INTRODUCTION
Norovirus is the predominant cause of gastroenteritis outbreaks worldwide and is responsible for more than 90% of viral gastroenteritis outbreaks [Reference Patel1]. Norovirus is transmitted by several routes, including person-to-person transmission and consumption of contaminated food or water. Prevalence studies in the general population have shown that totally asymptomatic infections may play an important role as the source of infection [Reference Hall2]. The infectious dose for norovirus has been reported to be as low as 18 viral particles [Reference Hall2], and thus norovirus is also one of the main agents responsible for transmission of nosocomial infections between staff and patients/residents [Reference Hall2]. Norovirus can be introduced from the community into healthcare facilities and nursing homes by staff, visitors and patients who might either be incubating or infected with norovirus upon admission [Reference Mattner3].
Social changes and an ageing population have, in recent decades, resulted in an increased number of adults with chronic disorders requiring frequent hospitalizations and care in specialized centres [Reference Godoy4]. This has facilitated the proliferation of social health centres and assisted-living nursing homes aimed mainly at the elderly. Residents often have a high degree of dependency, which may result in the transmission of nosocomial infections from staff to residents [Reference Lopman5]. The risk of nosocomial infection is increased by the degree of dependency and by difficulties in applying preventive protocols due to work overload or insufficient professional training. Therefore, the risk of nosocomial transmission and attack rates could be similar or even higher in hospitals than in nursing homes [Reference Hall2].
Although considered a benign infection, norovirus infections may be severe in the elderly and people with comorbidities and, like influenza infections, have been associated with increased hospitalizations and all-cause deaths [Reference Trivedi6] during periods of community transmission of the virus.
An increased incidence of outbreaks due to changes in surveillance systems or the appearance of emergent strains has also been reported [Reference Svraka7]. Periodic increases in norovirus outbreaks tend to occur in association with new GII.4 strains that evade existing immunity in the community, and may lead to greater transmission in hospitals and nursing homes [Reference Svraka7, Reference Zheng8].
The objective of this study was to estimate and compare the incidence of outbreaks of gastroenteritis due to norovirus in hospitals and nursing homes in Catalonia, Spain.
METHODS
A descriptive study of the incidence of norovirus outbreaks between 1 January 2010 and 31 December 2011 in Catalonia, an autonomous community in the northeast of Spain with 7·5 million inhabitants, was performed. In Catalonia, all suspected epidemic outbreaks must be notified to the epidemiological surveillance units (ESUs) of the Department of Health. An outbreak was defined as ⩾2 cases of gastroenteritis due to norovirus in one centre in a period of 48 h. Patients and healthcare workers met the case definition if they had new onset of vomiting and/or diarrhoea during the outbreak period.
Once a report was received, the ESU confirmed the existence of the outbreak and the clinical and epidemiological characteristics were studied (Fig. 1). Data was collected by ESU staff using a standardized questionnaire. A confirmed case of norovirus gastroenteritis was defined as ⩾2 loose stools and/or ⩾2 episodes of vomiting within 24 h and detection of norovirus in faeces. A probable case was defined as a patient with clinical criteria who was epidemiologically related to a confirmed case. The mechanism of transmission was determined through microbiological and statistical analysis for all outbreaks. Epidemiologists, with all the information available, determined the mechanism of transmission for each outbreak and for each patient involved. Therefore, in some outbreaks more than one mechanism of transmission was determined.
