INTRODUCTION
The survival of patients with solid tumours and haematological diseases has steadily improved over the last few decades. Despite advances in other therapies, cytotoxic chemotherapy remains one of the key therapeutic options. One of the main complications of cytotoxic chemotherapy is febrile neutropenia, which can lead to life-threatening adverse events such as severe sepsis. Antibiotics and granulocyte colony-stimulating factor prophylaxis as well as empirical treatment protocols have been shown to improve the management of febrile neutropenic patients [Reference Schelenz, Giles and Abdallah1–Reference Klastersky3].
Empirical treatment, with a combination of antibiotics at the onset of fever rather than after identification of the pathogen, reduces morbidity and mortality in these patients [Reference Pizzo, Mandell, Bennett and Dolin4]. From the 1970s to about the 1990s, standard therapy for febrile neutropenia was a combination of antibiotics, which treat a broad range of possible pathogens, achieve bactericidal serum concentrations, exert a synergistic effect, and prevent the emergence of resistant organisms [Reference Klastersky, Meunier-Carpentier and Prevost5, Reference Klastersky and Zinner6]. Thus, a combination of an aminoglycoside with an anti-pseudomonal β-lactam, with or without an anti-staphylococcal drug, was usually chosen as the treatment of choice for patients with febrile neutropenia, with particular attention paid to Gram-negative infections, which are predominant and life-threatening [Reference Hughes7]. With a worldwide decrease in the frequency of Gram-negative infections in neutropenic patients, and the availability of new antibiotics with an extended spectrum of activity, possible treatment of febrile neutropenia with a single antibiotic (monotherapy) became the preferred therapeutic option at the end of the 1990s [Reference Wade8–Reference Ramphal10]. However, since 2000, we have witnessed a further increase in Enterobacteriaceae isolates often resistant to β-lactam antibiotics as well as a greater number of isolates of Pseudomonas aeruginosa.
Most epidemiological and outcome analysis on febrile neutropenia has been described from clinical trials, but data from clinical practice is often lacking. The aim of this study was to determine the epidemiology and antimicrobial susceptibility of microorganisms isolated from bacteraemia in neutropenic patients over two decades in our setting, and to identify the independent prognostic factors of mortality.
PATIENTS AND METHODS
Setting and data collection
The Hospital Clinic in Barcelona, Spain is a 700-bed university tertiary centre that provides specialized and broad medical, surgical and intensive care for an urban population of 500 000 people. This hospital has followed a blood-culture surveillance programme since 1991. Briefly, an infectious disease specialist and a microbiologist review the charts of patients with positive blood cultures and recommend antibiotic therapy according to the clinical context and the results of identification and antimicrobial susceptibility tests of the organism. Patients were observed from the diagnosis of bacteraemia until 30 days afterwards. Data regarding episodes of bacteraemia are entered in a database designed specifically for the surveillance programme.
Study design and inclusion criteria
The type of study was based on an analysis of cases of bacteraemia in neutropenic patients prospectively collected through the surveillance programme from January 1991 to December 2012. Neutropenia was defined as an absolute neutrophil count of <500 cells/mm3. The Ethics Committee of the hospital approved the study.
Microbiological methods
Between 1991 and 1997, blood samples were processed by the BACTEC NR-730 system (Becton Dickinson, USA) and incubated for 7 days. Since 1998 we have used the BACTEC 9240 system (Becton Dickinson), with an incubation period of 5 days. Isolates were identified by standard techniques [Reference Murray, Baron and Jorgensen11] and antimicrobial susceptibility was determined by a microdilution system (Microscan, Dade Behring, USA), or Sensititre (Trek Diagnostic Systems, UK), or the Phoenix system (Becton Dickinson). Minimum Inhibitory concentrations determined with the three systems were interpreted according to CLSI guidelines [12]; microorganisms reported as intermediate were considered to be resistant to the tested drug.
