INTRODUCTION
Vibrio parahaemolyticus is a Gram-negative halophilic and mesophilic bacterium that occurs naturally in estuarine and marine environments worldwide [Reference Joseph, Colwell and Kaper1, Reference Kaneko and Colwell2]. It is an important human pathogen that causes acute gastroenteritis, generally associated with the consumption of raw or undercooked seafood [Reference Daniels3–Reference Iwamoto5]. Thermostable direct haemolysin (TDH), TDH-related haemolysin, and the type III secretion system, contribute to the pathogenicity of V. parahaemolyticus [Reference Shirai6–Reference Jones8]. This pathogen is the leading cause of seafood-associated gastroenteritis in the USA and a common cause of foodborne illness in many Asian countries, including China, Japan and India [Reference Alam9–Reference Raghunath11].
Most epidemiological information about V. parahaemolyticus infections in China comes from outbreak investigations. Meat and meat products are the most common vehicle of infection in reported outbreaks of V. parahaemolyticus infection. Of the 322 outbreaks of V. parahaemolyticus infection reported to China National Foodborne Diseases Surveillance Network from 2003 to 2008, meat and meat products were the most frequently implicated food and were associated with 58 (18·0%) outbreaks [Reference Wu12]. However, meat is unlikely to carry V. parahaemolyticus. Whenever meat is implicated in an outbreak, the meat has probably been cross-contaminated with other foods. Although implicated food vehicles were identified in outbreaks, food vehicles in sporadic cases are difficult to detect. However, as shown by data from Japan and the USA, most cases of V. parahaemolyticus infection are sporadic, with outbreaks less common [Reference Kubota13, Reference Scallan14]. Active surveillance data suggest that in agreement with previous studies, most V. parahaemolyticus cases in China are sporadic (data not shown). Epidemiological studies of sporadic V. parahaemolyticus infection are limited and there has been only one published case-control study of risk factors for sporadic V. parahaemolyticus infection in the USA [Reference Bean15]. Given the increasing prevalence of V. parahaemolyticus infection in China, it is important to elucidate the epidemiological characteristics of this pathogen in order to develop appropriate evidence-based prevention and control strategies. Therefore, we conducted a matched case-control study to identify risk factors that could provide leads for preventing sporadic cases of gastroenteritis attributable to V. parahaemolyticus.
METHODS
Sentinel hospital surveillance
Sentinel hospital surveillance was conducted at 11 sentinel sites in two coastal provinces, Shanghai (2) and Jiangsu (9), in east China from 1 July 2010 to 30 June 2011 (Fig. 1). The sentinel sites were selected based on their suitability, cooperation of local authorities and feasibility of completing the studies. The sentinel sites were, with the number of participating hospitals in parentheses: (a) Shanghai [Luwan (4) and Qingpu (4) district]; (b) Jiangsu [urban area (3) and Jiangyin county (4) of Wuxi prefecture; urban area (3) of Xuzhou prefecture; urban area (3), Changshu county (4) and Taicang county (4) of Suzhou prefecture; urban area (3) and Yizheng county (4) of Yangzhou prefecture; and urban area (3) of Taizhou prefecture]. The sentinel hospital surveillance area covered an estimated population of 14 180 386. Of the 39 hospitals participating in the study: 11 (28%) were tertiary hospitals, 10 (26%) were secondary hospitals, and 18 (46%) were primary hospitals.

Fig. 1. Map of the sentinel sites in Shanghai and Jiangsu, China.
Cases
Eligible case-patients were individuals aged ⩾1 year with diarrhoea who had V. parahaemolyticus isolated from stool samples, and resided in the surveillance area. Case-patients were excluded if their primary residence was outside the sentinel site, were not accessible (e.g. did not have a home telephone), or if they were identified as secondary cases arising from a single household. Case-patients from recognized outbreaks were not included.