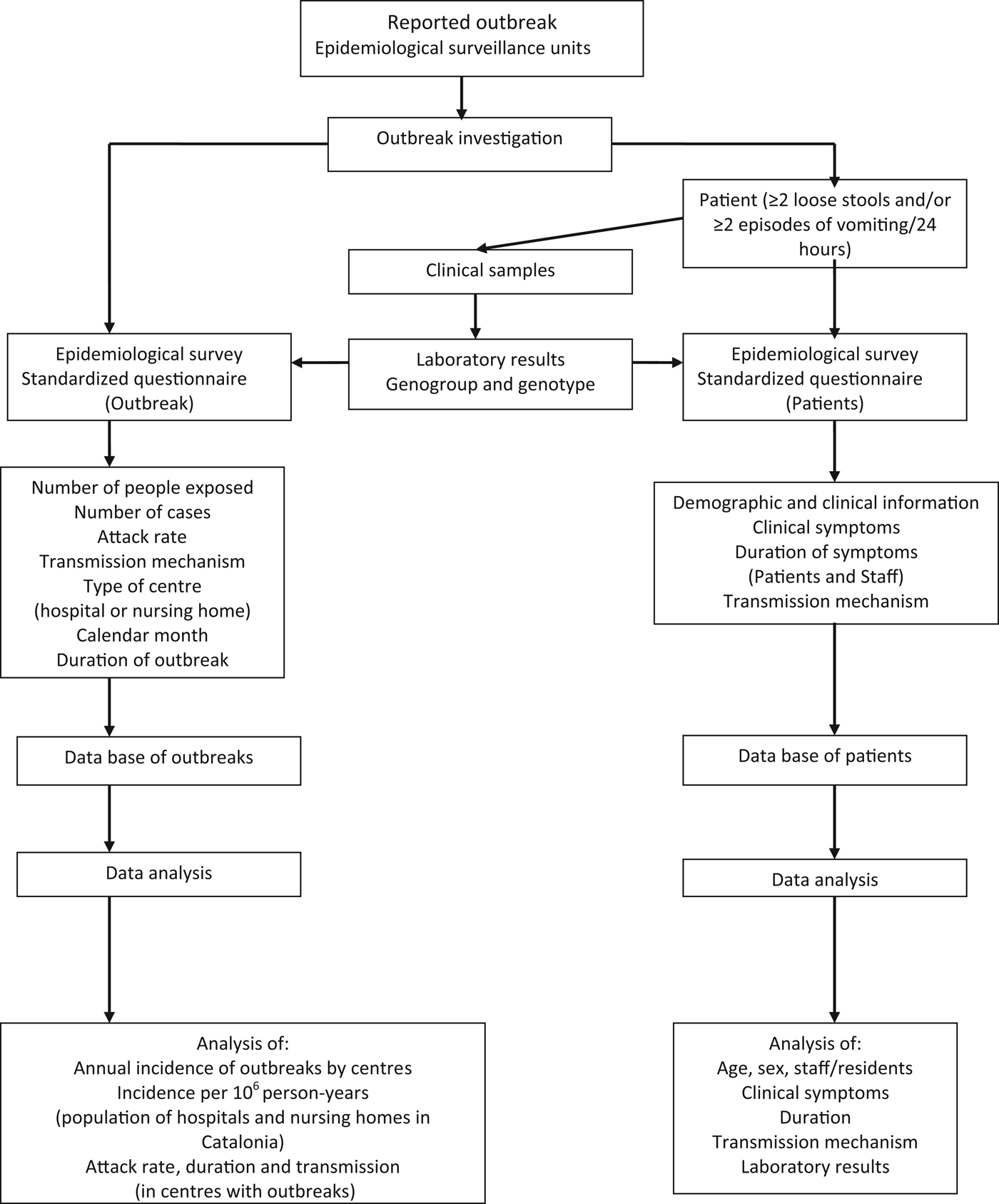
Fig. 1. Protocol scheme of the study of the incidence of norovirus GII.4 outbreaks in hospitals and nursing homes in Catalonia (Spain), 2010–2011.
An epidemiological survey was made for each outbreak studied, including the following variables: number of people exposed, number of cases, attack rate, mechanism of transmission, type of centre (hospital or nursing home), health region, calendar month and duration. Demographic and clinical information (clinical symptoms, duration of symptoms, transmission mechanism and results of laboratory study of clinical samples) was collected for each case. Clinical samples were collected and shipped to the corresponding reference microbiology laboratory (Public Health Agency Laboratory for the city of Barcelona and the University Hospital Vall d'Hebron Microbiology Laboratory for the other health regions of Catalonia). For each outbreak, all stool samples were investigated if there were fewer than ten cases, and a minimum of ten samples were studied in outbreaks with more than ten cases. Faecal samples were collected during the acute phase (first 3–5 days) of infection; a minimum of 1 g stool was collected in a sterile, plastic container without preservative. Stool samples were kept at 4°C for 2 days and frozen at −20°C until microbiological analysis, which was performed by the reference laboratories.