Patients' characteristics
The following data were obtained for all patients: age, sex, pre-existing comorbidities, prognosis of the underlying disease, prior antibiotic therapy, prior surgery (within the last month), current administration of ⩾20 mg corticosteroids per day, current administration of anti-neoplastic chemotherapy, source of bacteraemia, leucocyte count, origin of the infection (community-acquired or healthcare-associated, including last conventional hospitalization or outpatient visit), length of hospitalization before diagnosis of bacteraemia, intensive care unit (ICU) admission, need for mechanical ventilation, empirical and definitive antibiotic treatment, susceptibility to antibiotics of the isolates, presence of shock, and mortality.
Significant bacteraemia was defined as one or more blood cultures positive for one or more primary pathogens or two or more blood cultures positive for non-primary pathogens and clinically apparent signs and symptoms of sepsis [Reference Horan, Andrus and Dudeck13, Reference Annane, Bellisant and Cavaillon14]. The source of infection was determined by an infectious disease specialist and the attending physician who considered the patient's medical history, physical examination and the results of other microbiological tests and complementary imaging exploration. An intravenous catheter was considered to be the source of bacteraemia when, in the absence of any other clinically apparent focus, any of the following criteria were present: local inflammatory signs or suppuration at the insertion site; a positive culture of the catheter tip for a microorganism with the same susceptibility pattern as that isolated from peripheral blood; we required at least 2 h difference in the time of growth between catheter and blood culture [Reference Blot, Nitenberg and Brun-Buisson15, Reference Raad16]. When no focal infection could be demonstrated, the source was categorized as unknown.
Comorbidity other than neutropenia was defined as a disease or therapy that could predispose patients to infection, alter defence mechanisms or cause functional impairment, such as the following: diabetes, liver cirrhosis, renal failure, alcoholism (>100 g alcohol every day), active neoplastic disease, severe chronic obstructive pulmonary disease, severe cardiac disease with symptomatic heart failure and administration of immunosuppressive drugs (⩾20 mg corticosteroids per day on a regular basis or anti-neoplastic chemotherapy). The prognosis of the underlying disease was classified, according to the criteria of McCabe and Jackson, as rapidly fatal (death expected within ⩽3 months), ultimately fatal (death expected within a period of >3 months but <5 years) and non-fatal (life expectancy ⩾5 years) [Reference McCabe and Jackson17].
Prior antibiotic therapy was defined as the use of any antimicrobial agent for ⩾3 days during the month prior to the occurrence of the bacteraemic episode. According to the protocols of our hospital, patients with an expected neutropenia over 10 days received prophylaxis with a fluoroquinolone. Empirical antibiotic treatment for febrile neutropenia was decided according to hospital protocols based on published guidelines. Briefly, when a neutropenic patient became febrile, an empirical therapeutic regimen active against P. aeruginosa was started. During the first years, a cephalosporin plus an aminoglycoside was used. Since 1995, most patients have received monotherapy with a carbapenem, piperacillin-tazobactam, or cefepime; amikacin was added in the presence of severe sepsis or septic shock, when a Gram-negative pathogen was isolated, or fever did not respond. Vancomycin (or teicoplanin) was used when a Gram-positive pathogen was isolated from the blood, a catheter-associated infection was suspected, when fever persisted, and if patients presented with a severe mucositis.
Antibiotic treatment, either empirical (before) or definitive (after) species identity and susceptibilities were known, was considered appropriate if at least one of the antibiotics prescribed had in vitro activity against the isolate and the dose and route of administration were adequate. Shock was defined as a systolic pressure of <90 mmHg that was unresponsive to fluid treatment or required vasoactive drug therapy [Reference Annane, Bellisant and Cavaillon14]. Death was considered related to the bloodstream infection if it occurred before the resolution of symptoms or signs or within 7 days of the onset of bacteraemia, and if there was no other explanation; otherwise, death within 30 days of the beginning of bacteraemia was considered unrelated to the episode. Patients were observed from the diagnosis of bacteraemia until 30 days afterwards.
