INTRODUCTION
Human papillomavirus (HPV) infection has been established as the primary cause in the development of cervical intraepithelial neoplasia (CIN) and cervical cancer [Reference Schiffman1]. However, HPV infections are usually transient and mostly regress to a virus-free status or low-grade lesions [Reference Ho2]. By contrast, persistent infection with carcinogenic HPV genotypes causes CIN lesions and cervical cancer development [Reference Ho2, Reference Schiffman3]. HPV persistence has been reportedly associated with viral variants such as viral genotypes, multiple infections, and viral loads [Reference de Freitas4] as well as host genetic variability, especially in genes that control the immune response [Reference de Araujo Souza, Sichero and Maciag5]. Several host epidemic characteristics including older age [Reference Ho2, Reference Lai6], multiple sexual partners [Reference Ho2, Reference Schmeink7], smoking [Reference Schmeink7], individual immune responsiveness [Reference Hibma8], and oral contraceptive use [Reference Nielsen9] have also been reportedly associated with HPV persistence, but the results related to these epidemiological factors are highly controversial.
Alcohol is a potential risk factor of oral, oesophageal, colorectal, and liver cancers [10] and has been reported to increase the risk of breast, endometrial, and ovarian cancers by enhancing oestrogen levels and metabolism [Reference Rod11]. However, epidemiological evidence for cervical cancer is lacking and controversial [Reference Hjartaker, Meo and Weiderpass12]. Alcohol is known to be a potential risk factor of HPV infection (acquisition), but there are few studies on the association of alcohol consumption and HPV persistence [Reference Ho2, Reference Goodman13]. Furthermore, no studies have reported on specific alcohol consumption behaviours such as frequency, duration, and specific types of alcoholic beverages consumed, relative to HPV persistence.
The aim of our prospective study was to investigate the alcohol consumption characteristics (drinking status, frequency, duration of drinking, and the amount of alcoholic beverages consumed) in association with a high risk (HR)-HPV persistence according to the subjects' health screening data.
METHODS
Study design and population
A total of 10 444 Korean women who underwent health-screening examinations at the National Cancer Center from 2002 to 2011 and provided signed informed consent were included in this study. This health screening examinee study is part of the Korean Prospective Study for the Transition of Human Papillomavirus into Cervical Carcinoma (KOVIC). KOVIC is the prospective study established to identify host and environmental co-factors in the persistent HPV infection and its transformation into cervical carcinoma in Korean women. KOVIC is composed to two studies, a health examinee-based prospective study and a patient-based prospective study. There is an ongoing follow-up investigation. The study protocol was approved by the Institutional Review Boards and Ethics Committee of the National Cancer Center in Korea (NCCNCS-11-433). After excluding 558 women who did not participate in HR-HPV deoxyribonucleic acid (DNA) testing and 656 women who did not complete the questionnaire about detailed alcohol consumption behaviours, a total of 9230 women were available for HPV infection analysis. We collected HPV DNA data, cytology findings (Pap smear), and questionnaires about detailed alcohol consumption behaviours, sociodemographics, smoking history, parity, menopausal status, and oral contraceptive use for these 9230 women. Among them, 1284 and 543 women with a cytological result of low-grade squamous intraepithelial lesion (LSIL) or less for the study period were available for 1- and 2-year follow-up studies, respectively, after excluding six subjects. Five subjects and one subject had cytology findings of high-grade squamous intraepithelial lesion (HSIL) or worse in the 1- and 2-year follow-up, respectively.
Two definitions of HR-HPV persistence were used in this study. First, 1-year HPV persistence was defined as HR-HPV positivity in the enrolment year and the 1-year follow-up study year, and 2-year HPV persistence was defined as HR-HPV positivity in the enrolment year and both the 1- and 2-year follow-up study years. One- and 2-year HPV negatives were defined as HPV negativity in the 1-year follow-up study year and as HPV negativity in both the 1- and 2-year follow-up study years, respectively, after enrolment with HPV negativity. According to these definitions, 10·1% of the 9230 women were HPV positive (negative, 8301; positive, 929) at enrolment. In the follow-up study, 127 and 46 women were included in the 1- and 2-year HPV-persistence categories, and 949 and 373 women were included in the 1- and 2-year HPV-negative categories, respectively.