Samples were pre-screened using standard microbiological tests to rule out bacteria, parasites, rotaviruses and adenoviruses. Screening for norovirus was performed using two one-step qRT–PCR assays, depending on the laboratory making the analysis. Samples sent to the University Hospital of Vall d'Hebron Microbiology Laboratory were analysed using a duplex qRT–PCR assay based on the primers and hydrolysis probes described by Kageyama et al. [Reference Kageyama9]. Samples sent to the Public Health Agency of Barcelona Laboratory were analysed using the MutaREAL Norovirus Real Time RT–PCR kit (Immundiagnostik, Germany). Genogroups were assigned after amplification by semi-nested RT–PCR of the ORF1/ORF2 junction region (region C), as previously described by Pérez et al. [Reference Pérez10].
The annual incidence of outbreaks by centre in a natural year and the incidence/106 person-years for the population of hospitals and nursing homes were calculated. The 2011 population and the number of nursing homes and hospitals according to the official 2011 census were used as denominators. We also calculated the attack rate in centres that suffered outbreaks. The 95% CIs were calculated assuming a Poisson distribution. Epidemiological and statistical associations between the dependent variable, [type of centre (hospital or nursing homes)], and the other study variables were determined using ORs and 95% CIs, χ 2 test and Student's t test or Kruskal–Wallis test. The independent effect of each variable was determined using linear regression or logistic regression, as appropriate. Statistical significance was established as P < 0·05.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
RESULTS
Twenty-seven outbreaks were detected; 13 in hospitals (48·1%) and 14 in nursing homes (51·9%). According to the official census, the incidence of outbreaks in the population of hospitals and nursing homes was 156·6 outbreaks/106 person-years. The incidence in all centres in Catalonia was 1·3% per year and was greater in hospitals (2·6%) than in nursing homes (0·9%) (OR 2·6, 95% CI 1·5–2·4) (Table 1). A total of 81·5% (22/27) of outbreaks were due to person-to-person transmission, 11·1% (3/27) were due to foodborne and person-to-person transmission and 7·4% (2/27) were foodborne. Norovirus was confirmed as the agent in all outbreaks.
Table 1. Incidence, attack rate and duration of norovirus outbreaks in nursing homes and hospitals in Catalonia (Spain), 2010–2011

OR, Odds ratio; CI, confidence interval.
In centres with outbreaks, 2348 people were exposed, of whom 816 became cases (Table 1). Therefore, the global attack rate was 34·7, and was higher in hospitals than in nursing homes (OR 1·1, 95% CI 1·0–1·2). In addition, there were differences in the attack rate between staff (48·8%, 144/301) and residents of hospitals and nursing homes (32·8%, 672/2047). A total of 17·6% (144/816) of cases occurred in staff, who were advised to cease working until 3 days after recovery from the illness. The mean number of days between the first and last cases of each outbreak was 14·3 (s.d. = 10·0), and was higher in hospitals (mean = 15·6, s.d. = 11·3) than in nursing homes (mean = 13·1, s.d. = 8·8), but the differences were not statistically significant (P > 0·05).
Samples were analysed for 358 patients and the results were positive for norovirus in 45%. The most common symptoms were: diarrhoea 61·5%, vomiting 55·0%, abdominal pain 34·9%, nausea 33·8% and fever 20·2% (Table 2). In the bivariate analysis, female sex, younger age group, staff who were patients, person-to-person transmission, nausea, abdominal pain, fever and diarrhoea were more common in hospital patients. The mean duration of symptoms was 2·24 (s.d. = 1·5) days and was greater in hospital patients (2·56, s.d. = 1·7) than in nursing home residents (2·05, s.d. = 1·4) (P < 0·001). In the multivariate regression analysis, the ⩽65 and 65–74 years age groups, staff who were patients, abdominal pain and diarrhoea were associated with hospital patients (Table 3).