Statistical analysis
Statistical analyses were performed using the SPSS software v. 18.0 (SPSS Inc., USA). Continuous variables were expressed as mean±s.d. or median (range) according to their homogeneity and categorical variables were compared using the χ 2 test or Fisher's exact test. Quantitative variables were compared using the Student's–Fisher t test or analysis of variance (ANOVA). Non-parametric tests were used where required. Statistical significance was defined as a two-tailed P value ⩽0·05. Variables with P ⩽0·2 in the univariate analysis were further analysed by using a stepwise non-conditional (logistic regression) multivariate analysis to find the independent factors associated with mortality; related and unrelated mortality (within 30 days of bloodstream infection) were considered together.
RESULTS
Demographic and clinical data
We identified 2092 bacteraemia episodes in neutropenic patients during the period of study. Demographic and clinical data are presented in Table 1. The average age was 50 years (s.d.= 17). Acute leukaemia was the most frequent underlying comorbidity (38%). The focus of bacteraemia was unknown in 1030 (49%) cases followed by catheter-related bacteraemia and pneumonia (33% and 8%, respectively). Bacteraemia was polymicrobial in 10% of cases. There were 299 (14%) cases with shock on presentation and mortality accounted for 349 (17%) cases.
Table 1. Demographic and clinical data of bacteraemic and neutropenic patients
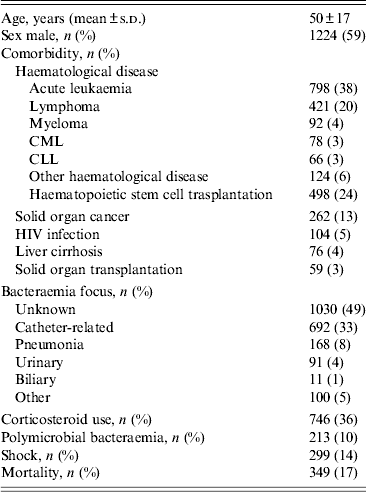
CML, Chronic myeloid leukaemia; CLL, chronic lymphocytic leukaemia.
Microbiological data
Analysis of positive blood cultures distributed over four time periods of the total study showed a predominance of Gram-positive infections (Table 2) and the most common isolates were coagulase-negative staphylococci (CoNS; 654, 30%), followed by Escherichia coli (468, 22%) and P. aeruginosa (235, 11%). However, the prevalence of different species changed over the study period, with a tendency towards a decreased incidence of CoNS (34% vs. 25%, P < 0·001) and an increase in E. coli (16% vs. 27%, P < 0·001) and P. aeruginosa (10% vs. 16%, P < 0·001) in the later years of the study. Regarding antibiotic resistant Gram-negative organisms, 291 (62%) fluoroquinolone (ciprofloxacin)-resistant (FQ-R) and 54 (19%) cefotaxime-resistant (CTX-R) E. coli strains were identified. An increase in Enterobacteriaceae (E. coli and Klebsiella spp.) resistant to antibiotics (FQ or CTX) was also noted in the last study period (2006–2012 years), with 170 of these strains being detected from 567 (30%) episodes of bacteraemia compared to 6% (P < 0·001) in the first period (1991–1995) of the study. We analysed these two classes of antibiotics as they were the marker drugs with the most frequent rates of resistance in our environment. Resistance to aminoglycosides and carbapenems was very low in our series, even in the last years of the study. The resistance of Gram-positive organisms remained stable during the study years with relatively few MRSA isolations (Table 2).