An additional analysis was performed to compare the statuses of those cleared of HR-HPV and those still infected by HR-HPV for the two follow-up years. Two-year consecutive or alternate HR-HPV persistence was defined as HPV positivity/HPV positivity, as HPV positivity/HPV negativity, or as HPV negativity/HPV positivity in both the next year and the subsequent year, respectively, after enrolment with HPV positivity. Two-year consecutive HR-HPV clearance was defined as HPV negativity in both the next year and the subsequent year after enrolment with HPV positivity. By this definition, 140 women (2-year consecutive or alternate persistence: 89, 2-year consecutive clearance: 51) were included in the second analysis.
Questionnaires related to alcohol consumption behaviours
General characteristics were analysed with the questionnaire that included age, height, weight, marital status, number of children, menopausal status, oral contraceptive use, education level, income level, and cigarette smoking status. A detailed questionnaire about alcohol consumption included drinking status (current, former, never), the frequency of alcohol consumption (once/month, 2–3 times/month, once/week, 2–3 times/week, 4–5 times/week, every day, twice/day), duration of drinking habit (<5 years, ⩾5 years), and usual consumption per drinking occasion of two alcoholic beverages, beer and soju at 200 cc and 50 cc, respectively (1 glass, 2 glasses, ⩾3 glasses). Soju is a distilled beverage native to Korea and its alcohol content is usually about 20% ethanol by volume.
HPV DNA detection and Pap smear
HR-HPV DNA detection was performed with the commercially available Hybrid capture II system (HC-II, Digene Co., USA). A chemiluminescent HPV DNA test was measured as relative light units (RLU) with a probe designed for 13 types of HR-HPV (HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68). The test results were read as positive at concentrations of ⩾1 pg/ml of RLU/cut-off ratio (RLU of specimen/mean RLU of two positive controls). The cytological grades for Pap smear reports were based on the Bethesda classification system [Reference Solomon14]. Types of results in this system included normal cells, atypical squamous cells, LSIL, HSIL, squamous cell carcinoma, atypical glandular cells, and adenocarcinoma in situ.
Statistical analysis
The χ 2 test and t test were used to analyse differences in the distributions of categorical and continuous general variables, respectively. Multivariate logistic regression models were used to estimate the odds ratios (ORs) and corresponding 95% confidence intervals (CIs). A regression analysis was performed to assess the association between several characteristics related to alcohol consumption and HR-HPV infection (vs. HPV negativity) and between those and 1- and 2-year HR-HPV persistence groups (vs. 1- and 2-year HR-HPV negative groups, respectively). The model was adjusted for age, body mass index (BMI), marital status, parity, menopausal status, oral contraceptive use, education level, income level, and smoking habit as categorical variables indicated in Table 1. Another multivariate logistic regression analysis was performed to estimate the risk of 2-year consecutive or alternate HR-HPV persistence (vs. HR-HPV clearance) associated with the characteristics related to alcohol consumption after adjustment for age, BMI, parity and education level as categorical variables. The risk estimates were calculated using the non-drinkers or non-alcoholic characteristics as the non-exposure category. The statistical analysis and graphical design were performed with SAS v. 9·1 (SAS Institute, USA) and Stata v. 12·0 (Stata Corp., USA) software packages.
Table 1. General characteristics of study subjects at baseline (n = 9230)
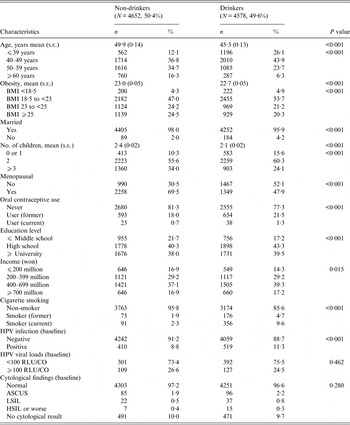
BMI, Body mass index; RLU/CO, relative light units/cut-off; ASCUS, atypical squamous cells of undetermined significance; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion.
Data in this table indicates the number of subjects (n) and the percentage of subjects (%) among the enrolled non-drinkers and drinkers.
P values for the categorical and continuous variables were obtained from χ 2 test and t test, respectively.