Table 2. Characteristics of patients with norovirus gastroenteritis in outbreaks in nursing homes and hospitals in Catalonia (Spain), 2010–2011
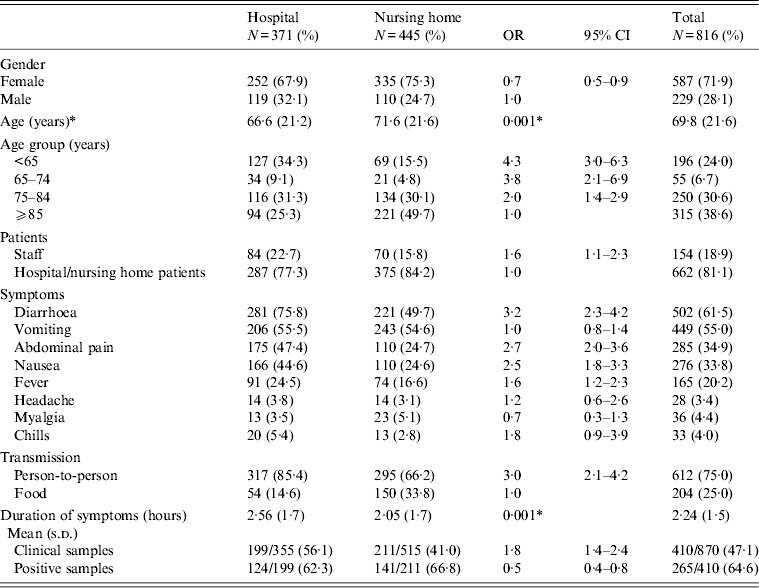
OR, Odds ratio; CI, confidence interval.
* P value for Student's t test.
Table 3. Variables associated with hospital patients with norovirus gastroenteritis in outbreaks in nursing homes and hospitals in Catalonia (Spain) in the multivariate regression analysis, 2010–2011
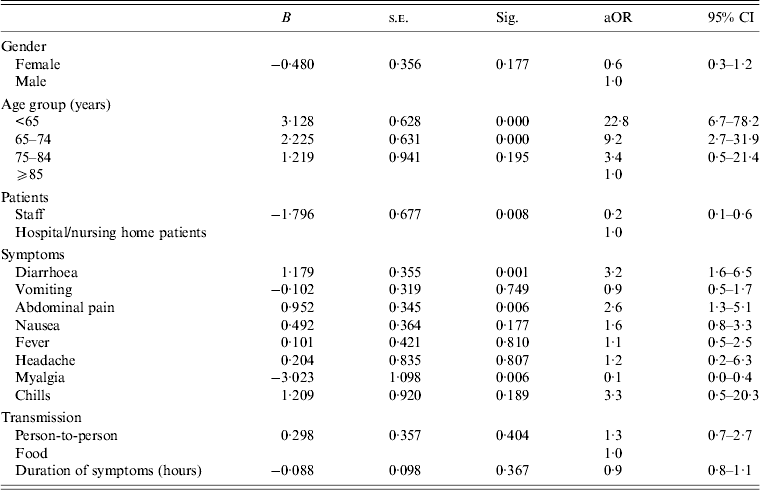
aOR, Adjusted odds ratio in the multivariate regression model according to the variable in the table; CI, confidence interval.
There were two deaths, which occurred in females aged 80 and 101 years, respectively. One died in hospital and the other in a nursing home. The case-fatality rate was 0·25% (2/816), but was higher in patients aged ⩾75 years (0·35%, 2/561).
Most outbreaks (74·1%, 20/27) occurred between December and March (Fig. 2). The genogroup was determined in 55% (15/27) of outbreaks. The most frequent genogroup was GGII, which was found in 86·7% (13/15) of outbreaks. The most frequent genotype was GII.4 which was detected in 66·7% (10/15) of outbreaks. The remaining outbreaks were due to genotypes GII.1, GII.12, GII.6, and GI.7. One outbreak was due to two genotypes (GI.6 and GII.7). The genotypes detected were GII.4 (8/12), GII.6 (1/12), GI.7 (1/12), GII.12 and GI6–GII.7 (1/12) in the winter months, and GII.4 (2/3) and GII.1 (1/3) in the remaining months.

