Halloysite was mechanically ground in the presence of NaCl, KCl, RbCl, RbCl and CsCl, and i.r. spectra were recorded after various periods of grinding, up to 10 min. Changes in the wave-numbers and sharpness of absorption bands were interpreted using assignments accepted for kaolinite. Diminution of absorption bands of OH groups and the appearance of new bands indicate formation of complexes of halloysite with KCl, KBr, RbCl, and CsCl. Water was essential in the complex formation, which may be formulated:
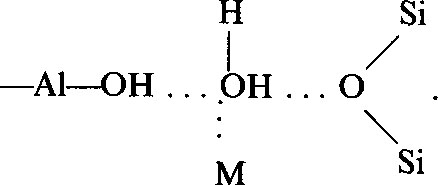
The amount of the complex formed depended on the salt and the time of grinding. Complex formation requires that the cation be large enough to serve as a “structure breaker” of the interlayer water sheet. Complex formation also requires that the cation be a weak electron acceptor. Grinding also led to changes in the fine structure of the tactoid.
The possibility of using the i.r. spectrum of halloysite as a finger print for identification of the mineral is discussed. It was concluded that NaCl is the most reliable dispersing agent to determine whether or not a mineral sample belongs to the kaolinite group. Disks in RbCl may be used to differentiate between kaolinite and hydrated halloysite.