RESULTS
General characteristics of the study subjects at baseline
As shown in Table 1, about 50% of the 9230 enrolled subjects were drinkers (4578). The mean ages of the non-drinkers and drinkers were 50·1 and 45·5 years, respectively. The drinkers were younger (~5 years) and less obese than the non-drinkers. Most of the subjects were married, and slightly more of the drinkers were single. Fewer children (2·4 of non-drinkers vs. 2·1 of drinkers) and greater rates of oral contraceptive use were observed in the drinkers (18% of non-drinkers vs. 23% of drinkers). The drinkers were more educated than the non-drinkers, and more of the drinkers were also smokers (4% of non-drinkers vs. 15% of drinkers). The drinkers had a higher rate of HR-HPV infection than the non-drinkers (9% and 11% of non-drinkers and drinkers, respectively).
Alcohol consumption and the risk of HR-HPV infection
Current drinkers (OR 1·21, 95% CI 1·07–1·41) and those with a higher frequency of drinking (2–4 times/month: OR 1·28, 95% CI 1·04–1·56; ⩾2 times/week: OR 1·31, 95% CI 1·05–1·64; P for trend = 0·049) had higher risks of HR-HPV infection than the non-drinkers (Table 2). Women with a drinking habit for ⩾5 years had a higher risk of HR-HPV infection (OR 1·20, 95% CI 1·01–1·36) than those with a <5-year drinking habit. Increased consumption of the alcoholic beverages beer and soju was associated with increased risk of HR-HPV infection (P for trend <0·001 and <0·006 for glasses of beer or soju normally consumed; ⩾3 glasses of soju: OR 1·24, 95% CI 1·03–1·68).
Table 2. Odds ratios for persistent infection of high risk-human papillomavirus (HPV)

The subject number for each characteristic is based on the available data rather than all 9230 subjects who answered the indicated questions.
* Enrolment prevalence indicates the HR-HPV infection at the time of enrolment (vs. HR-HPV negativity at enrolment).
† 1-year and 2-year HR-HPV persistence were defined as HPV positivity in the 1-year follow-up study year and as HPV positivity in both the 1- and 2-year follow-up study years, respectively, after enrolment with HR-HPV positivity (vs. 1- and 2-year HR-HPV negatives defined as HR-HPV negativity for 1 and 2 consecutive years, respectively, after enrolment with HR-HPV negativity).
‡ Odds ratios and 95% confidence intervals were calculated using a multivariate logistic regression analysis (adjusted for age, BMI, marital status, number of children, menopausal status, oral contraceptive use, education level, income level, and smoking status as categorical variables).
§ The P value is for the linear trend of multivariate odds ratios (adjusted for age, BMI, marital status, number of children, menopausal status, oral contraceptive use, education level, income level, and smoking status as categorical variables).
¶ Usual amount is defined as the amount of beer or soju (in glasses) normally consumed per occasion.
|| Soju is a popular alcoholic beverage in Korea with a pure alcohol content (%) about 4–5 times greater than that of beer.
Alcohol consumption and 1- and 2-year HPV persistence
Compared to non-drinkers, the current alcohol drinkers had higher risks of 1- and 2-year HR-HPV persistence; the OR of current drinkers was 1·56 (95% CI 1·09–2·24) for the risk of 1-year HR-HPV persistence (vs. 1-year HR-HPV negative) and 2·49 (95% CI 1·32–4·71) for the risk of 2-year HR-HPV persistence (vs. 2-year HR-HPV negative) (Table 2). Drinkers who consumed alcohol ⩾2 times/week had a higher risk of 1-year HR-HPV persistence (OR 1·80, 95% CI 1·01–3·36) than non-drinkers. Drinkers with a ⩾5-year drinking habit had higher risks of 1-year HR-HPV persistence (OR 1·77, 95% CI 1·19–2·62) and 2-year HR-HPV persistence (OR 2·33, 95% CI 1·17–4·63) than women with a <5-year drinking habit. The risk of 2-year HR-HPV persistence showed an increasing trend with more glasses of beer consumed (P for trend = 0·025), and in particular, drinkers who consumed ⩾3 glasses of beer had a threefold increased risk of 2-year HR-HPV persistence, compared to non-drinkers (OR 3·62, 95% CI 1·35–9·75).