Fig. 2. Outbreaks of norovirus in hospitals and nursing homes in Catalonia (n = 27), 2010–2011.
There were differences between person-to-person and foodborne outbreaks in the attack rate (32·3%, 646/1997 vs. 48·4%, 170/351) and the size of the outbreak (mean = 29·4, s.d. = 4·3 vs. mean = 34·0, s.d. = 52·2). Genotype GII.4 caused more person-to-person outbreaks (72·7%) than foodborne outbreaks (50%).
DISCUSSION
Our results show that the annual estimated incidence of nosocomial norovirus outbreaks in health centres (1·3%) and in the population of hospitals and nursing homes (156·6 outbreaks/106) was very high and was greater in hospitals than in nursing homes. Lopman et al. [Reference Lopman11] found an incidence of 3–7/106 for all types of outbreaks in Europe, including community outbreaks. Surveillance of outbreaks in the USA [Reference Hall12] and Europe [Reference Lopman11] show that the incidence, the location and even the seasonality may vary from one year to another. It is unclear whether an increase in outbreaks was due to improved surveillance or to the emergence of new strains. As the same surveillance methods were used throughout this study, it seems likely that the increase and later reduction in outbreaks in Catalonia was due to the emergence of a new strain or to greater seasonal activity of the virus [Reference Zheng8, Reference Leshem13]. In addition, the gradual reduction in outbreaks suggests that the population may acquire immunity against the predominant strain [Reference Lindesmith14, Reference Cannon15]. Norovirus transmission and epidemiology has been compared to that of influenza A virus. New antigenic variants of influenza A virus emerge every 2–4 years and are associated with epidemics of infection [Reference Hall2, Reference Bull16–Reference Fonager, Barzinci and Fischer19]. In comparison, pandemic variants of norovirus GII.4 emerged in 2002, 2004, 2006, 2009 and 2012 [Reference Hall2, Reference Bull16–Reference Fonager, Barzinci and Fischer19].
As in other studies in nursing homes and health centres, most outbreaks were due to GII.4 [Reference Svraka7, Reference Blanton20, Reference Matthews21], which is the most common genotype in Europe and has been associated with highly recombinant emergent strains [Reference Siebenga22] and with an increase in outbreaks in hospitals and nursing homes [Reference Matthews21, Reference Siebenga22]. Most outbreaks occurred between December and March: this implies a greater risk of transmission during the cold months due to the greater risk of hospitalization of the elderly and because elderly people tend to spend more time indoors, which may result in greater overcrowding.
The mean duration of symptoms was only 2·24 days, but was longer in hospital patients than in residents of nursing homes. In addition, some symptoms (abdominal pain and diarrhoea) were more frequent in hospital patients in the multivariate analysis. Other studies also have found greater severity and length of symptoms in patients affected by other processes [Reference Lopman23]. As in other nosocomial outbreaks, healthcare workers were also affected and, as they had to stop work until 3 days after recovering, this resulted in significant loss of income and increased use of resources [Reference Johnston24, Reference Lopman25].
Most outbreaks were caused by person-to-person transmission and, once a centre was affected, the final attack rates were high (34·7%), showing the difficulty of controlling transmission due to the diversity of transmission mechanisms [Reference Wu26, Reference Weber27] and low compliance with hand washing [Reference Cooper and Blamey28]. The incidence of outbreaks and the attack rates were higher in hospitals than in nursing homes, suggesting that transmission of the virus may be greater in hospitals and that nosocomial infection protocols may be more difficult to apply. No significant differences were observed in the duration of outbreaks in the two types of centres and the global duration was similar to that observed by Harris et al. [Reference Harris, Lopman and O'Brien29] in a systematic review of the duration of outbreaks in centres with control measures, both documented and undocumented (16 vs. 14 days). The attack rate of 34·7% observed was in the high range of the rates found by a systematic review of outbreaks by Matthews et al. [Reference Matthews21], which estimated a rate of 27% (95% CI 12–46) in person-to-person outbreaks and 20% (95% CI 6–34) in outbreaks in health centres. It has been reported that elderly people with comorbidities shed a greater amount of the virus and for a longer time and this would facilitate not only the transmission but also the longer duration of outbreaks [Reference Matthews21, Reference Rockx30, Reference Cieslak31].
