INTRODUCTION
Hepatitis E virus (HEV) is transmitted predominantly by the faecal–oral route. In areas where hepatitis E is highly endemic, the virus is mainly transmitted through contaminated water. Acute hepatitis E is characterized by a high case-fatality ratio among pregnant women and is responsible for large outbreaks in Asia, Africa, Middle East and Central America [Reference Labrique1]. The treatment remains symptomatic. While a human vaccine is effective, it will not be available for wider use for some time [Reference Shrestha2, Reference Krawczynski3]. In the meantime, prevention and control remains based upon sanitation and provision of safe drinking water.
Several hepatitis E outbreaks have been reported in India [Reference Vishwanathan4–Reference Singh17]. Most of these have been attributed to contamination of the drinking water, including pipelines crossing sewage lines. Investigators generally investigated outbreaks using a descriptive approach. Use of analytical methods to identify a source of infection more definitively has been less common. Identification of specific source of infection is of importance to control the outbreak as standard control measures like chlorination of water may not be effective [Reference Guthmann18, 19]. In the absence of targeted, evidence-based, prevention measures, outbreaks may continue until they die out in their natural course through an exhaustion of susceptible individuals or through the interruption of the source of infection.
Hyderabad, the capital of the state of Andhra Pradesh, Southern India, had an estimated population of 4 036 347 in 2005 projected from the census data of 2001. This population was distributed in seven municipal divisions, called ‘circles’. In March 2005, health officials received reports of an increase in the number of cases of acute hepatitis from the old city. A large open sewage drain crossed the old city of Hyderabad. In several places, the water pipelines that were not buried in the ground crossed this drain. We investigated this suspected outbreak with the objectives of (1) identifying the aetiological agent, (2) finding the source of infection, and (3) implementing control measures.
METHODS
Descriptive epidemiology
We defined a case of acute hepatitis as an acute illness with icterus, dark-coloured urine, fever, loss of appetite and right upper quadrant pain in a resident of Hyderabad city between 14 March 2005 and 31 December 2005. We received notifications of cases admitted at the Fever Hospital (Sir Ronald Ross Institute of Tropical and Communicable Diseases), the main infectious diseases hospital in the city that caters to the whole of Hyderabad. We obtained information regarding demographic characteristics, area of residence and date of admission from all case-patients. To generate hypotheses with respect to the source of the outbreak, we interviewed a sample of case-patients to collect qualitative information, including the source of drinking water and important events before the onset of illness.
We calculated attack rates of acute hepatitis by age and sex using the Hyderabad population as denominator. Administratively, the seven municipal circles of Hyderabad city were further divided in 35 ‘wards’ and 236 ‘blocks’. We plotted the geographical location of cases with reference to water pipelines and sewage lines on maps of circles 1 and 2 that had the highest attack rates. We constructed an epidemic curve to study the dynamics of the epidemic.
Environmental assessment
We formed a joint working group comprising staff from (1) the Department of Health, (2) the quality assurance wing of the Hyderabad Metropolitan Water Supply and Sewerage Board (referred to in the remainder of this paper as the ‘water board’) and (3) the Institute of Preventive Medicine of Hyderabad. The joint working group visited water reservoirs, water pipelines, drains and sewerages to review water treatment and sanitation practices. The water board provided geographical system information on the location of water and sewerage lines. We examined this information in light of the distribution of the cases. The joint working group collected water samples from different reservoirs, final distribution taps from affected and unaffected blocks for water quality assessment. They sampled all the 15 supply reservoirs and 164 tap water samples from the old city.
Laboratory investigations
We tested sera from the case-patients for hepatitis B surface antigen (HBsAg) and IgM antibodies against hepatitis A and E virus using commercially available ELISA kits (Immunovision, Springdale, AR, USA). The cost of laboratory reagents and the large number of patients prevented the testing of all case-patients. Accordingly, we tested sera samples from case-patients admitted during entire month of April 2005 and subsequently all the new case-patients admitted in the second week of every month. We examined water samples collected during the environmental investigation for residual chlorine using the Ortho-toluidine test [20] and for bacteriological characteristics. We screened water samples for presence of coliform bacteria using the most probable number (MPN) method and positive samples were further examined for the presence of Escherichia coli by culture. We considered water as bacteriologically satisfactory if it did not contain more than 10 coliforms in a 100-ml sample and if it did not contain any E. coli in a 100-ml sample [21].