Alcohol consumption and 2-year consecutive or alternate HR-HPV persistence
As shown in Figure 1, compared to non-drinkers, the alcohol drinkers had a higher risk of 2-year consecutive or alternate HR-HPV persistence (OR 2·68, 95% CI 1·10–6·51) (vs. 2-year consecutive HR-HPV clearance). Women who normally consumed ⩾2 glasses of beer or soju in a single occasion had a higher risk of 2-year consecutive or alternate HR-HPV persistence compared to those who consumed ⩽1 glass of these alcoholic beverages (OR 2·90, 95% CI 1·06–7·98). The drinking duration (⩾5 years) and frequency (⩾2 times/month) demonstrated an increasing trend for the risk although these differences were not significant (OR 1·87, 95% CI 0·71–4·92 and OR 2·60, 95% CI 0·80–8·42, respectively).

Fig. 1. Odds ratios for 2-year consecutive or alternate high-risk human papillomavirus persistence associated with the characteristics related to alcohol consumption such as ‘alcohol drinkers’ (non-drinkers vs. drinkers); ‘usual amount of alcoholic beverages’ [2 glasses (50 ml/glass) of beer or soju vs. ⩾2 glasses]; ‘duration of drinking’ (<5 years vs. ⩾5 years); and ‘frequency of drinking’ (<2 times/month vs. ⩾2 times/month). A multivariate logistic regression analysis was performed after adjustment for age, body mass index, education level, and the number of children.
DISCUSSION
Our findings demonstrated that alcohol consumption and its associated behaviours, including the frequency of alcohol consumption, duration of drinking habit, and amount of alcoholic beverages consumed increased the risk of 1- and 2-year HR-HPV persistence (vs. 1- and 2-year HR-HPV negatives). Alcohol consumption and a high regular consumption of alcoholic beverages increased the risk of 2-year consecutive or alternate HR-HPV persistence (vs. 2-year consecutive HR-HPV clearance after enrolment with HR-HPV positivity).
Although alcohol has been reported as a potential risk factor of cervical cancer and HPV infection [Reference Ho2, Reference Goodman13], no studies have investigated the association between specific alcohol behaviours and HPV persistence in the cervix. Our study is the first to demonstrate a significant association between cervical HPV persistence and alcohol consumption. In a few previous studies reporting on epidemic co-factors in natural HPV infection histories, alcohol consumption increased the risk of 36-month HPV infection in college-aged young women, but alcohol was not presented as a risk factor for persistent HPV infections at ⩽6 months [Reference Ho2]. Goodman et al. reported that cervical HPV acquisition increased with alcohol consumption among sexually active multi-ethnic women, aged 18–85 years, who were recruited from Hawaii, but cervical HPV clearance did not correlate with alcohol consumption [Reference Goodman13]. In contrast to the lack of studies on alcohol and HPV persistence, several cervical cancer cohort studies have been conducted with respect to alcoholics or alcoholism [Reference Sigvardsson15–Reference Adami17]. A single large-scale population-based cohort study assessed the association between moderate alcohol intake and the total incidence of cancer among women in the UK, but no significant association was found with cervical cancer [Reference Allen18].
A critical point is that the drinkers in our cohort had a light-to-moderate level of alcohol consumption; the mean daily alcohol amount consumed by the drinkers (6·0 ± 9·5 g/day) was within the range of light-to-moderate alcohol consumption for Korean women (1·0–14·9 g/day [Reference Kim, Ko and Han19]). Additionally, drinking of beer, a mild type of alcohol (the rates of pure alcohol in beer and soju are 4·5% and 21%, respectively) showed the highest risk of HPV persistence with a regular consumption of ⩾3 glasses of beer. Daily alcohol consumption had a relatively high level in our subjects, but this is included in a moderate alcohol consumption level. The benefits of moderate alcohol consumption have been suggested by several health outcomes, but to date, controversies and inconsistent findings exist [Reference Nova20]. As reported in a recent meta-analysis that investigated the relationship between alcohol consumption and the mortality risk of all cancers, a J-shaped association was found in men but not in women [Reference Jin21]. In a large-scale prospective study of Korean men and women, light-to-moderate alcohol consumption was associated with lower mortality risks from all causes and from cancer in men, but did not show favourable effects on the risks in women [Reference Kim, Ko and Han19]. Members of Asian populations frequently present with a slow-metabolizing acetaldehyde dehydrogenase variant [Reference Druesne-Pecollo22]. Furthermore, there are gender differences in alcohol pharmacokinetics and alcohol-related comorbidities [Reference Baraona23]. Therefore, the genetic susceptibility of Asian women to alcohol might differ from that of other populations with respect to defence against viral infection or viral clearance.