The mortality rate was 0·25%, which was similar to that observed by other studies. A review of reported outbreaks by Desai et al. [Reference Desai32] found a mortality rate of 0·39% in health centres. More recently, a study of the surveillance of outbreaks due to the new emerging strain by Leshem et al. [Reference Leshem13] found a mortality rate of 0·2–0·4%. A cohort study by Trivedi et al. in nursing homes affected by outbreaks [Reference Trivedi6] found the risk of death was 1·14 times higher in the outbreak period compared to periods without outbreaks (P< 0·001).
The main strength of this study is that it documents the importance of norovirus outbreaks in Catalonia according to incidence by centre, the population at risk in hospitals and nursing homes and the attack rate in the centres affected. Notably, all outbreaks were confirmed microbiologically, clinical samples were available from a large number of patients and all outbreaks were investigated by the same experienced epidemiological staff using the same protocol.
The study has some limitations. Due to the mildness of the symptoms and the clinical course of the disease, some outbreaks may not have been detected. In addition, outbreaks due to person-to-person transmission are less likely to be detected than those due to other types of transmission and might have been underdetected. However, most outbreaks studied were due to person-to-person transmission. The validity of the symptoms and duration of the clinical course could be questioned in some elderly people with cognitive disorders, especially residents of nursing homes. Similarly, the intensity and duration of symptoms might be less evident in hospitalized patients due to the more-intensive treatment administered. Therefore, the differences found between hospital patients and nursing home residents might also have been underestimated.
CONCLUSIONS
The incidence of norovirus outbreaks in hospitals and nursing homes in Catalonia was very high and was associated with significant mortality. Most outbreaks were caused by genotype GII.4 and were due to person-to-person transmission. The significant disruption of patient care and the cost of nosocomial outbreaks support aggressive efforts to prevent norovirus transmission in healthcare settings and nursing homes [Reference Wu26–Reference Cooper and Blamey28]. Primary control measures of norovirus outbreaks, such as environmental decontamination with solutions of hypochlorite at 1000–5000 ppm, the prevention of food contamination, the exclusion of sick workers, the cohorting of infectious patients and ensuring hand washing or the use of alcoholic solutions among healthcare workers, should be improved [Reference Hall2, Reference Weber27].
APPENDIX
The other members of the Working Group for the Study of Outbreaks of Acute Gastroenteritis in Catalonia are:
M. Alsedà, J. Alvarez, C. Arias, A. Artigues, I. Barrabeig, P. J Balanyà, N. Camps, M. Company, M. Carol, G. Ferrús, N. Follia, A. Martínez, S. Minguell, I. Parrón, A. Rovira, M.R. Sala, N. Torner, R. Torra, J. Torres (Public Health Agency of Catalonia. Barcelona, Spain), M. de Simón, D. Ferrer, A. Moreno, M. Sanz (Public Health Agency of Barcelona, Barcelona, Spain), R. Bartolomé, T. Cornejo (Microbiology Department. University Hospital Vall d'Hebron. Barcelona, Spain), A. Bosch, S. Guix, R Pintó (Enteric Virus Department. University of Barcelona, Barcelona, Spain) and S Broner (CIBER Epidemiology and Public Health, CIBERESP, Spain).
ACKNOWLEDGEMENTS
This project was funded by Instituto de Salud Carlos III (FIS PS0902516 and CIBERESP) and the Catalan Agency for the Management of Grants for University Research (AGAUR Grant no. 2009/ SGR 42).
DECLARATION OF INTEREST
None.