Controls
For each case-patient enrolled, two controls matched for gender, age group and residential area (city block in the urban setting and village in the rural setting) were selected. We chose these factors to eliminate them as potential confounders. Controls were matched to case-patients using the following age groups: 1–5, 6–17, 18–39, 40–59, and ⩾60 years. Potential controls were excluded if they had reported diarrhoea, nausea, vomiting and abdominal pain in the 30 days prior to interview. Controls were interviewed as soon as possible and within 28 days of the case-patient's interview.
Questionnaire
Interviews were performed by trained health workers using a standard questionnaire. The questionnaire was validated and developed specifically for the purposes of this study. It was at the discretion of the parent or guardian as to whether an individual aged 12–18 years was interviewed directly. Information from individuals aged <12 years was obtained from the parent or guardian who was most familiar with their diet and behaviour. Verbal informed consent was obtained from all respondents before the interview. Surveys were conducted in Chinese. The questionnaire and study protocol were reviewed and approved by the Committee on Human Experimentation of the National Institute for Nutrition and Food Safety, Chinese Center for Disease Control and Prevention (CDC).
For case-patients, all questions related to the 5-day period preceding their onset of diarrhoea, except for prior use of antibiotics or antidiarrhoeals, which were based on the preceding 4 weeks. Controls were asked about the 5 days or 4 weeks prior to interview. Data collected included demographics; pre-existing illness; previous medication use; travel history (travel to other city/province); animal contact; refrigeration of certain aquatic products (i.e. fish, shrimp, crab and shellfish, whether of freshwater or saltwater origin) at home; other family kitchen practices; food consumption; meals eaten outside of the home; and drinking water source. Case-patients were also asked questions about their illness.
Laboratory methods
Hospitals were responsible for collecting stool specimens from patients with diarrhoea, and CDC laboratories for testing stool specimens for V. parahaemolyticus. Rectal swabs or stool specimens were enriched in alkaline peptone water or sodium chloride Violet Purple enrichment broth (6/11 local CDC laboratories used the enrichment broth to increase the sensitivity of stool culture), followed by subcultivation onto thiosulfate citrate bile salts sucrose agar, sodium chloride sucrose agar, or CHROMagar Vibrio agar (CHROMagar, France) for the isolation of V. parahaemolyticus. The plates were incubated at 37°C for 18–24 h.
Sample size
The sample size of the study was calculated using shellfish consumption as the most important exposure to be assessed. The expected number of cases in the study region for a 12-month period was about 68. Based on a case-control ratio of 1:2, this number would allow the detection of an association with an odds ratio (OR) of 3, at the 5% significance level with 80% power (assuming 10% of controls are exposed). Power calculations were performed using PS 3·0 [Reference Dupont and Plummer16].
Data analysis
Interview data were entered into EpiData v. 3.1 (EpiData Association, Denmark). All the data analyses were performed using the software package SPSS v. 16.0 (SPSS Inc., USA).
The data were analysed using conditional logistic regression for matched case-control studies [Reference Rothman, Greenland and Lash17]. ORs and 95% confidence intervals (CIs) of individual factors were obtained. Statistical significance was assessed using the likelihood ratio test. Variables significantly associated with infection at a P value <0·10 by univariable analysis were included in a multivariable conditional model. If two or more independent variables were significantly associated with each other (P < 0·05), the more biologically plausible variable was included in the model and the other was discarded. In multivariable analysis, to determine the variables in the model we used forward elimination method. Variables with P < 0·05 were included in the final model. Interactions among the factors were examined in the logistic models.
Population-attributable fraction (PAF)
The PAF is the proportion of all cases attributable to a particular risk factor. We estimated the PAF for the factors from the results of the multivariable logistic regression models, using the formula: p(OR – 1)/OR, where p is the proportion exposed among cases [Reference Rockhill, Newman and Weinberg18].
