was lower in our earlier comparison-model cohort (38%) and even lower in our 2022 cohort (24%) and few patients in either cohort were anticoagulated with a vitamin K antagonist for atrial arrhythmias alone, likely due to the increased use of direct-acting oral anticoagulants for these indications. The use of warfarin anticoagulation for indications other than mechanical valve replacement decreased from 50% in the model comparison cohort (2011–2014) to 32% in the 2022 cohort. This decrease was expected based on the increasing body of evidence and comfort using direct-acting oral anticoagulants in both adult and paediatric patients with CHD. Furthermore, we expect warfarin use in these specific patients will further decrease as direct-acting oral anticoagulants become more widely accepted and utilised.
In contrast, the percentage of patients with mechanical valve replacements in our cohorts increased from 50% (2011–2014) to almost 70% (2022) without a decrease in number of overall patients. Current guidelines for this population recommend anticoagulation with vitamin K antagonists and against the use of direct-acting oral anticoagulants highlighting the importance of quality anticoagulation in this high-risk population. Reference Otto, Nishimura and Bonow3 Our programme’s patient population also consisted of more paediatric patients than the previous report in CHD. Reference Basmaji, Samuel and Shohoudi7 Although there is limited literature regarding time in therapeutic range in paediatrics, a recent randomised control trial comparing warfarin to edoxaban in paediatric cardiac patients reported a time in therapeutic range of 45% in the warfarin arm, which is considerably lower than demonstrated by our Anticoagulation Program.
Most non-physician-driven warfarin dosing programmes are protocol driven allowing medication changes to be made by standardised weekly percentages based on international normalised ratio results. These protocols are based primarily on adult data related to dosing, pharmacokinetics, and pharmacodynamics. Applying these protocols to paediatric patients is difficult. Factors such as diet, administration-related challenges, and frequent illness in school-age children complicate warfarin management and lead to increased risk for labile international normalised ratios. In addition, drug metabolism differs in children as compared to adults, and warfarin is metabolised in the liver via the cytochrome P450 enzyme systems. Routes of administration also differ in that most adults can swallow whole tablets whereas paediatric patients may require fractions of tablets and/or may require administration via a tube (gastric, nasogastric, jejunum). There are also many dietary considerations specific to children such as use of formula or breast milk, which vary significantly in vitamin K content, and there are usually frequent changes in intake throughout development. Allowing a pharmacist with expertise in this area to manage warfarin adds an additional benefit as pharmacists have specialised training in medications, including specific knowledge of the pharmacokinetic and pharmacodynamic properties of drugs. With warfarin being a narrow therapeutic index medication, having this specialised knowledge and experience helps maintain a high time in therapeutic range, thereby maximising efficacy and minimising adverse events.
The importance of a centralised, consistent anticoagulation team particularly at a large institution is emphasised by our data showing that twenty-three cardiologists had patients enrolled in our programme in the 2022 cohort with most physicians functioning as the primary cardiologist for only one or two patients. The transition from the previous model where each physician managed a small number of patients on warfarin without a structured or consistent approach to the new model utilising a consistent provider, a pharmacist with specialised training and expertise, was a key intervention resulting in the improvement in time in therapeutic range and ability to maintain high-quality anticoagulation since inception of the programme. In addition, we believe a dedicated, consistent registered nurse for the Anticoagulation Program responsible for structured follow up and management was crucial to the success of the programme.
When possible, the Anticoagulation Program facilitates the use of home international normalised ratio point-of-care testing, and fifty percent of the 2022 cohort utilised this method of testing. However, insurance coverage is the most common barrier for use. The high utilisation of home monitoring by our programme allows for closer monitoring in this high-risk population and makes it substantially easier for families, avoiding the need for transportation or traumatic venipunctures. Numerous studies have established no difference or improved clinical outcomes for patients utilising home monitoring versus usual medical care with significant improvements in quality of life and patient satisfaction. Reference van Zyl, Wysokinski, Jaeger, Casanegra, Gersh and McBane24–Reference Matchar, Jacobson and Dolor28
There are several limitations to this analysis including the retrospective design. Our centre also utilises a pre-programmed expanded international normalised ratio goal range of ± 0.2 to calculate time in therapeutic range. This approach has been described in several studies and use of this expanded range for dose modifications was shown to result in a higher time in therapeutic range. Reference Rose, Ozonoff, Berlowitz, Henault and Hylek29,Reference Hou, Yang, Ye, Wang, Liu and Cui30 However, there is no standard benchmark time in therapeutic range specific to this expanded international normalised ratio goal or the paediatric population. Literature assessing time in therapeutic range has mostly evaluated adult patients with atrial fibrillation with a goal international normalised ratio of two to three. In contrast, a large percentage of patients in our analysis was anticoagulated with warfarin for mechanical heart valves with a higher international normalised ratio goal of 2.5–3.5. Some studies have suggested an increased risk of bleeding and lower time in therapeutic range in patients with higher international normalised ratio goals. Reference van Zyl, Wysokinski, Jaeger, Casanegra, Gersh and McBane24,Reference Labaf, Svensson, Renlund, Jeppsson and Sjalander31,Reference Eikelboom, Connolly and Brueckmann32 Despite some of these differences, applying a time in therapeutic range of greater than 65% as a standard for quality anticoagulation control in this study population is likely applicable based on evidence for its utility in general practice. In addition, a limitation of our study was the inability to evaluate the direct impact of the transition to a pharmacist-managed model on thrombotic events, bleeding events, hospital admissions, or emergency department visits. This data was not consistently available or documented for all patients over the time period analysed. However, other studies have demonstrated improvement in time in therapeutic range as an accurate predictor of reducing adverse events. Reference Faircloth, Miner and Alsaied6,Reference Van Den Ham, Klungel, Leufkens and Van Staa33–Reference Wan, Heneghan and Perera35
Warfarin management is challenging even in the most ideal environment but many factors including large variation in practice, volume of providers, patient age, and lack of specific literature or guidelines contribute to difficulties at paediatric institutions and in patients with CHD. However, evidence highlights that improving time in therapeutic range should improve clinical outcomes. Therefore, resources should be utilised to improve anticoagulation management in these patients. This study demonstrated a significant increase in time in therapeutic range and the ability to consistently maintain this high-quality anticoagulation management through a practice change incorporating a collaborative, centralised, pharmacist-managed model.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Competing interests
None.