Comparison of attack rates by blocks
Based on the findings of the descriptive study, we suspected that the old pipelines crossing sewerage drains could have been the source of the outbreak. To test this hypothesis, we calculated the attack rate of acute hepatitis in different blocks of Hyderabad city with respect to the approximate number of water supply lines crossing open sewage drains (water board data). We categorized the blocks in four levels of exposure depending on the number of household water connections passing through open sewage drains and calculated the attack rate in the blocks according to these exposures. We calculated the χ2 for trend to assess the linear increase in attack rate in the blocks according to the four different levels of exposures.
RESULTS
Descriptive epidemiology
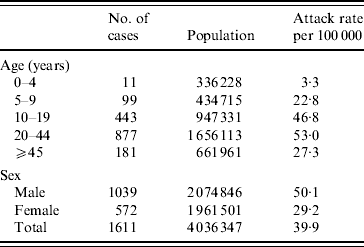
In total, 1611 cases of acute hepatitis from Hyderabad were admitted to the Fever Hospital from 14 March 2005 to 31 December 2005 (attack rate 40/100 000, Table 1). Three deaths were reported (case-fatality ratio 0·2%). Cases were reported among all age groups and both sexes. However, the highest incidence was observed among males and younger adults (Table 1). The number of cases of acute hepatitis started to rise from 19 March 2005, reached a peak between 22 and 29 March 2005 and continued as a plateau thereafter until June 2005. Subsequently, the number of cases slowly declined from the first week of July 2005 (Fig. 1). Of the 1611 cases reported, 824 (51·1%) were from circle 1 and 541 (34%) were from circle 2. These two circles in the old city accounted for 85% of all cases. In circles 1 and 2, the attack rates by block ranged from 0 to 345/100 000 (Fig. 2). The distribution of cases in the old city was not consistent with a contamination of the water supply before the main entry point or of any of the intermediate supply reservoirs. However, the blocks with the highest attack rates were located close to a main sewerage drain (Fig. 2). The cases within affected blocks clustered around water pipelines that had crossed the main sewerage drain.

Fig. 1. Cases of acute hepatitis by week of occurrence, Hyderabad, Andhra Pradesh, India, 2005.

Fig. 2. Attack rate of acute hepatitis in the old city of Hyderabad (circles 1 and 2), Andhra Pradesh.
Table 1. Attack rates of acute hepatitis by age and sex, Hyderabad, Andhra Pradesh, India, 2005
Laboratory studies
A total of 565 sera from case-patients with acute hepatitis were tested for IgM antibodies against HEV. Of these, 482 (81%) were positive. The proportion of samples positive for HEV did not vary throughout the duration of the outbreak. IgM antibodies against HAV were detected in 13% (n=79) of the 553 samples tested. The proportion of case-patients positive for IgM anti-HAV applied to the total number of reported cases suggested that on average, each month, 21 cases of acute hepatitis were caused by HAV during the course of the outbreak. This estimate was close to the average number of cases (25–30) reported each month in 2004 in the city, suggesting that the hepatitis A cases corresponded to the background endemicity. None of the samples were positive for HBsAg.
Environmental assessment
The old city of Hyderabad (circles 1 and 2) was supplied with water from the Krishna river that was pumped against gravity 60 km away from the city. At the main entry point (Santosh Nagar, located in the circle 1), the water was treated at an automated chlorination plant. From there, the water was distributed to different supply reservoirs where it was chlorinated again. Water was then supplied intermittently for 2 h every other day in the old city area, making the system vulnerable to development of negative pressure in the water pipelines. Residual chlorine levels were consistently above 1 ppm in all 13 of 15 supply reservoirs in old city. In contrast, 34% of the 117 tap water samples from the blocks with high attack rates had no residual chlorine level and were not satisfactory bacteriologically. On the other hand, 17% of the 47 water samples from low attack areas were also unsatisfactory bacteriologically. A large open sewage drain passes through the old city (Fig. 2). In several places, water supply lines passed through the open sewage drain. Such pipelines were not buried underground. Water supply lines that passed through the open sewage drain were more than 20 years old, had signs of corrosion and leaked. Some of the leaks on the pipelines had been tied with plastic sheeting (Fig. 3). Thus, on the basis of the geographical distribution of cases and of the environmental assessment, we suspected that (1) the main sewerage drain was the source of the infection and that (2) the old, damaged pipelines crossing the sewerage drain sucked raw sewage from the drain during periods of negative pressure, thereby distributing contaminated water to the population in the area.