Table 2. Distribution of microorganisms and significant antimicrobial resistance from bacteraemia over the study period
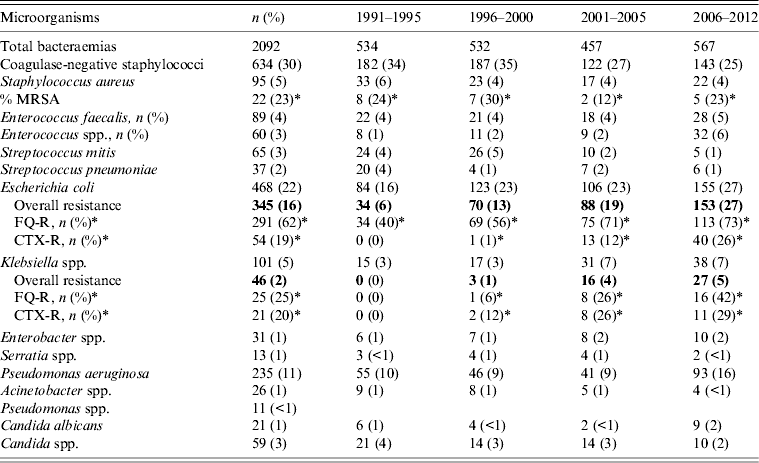
FQ-R, Fluoroquinolone (ciprofloxacin) resistant; CTX-R, cefotaxime resistant.
* Percentage of resistant strains of specific microorganisms.
Mortality analysis
Table 3 shows that by univariate analysis several factors were associated with higher mortality in bacteraemic patients, notably: age, acute leukaemia, haematopoietic stem cell transplantation, solid organ cancer, HIV infection, liver cirrhosis, rapidly fatal prognosis of underlying disease, pneumonia as focus of bacteraemia, corticosteroid use, shock on presentation and inappropriate empirical therapy. Among the organisms isolated from blood culture Enterococcus spp., Streptocccus pneumoniae and P. aeruginosa were the species found to be most significantly associated with mortality; although relatively low in number, 22 deaths were associated with recovery of Candida spp. (P = 0·002).
Table 3. Univariate analysis of risk factors for mortality in neutropenic patients
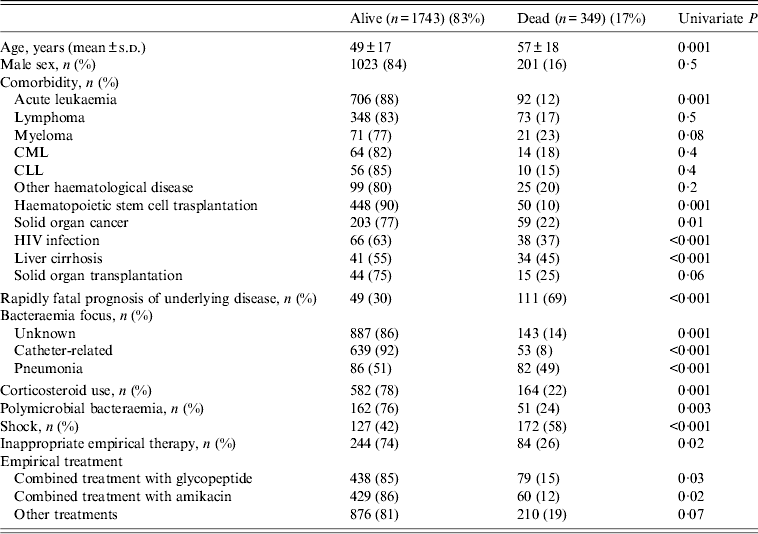
CML, Chronic myeloid leukaemia; CLL, chronic lymphocytic leukaemia.
By multivariate analysis, the independent risk factors most associated with 30-day mortality were shock on presentation [odds ratio (OR) 15·17, 95% confidence interval (CI) 8·39–27·45, P < 0·001] and rapidly fatal prognosis of underlying disease (OR 3·15, 95% CI 1·40–7·13, P = 0·006). Other factors such as age, costicosteroid use, and polymicrobial bacteraemia were less associated with higher mortality but, by contrast, better survival was predicted by isolation of CoNS from blood (OR 0·38, 95% CI 0·20–0·73, P = 0·004), and empirical therapy with amikacin (OR 0·50, 95% CI 0·29–0·88, P = 0·016).