RESULTS
Sentinel hospital surveillance
From July 2010 to June 2011, the isolation of V. parahaemolyticus from stool specimens was: Shanghai, 1·1% (48/4548); Jiangsu, 2·2% (52/2328). Of the 100 V. parahaemolyticus gastroenteritis cases reported from the catchment area, 29 were excluded from the study because the subjects were not accessible (22 from Shanghai and seven from Jiangsu). The case-control study recruited 71 case-patients of V. parahaemolyticus gastroenteritis and 142 matched controls over the 12-month period. The education level and income of cases and controls were similar (Table 1).
Table 1. Socioeconomic characteristics of case-patients and controls enrolled in a population-based case-control study to identify risk factors for V. parahaemolyticus gastroenteritis, China, July 2010–June 2011
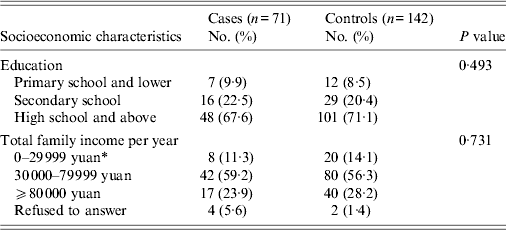
* 10 yuan = 0·95 British pound sterling.
Clinical characteristics
Case-patients were interviewed with a mean delay of 7 days (range 3–18 days) from the onset of symptoms. Controls were interviewed with a mean delay of 5 days after case interview (range −1 to 24 days). One case-patient was interviewed 1 day later than the matched controls. Forty-one (57·7%) case-patients were female; median age was 34 years (range 2–77 years). Only one case-patient was aged <5 years. Seven case-patients had taken an antibiotic in the 4 weeks before onset of illness. Seven reported underlying medical conditions: chronic gastrointestinal illness (3), hypertension (3), haemorrhoids (1). Dates of onset of illness of case-patients were distributed from April to October, with the peak in August (Fig. 2).
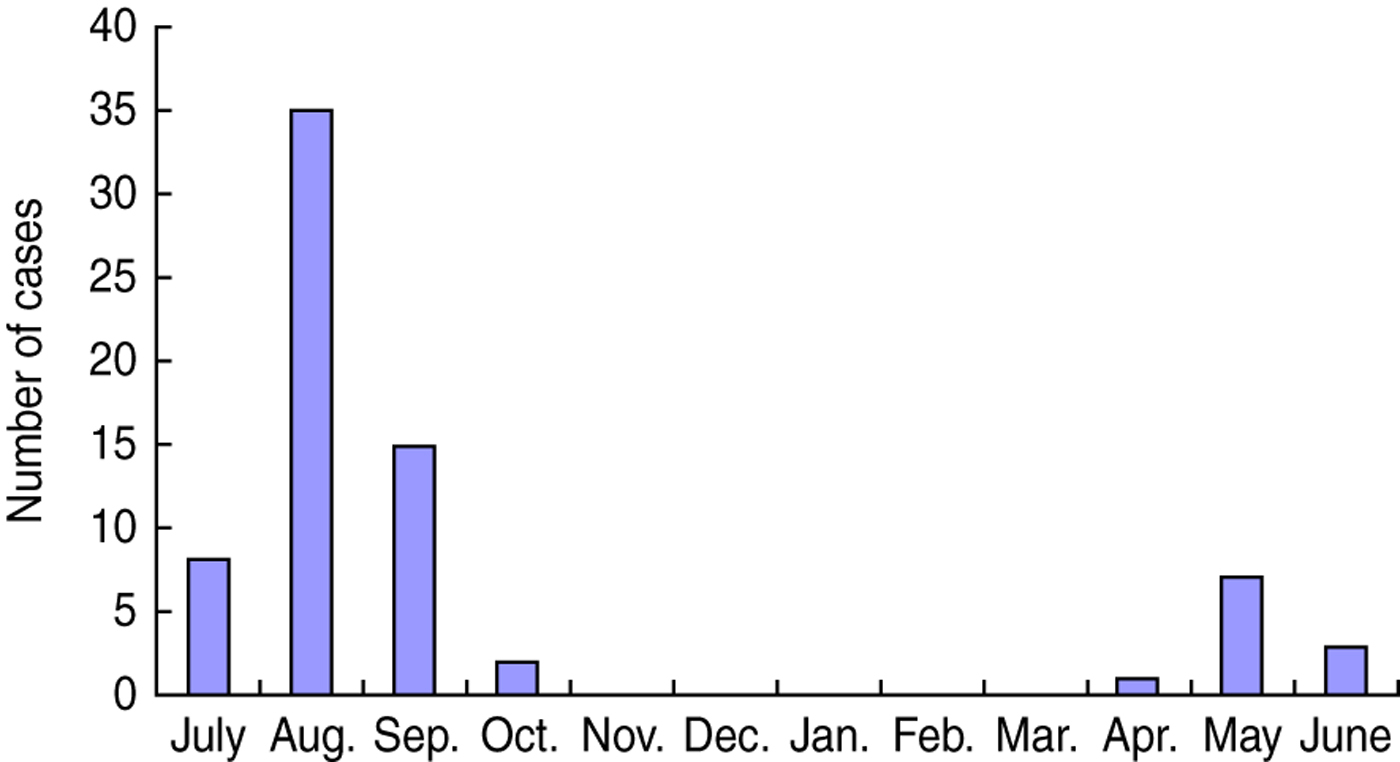
Fig. 2. Date of onset of V. parahaemolyticus gastroenteritis by month, China, July 2010–June 2011.
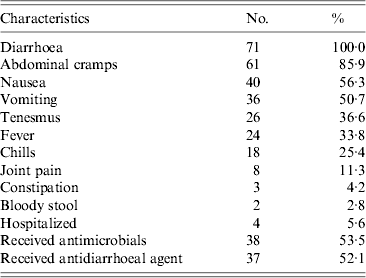