There are potential direct mechanisms to support a positive association between alcohol and HPV persistence. Alcohol can induce folate deficiency by reducing the absorption of folate in the colon and thus induce DNA hypomethylation [Reference Sanjoaquin24]. In fact, folate is a plausible protective factor against cervical cancer [Reference Powers25] and high levels have potential effects against the initiation of HPV-related dysplasia [Reference Piyathilake26, Reference Ferguson, Svoboda-Newman and Frank27]. Additionally, alcohol activates cytochrome P450 2E1 which leads to the production of reactive oxygen species (ROS) [Reference Poschl28], and various antioxidant enzymes and detoxifying pathways are consistently associated with HPV-transformed cells [Reference De Marco29]. As reported in several studies, cervical ROS could contribute to a divergent host response against the viral infection because of highly variable concentrations of amines and amine oxidases in the cervical mucus [Reference Fernandez30]. Moreover, women with high ferritin levels were less likely to clear HR-HPV due to the increased generation of ROS than those with lower levels [Reference Siegel31]. The immune system is important in viral disease, but the mechanisms that influence specific host immunity against HPV remain unclear [Reference Einstein32].
In our study, the frequency of current drinkers in the subjects (45·4%) was higher than the overall frequency of drinkers (20·3%) among Korean women [Reference Kim, Ko and Han19]. The reason for the higher rate of light-to-moderate alcohol drinkers in this study might be the higher socioeconomic status of our subjects, as determined by factors such as household income. Compared to the mean monthly household income in Korea (about 199 and 386 million won in 2001 and 2011, respectively) [33], our subjects had a higher income level (about 390 million won). It is a known fact that lower socioeconomic groups are at a higher risk of binge- or heavy drinking, while higher socioeconomic groups are associated with a higher frequency of light drinking [Reference Crum34, Reference Thun35]. Furthermore, the drinkers in our subject cohort were characterized as younger, more likely to smoke, and more likely to use oral contraceptives compared to non-drinkers. Although a regression analysis was performed after sufficiently adjusting for these characteristics, undisclosed social factors of the moderate drinkers or the complex natures of these factors could influence the risk of HPV persistence.
This study has some limitations. Sexual activity could not be considered as a confounding factor for HPV infection because of the absence of sexual behaviour-related questions in the health screening questionnaire. The mean age of our study subjects was 48 years (⩾40 years: 81%). A reduced sexual interest and desire along with a reduced frequency of sexual intercourse have been reported among middle-aged women [Reference Park36]. About 89% and 71% of Korean women aged 40–49 and 50–59 years, respectively, reported having an inactive sexual life and the highest sexual intercourse frequency was once/month in those aged 40–49 years [Reference Yoon37]. However, the incidence of cervical cancers in Korea was highest in middle-aged women (35–64 years). Sexual behaviours in this population might not greatly affect the association found in the study. Second, persistent HPV infection was defined according to the HC-II HPV genotype pooling method rather than HPV genotype-specific detection. However, it was reported that 1-year HPV persistence as detected by HC-II is more sensitive and predictive for CIN-3 than determination of genotype-specific persistence by linear blot assay [Reference Gage38, Reference Marks39]. The pooled detection of multiple oncogenic HPV genotypes can also minimize false-negative errors [Reference Marks39]. Furthermore, about 86·3% of Korean women had one or two sexual partners in their lifetimes [Reference Kim40, Reference Yoo41] and 91·3% did not report having new sexual partners in the past 6 months [Reference Yoo41]. Persistent HPV infection with the same genotype is highly probable.
In conclusion, this study demonstrates that light-to-moderate alcohol consumption or related behaviours may increase the risk of cervical HR-HPV persistence and infection. These increases may be caused by the direct harmful effects of alcohol on the cervix, or the combination of alcohol consumption, and the social behaviours of female moderate drinkers. We suggest that more studies that investigate the correlation between moderate alcohol consumption and HR-HPV persistence should be performed, particularly in Korean women.
ACKNOWLEDGEMENTS
We are grateful to Dr Jung Sun Kim for supplying data from the health screening examinees and sincerely appreciate the 10440 women who participated in this study. This research was supported by grants (1110320, 1310360, 0910221) from the National Cancer Center in Korea.
DECLARATION OF INTEREST
None.






