Volume 19 - Issue 4 - September 1984
Research Article
Ferrous-iron-bearing vermiculite-smectite series formed during alteration of chlorite to kaolinite, Otago Schist, New Zealand
-
- Published online by Cambridge University Press:
- 09 July 2018, pp. 509-520
-
- Article
- Export citation
The influence of aluminium on iron oxides: X. properties of Al-substituted goethites
-
- Published online by Cambridge University Press:
- 09 July 2018, pp. 521-539
-
- Article
- Export citation
Diffraction effects calculated for structural models of K-saturated montmorillonite containing different types of defects
-
- Published online by Cambridge University Press:
- 09 July 2018, pp. 541-561
-
- Article
- Export citation
Mode d'empilement des feuillets dans la vermiculite sodique hydratee a une couche (phase a 11·85 Å)
-
- Published online by Cambridge University Press:
- 09 July 2018, pp. 563-578
-
- Article
- Export citation
Hydrothermal clay mineral formation in a biotite-granite in northern Switzerland
-
- Published online by Cambridge University Press:
- 09 July 2018, pp. 579-590
-
- Article
- Export citation
The rapid formation of crystalline double hydroxy salts and other compounds by controlled hydrolysis
-
- Published online by Cambridge University Press:
- 09 July 2018, pp. 591-603
-
- Article
- Export citation
Etude par spectrographie infrarouge d'un encroutement calcaire sous galet. Mise en evidence et modelisation experimentale d'une suite minerale evolutive a partir de carbonate de calcium amorphe
-
- Published online by Cambridge University Press:
- 09 July 2018, pp. 605-614
-
- Article
- Export citation
Comportement des ions aluminiques et de la silice en solution: Etude de la formation de la kaolinite
-
- Published online by Cambridge University Press:
- 09 July 2018, pp. 615-627
-
- Article
- Export citation
L'‘halloysite’ blanche riche en fer de vate (vanuatu)—hypothese d'un edifice interstratifie halloysite-hisingerit
-
- Published online by Cambridge University Press:
- 09 July 2018, pp. 629-643
-
- Article
- Export citation
Las bentonitas sedimentarias de la formacion fardes, Granada, España
-
- Published online by Cambridge University Press:
- 09 July 2018, pp. 645-652
-
- Article
- Export citation
Re-examination of the kinetics of the thermal dehydroxylation of kaolinite
-
- Published online by Cambridge University Press:
- 09 July 2018, pp. 653-661
-
- Article
- Export citation
Note
The use of nuclear magnetic resonance (NMR) for the determination of tetrahedral aluminium in montmorillonite
-
- Published online by Cambridge University Press:
- 09 July 2018, pp. 663-667
-
- Article
- Export citation
Constitution of and relationships among volkonskoites
-
- Published online by Cambridge University Press:
- 09 July 2018, pp. 669-671
-
- Article
- Export citation
Notes
Surface acidity and catalytic activity of a modified sepiolite
-
- Published online by Cambridge University Press:
- 09 July 2018, pp. 673-676
-
- Article
- Export citation
Book Review
V.A. Frank-Kamenetskii, N.V. Kotov & E.A. Goilo Transformatsionnye Preobrazovaniya Sloistykh Silikatov pri Povyshennykh p-T-Parametrakh [Transformational Reorganization of Layer Silicates Under Elevated p-T Conditions]. Izd. Nedra, Leningrad, 1983. 151 pp., 11 Tables, 51 Figures. Price Rbl. 1·80 (£1·60).
-
- Published online by Cambridge University Press:
- 09 July 2018, p. 677
-
- Article
- Export citation
Front matter
CLM volume 19 issue 4 Cover and Front matter
-
- Published online by Cambridge University Press:
- 09 July 2018, p. f1
-
- Article
-
- You have access
- Export citation

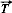 selon l'axe 0
selon l'axe 0