Fig. 3. Leakage in water pipeline tied with plastic sheet, Hyderabad old city, 2005.
Comparison of attack rates by blocks
To test the hypothesis that the old pipelines crossing the sewerage drain were the vehicle of the outbreak, we compared the attack rates by neighbourhood block according to the proportion of sewerage crossing pipelines. The attack rate was significantly higher in blocks supplied with water supply lines that crossed open drains (963/473 643=203/100 000) than in others (402/1 049 451=38/100 000; χ2=990·6, P<0·00001). The 10 blocks with water supply lines crossing open sewage drains accounted for 963 (70%) of the 1365 cases reported in the 87 blocks of circles 1 and 2. Furthermore, there was a linear trend for an increase in the attack rates from 38/100 000 in blocks where no pipelines crossed open sewage drains to 347/100 000 in those where 40–60% of connections crossed sewage drains (χ2 for trend=1336·7, P<0·0000, Table 2).
Table 2. Attack rate of hepatitis according to the proportion of population exposed to unsafe water supply, circles 1 and 2, Hyderabad, Andhra Pradesh, India, 2005
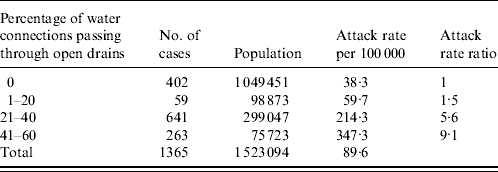
χ2 for trend 1336·7, P<0·0000.
Control measures
Water board authorities super-chlorinated supply reservoirs as a part of initial control measures during the outbreak. Subsequently, from June 2005 onward, identification of the possible vehicle of the outbreak by the joint working group allowed targeted repair (Fig. 1).
DISCUSSION
A large outbreak of hepatitis E occurred in the old city of Hyderabad, Andhra Pradesh from March to December 2005. An investigation that combined a review of the descriptive epidemiology and an environmental assessment led us to suspect that water supply lines passing through open sewage drains could have been the vehicle of the outbreak. A comparison of the attack rates in the blocks affected by these at-risk pipelines supported that hypothesis and directed prevention measures.
During this outbreak, identification of the source and of the vehicle of infection was challenging. Traditionally, in field epidemiology, outbreak investigations include a first descriptive step of hypotheses generation and a second analytical step of hypothesis testing. The second, hypothesis-testing step usually takes the form of a case-control or cohort study [Reference Gregg22] which uses individuals as the sampling unit. Our hypothesis-generation process was complex, time consuming and combined two approaches. First, it involved a study of the geographical distribution of the cases (a) among blocks (Fig. 2) and (b) within few affected blocks. This spatial analysis pointed to specific pipelines that had crossed the sewerage drain and that distributed water to specific neighbourhoods within affected blocks. Second, it involved an environmental assessment of these pipelines and of the water distribution system. This suggested that the leaks on the pipelines and the negative pressure could combine to direct raw sewage into pipelines that crossed the sewerage drains. A case-control study may have helped to test this hypothesis. However, this case-control study would have been limited by a low power: most people drank the municipal water supply. Hence, instead of conducting a case-control or cohort study, we compared the attack rates by blocks according to the proportion of the blocks that were supplied by the at-risk pipelines. This comparison supported our hypothesis and suggested that a majority of cases occurred in the blocks where at-risk pipelines had been observed. Similar mechanisms of water contamination due to old pipelines sucking in the sewage flowing above the water supply lines have been observed to be associated with other hepatitis E outbreaks [Reference Bandyopadhyay11, Reference Gupta14, Reference Singh16, Reference Kane23, Reference Malik24].