DISCUSSION
Knowledge of the species identity of microorganisms and their antimicrobial susceptibility profile is essential to inform empirical treatment of patients presenting with febrile neutropenia. Over the two decades of the study we observed a progressive decrease in CoNS isolates and a corresponding increase in isolations of Enterobacteriaceae (mainly E. coli and Klebsiella spp.), and P. aeruginosa. In the latter period 2006–2012, while CoNS represented 25% of isolates, E. coli and Klebsiella spp., together accounted for 34% and P. aeruginosa for 16% of all solates. In the last period of the study we also detected an increase in the frequency of Gram-negative strains resistant to antibiotics, specifically to fluoroquinolones (73%) and cefotaxime (26%) for E. coli. These results are consistent with those recently described in a systematic review of epidemiology and antibiotic resistance of microorganisms from bacteraemia in cancer patients where an increase in the frequency of Gram-negative bacteria and the emergence of antimicrobial-resistant strains was associated with an increased risk of morbidity, mortality, and cost [Reference Montaisser18]. We believe that the increased use of carbapenems is a consequence rather than a cause of the increased number of isolates resistant to cephalosporins. Owing to increasing resistance and the very limited arsenal of new agents, especially against Gram-negative pathogens, antibiotic regimens for febrile neutropenic patients should be carefully designed. All experts agree that initial empirical antibiotic treatment should reflect among others, the department/unit epidemiology, and the patient's risk factors for emergence of resistant bacteria and for a complicated clinical course [Reference Averbuch19]. These guidelines [Reference Averbuch19] recommend the avoidance of empirical broad-spectrum antibiotics in the absence of knowledge of prior colonization or infection with resistant pathogens, the presence of such organisms endemic in a centre and/or uncomplicated clinical presentation.
The multivariate analysis identified treatment with amikacin as a factor associated with lower mortality. The protocol of our centre indicates the use of amikacin in patients with severe sepsis or septic focus. This is significant because, according to results from a previous meta-analysis, monotherapy was as effective as aminoglycoside-containing combinations for empirical treatment of febrile neutropenia [Reference Furno, Bucaneve and Del20]. However, the findings in the present study do not support the widespread use of empirical single therapy as a routine treatment for febrile neutropenic patients because of the contribution of other significant factors such as the patients' risk level for infection, local epidemiology, resistance patterns, and potential for development of resistance in the long term, which should also be considered when choosing one of the valid alternatives available for the treatment of these critically ill individuals. Similar to the above, we observed in a previous analysis of a large series of patients with Gram-negative bacteraemia that mortality was lower in the subgroup of neutropenic patients treated empirically with a combination of a ß-lactam and an aminoglycoside [Reference Martínez21]. In patients with neutropenia, certain antibacterial properties of combination therapy, such as synergy or lower endotoxin release could be the reason for the major association of combination therapy with survival. It is possible that the administration of amikacin to our patients in a single dose (once daily) and high dose (20 mg/kg) could explain partially the benefits that we found [Reference Romano22].
The results of this study should be interpreted in the light of some limitations. First, we have reported the results of a retrospective analysis of a database prospectively collected for more than 18 years. Second, we considered related and unrelated mortality together. This is an analysis option used almost always in bloodstream infection outcome studies, but the factors associated with mortality in the first week after bacteraemia diagnosis could be different from those associated with a fatal outcome in the following 30 days. Finally, the status of the underlying disease (initial diagnosis, remission, progression on treatment or relapse), the proportion of subjects with acute leukaemia with unfavourable cytogenetic characteristics and the presence of oral and gastrointestinal mucositis induced by cytotoxic therapy are variables not specifically reported in our bloodstream infection database.
In conclusion, because of the progressive increase of Gram-negative pathogens resistant to antibiotics, therapeutic regimens for febrile neutropenic patients should be carefully designed. Herein lies the importance of studies such as ours in which epidemiological data are obtained through continuous and updated monitoring of local pathogens and their resistances. In our experience, the addition of once daily and high dose of amikacin could be beneficial, particularly in patients with severe sepsis or septic shock.
ACKNOWLEDGEMENTS
The authors thank Fundación Privada Máximo Soriano Jiménez for administrative support.
DECLARATION OF INTEREST
None.