According to the United Nations Millennium Development Goals (UNMDG), it is proposed to halve the proportion of people without sustainable access to safe drinking water and basic sanitation between 1990 and 2015 [25]. According to the Census of India 2001, if a household has access to drinking water supplied from taps, hand-pumps or tube wells within or outside the premises, it is considered to have access to safe drinking water. In 2001, it was estimated that 78% of the households in India and 90% households in urban areas in the country had access to safe drinking water [26]. However, piped water can also lead to outbreaks. The growth of the Hyderabad city population has been particularly striking in recent years, having risen from 1 683 000 in 1971, to 3 145 939 in 1991 and 3 640 368 in 2001. The density of the population increased from 7754/km2 in 1971 to 14 497/km2 in 1991. Water resources are scarce. Thus, currently, Hyderabad is supplied with water from sources that are 60 km away. In the old city water is available only for 2 h every alternate day through pipelines that require constant maintenance. Such a situation exposes residents to waterborne outbreaks, including hepatitis E.
Determinants of the time-course of hepatitis E include duration of the water contamination and the impact of corrective measures taken [Reference Aggarwal and Naik27]. The 1955–1956 Delhi outbreak resulted from severe, short-lasting faecal contamination of the Jamuna river. It had a steep rise and a fall with a clear unimodal peak [Reference Vishwanathan4]. In contrast, several other Indian outbreaks [Reference Sreenivasan5, Reference Tondon6, Reference Naik8] lasted for a prolonged period of time because of continued water contamination in the absence of effective control measures. The present outbreak lasted for about 9 months. Initial control measures proposed in the absence of evidence, including the super-chlorination of supply reservoirs were not effective. Subsequently, from June 2005 onwards, following the identification of the possible vehicle of the outbreak and targeted repair measures, the number of cases of acute hepatitis declined. Following the completion of all repair work in October, the monthly number of cases returned to the pre-outbreak baseline and remained below this baseline from December 2005 to April 2006.
The present investigations suffer from a number of limitations. First, we were unable to identify the original source of the outbreak. The damaged pipelines had been crossing the open sewage drains in several parts of the city for several years and might have caused other waterborne infections in the past. The simultaneous occurrence of hepatitis E in the whole old city pointed to a sudden contamination of the main sewerage drain by HEV. However, we were unable to determine when, why and how such a contamination could have occurred. Second, the overlap between the investigation and the repair work made it impossible to evaluate their exact impact on the dynamic of the outbreak. However, the results of our analysis indicated that hepatitis E was associated with these at-risk pipelines and the repair of the at-risk pipelines was followed by a decline in number of cases of cases of hepatitis (Fig. 1). Third, we were unable to isolate the virus from the case-patients, from the water supply or from the sewage. This prevented the molecular biological analysis that could have traced back the contamination of the water supply to its source. Fourth, given the limited number of deaths, we could not study the risk factor for fatality.
Hepatitis E outbreaks remain a serious public challenge for old, large Indian cities like Hyderabad, which are experiencing rapid population growth. The methods we used to direct prevention measures during this investigation required a close collaboration between city water board engineers and public health professionals. From a longer-term perspective, additional investment is needed for India to meet the UNMDG in terms of water and sanitation and prevent episodes such as this one. These measures should complement ongoing efforts to develop and administer vaccines against hepatitis E [Reference Labrique1, Reference Shrestha2]
ACKNOWLEDGEMENTS
We thank Dr Satyavathy (District Medical and Health Officer, Hyderabad), Dr Quadri (Municipal Health Officer) and Dr Nakshbandi for their help in the investigation. We are grateful to the Divisional Manager, Hyderabad Metropolitan Water Supply and Sewerage Board, Mr Narasappa, Deputy Director (Quality Control) and Mr Chenna Kesavulu, Chief Water Analyst and his team for their cooperation during the environmental investigations.
DECLARATION OF INTEREST
None.