The most frequently reported symptoms besides diarrhoea were abdominal cramps and nausea (Table 2). Among case-patients, 14% reported having had ⩾10 stools per 24-h period. The median duration of illness was 2 days (range 1–7 days). Hospitalization was reported by 6% of the case-patients; the median duration of hospital stay in this group was 2 days. None of the case-patients died. Use of antimicrobial medications and antidiarrhoeal agents was common; 54% (38/71) of case-patients reported taking an antimicrobial medication as a result of their illness, and 52% (37/71) used an antidiarrhoeal agent. Fluoroquinolones were the most commonly used class of antibiotics; 78% of case-patients who took an antibiotic for their illness reported receiving a fluoroquinolone. Of 38 case-patients who took antibiotics for their V. parahaemolyticus infection, three (8%) also reported taking antibiotics during the 4 weeks prior to illness.
Table 2. Clinical characteristics of case-patients infected with V. parahaemolyticus, China, July 2010–June 2011
Of 31 case-patients who were followed in the 3 months after V. parahaemolyticus gastroenteritis, none developed symptoms of irritable bowel syndrome or reactive arthritis as diagnosed by their doctor.
Univariable analysis
In univariable analysis, prior antibiotic use was highly associated with illness (OR 7·0, 95% CI 1·5–33·7). Case-patients were more likely than controls to have reported eating outside the home. Overall, shellfish consumption was associated with increased risk (Table 3). In August, case-patients were four times more likely to eat shellfish compared to controls (OR 4·0, 95% CI 1·2–13·3). However, no association was observed between illness and shellfish consumption in other months (OR 2·0, 95% CI 0·4–9·9). Consumption of shellfish was reported by 14 (20%) of 71 patients and 13 (9·2%) of 142 controls. Only one case-patient (1%) reported eating raw shellfish. Factors associated with decreased risk were keeping the aquatic products refrigerated; and consumption of poultry other than chicken, pasteurized milk, pork, eggs, and nuts.
Table 3. Univariable analysis of risk factors associated with V. parahaemolyticus gastroenteritis, China, July 2010–June 2011
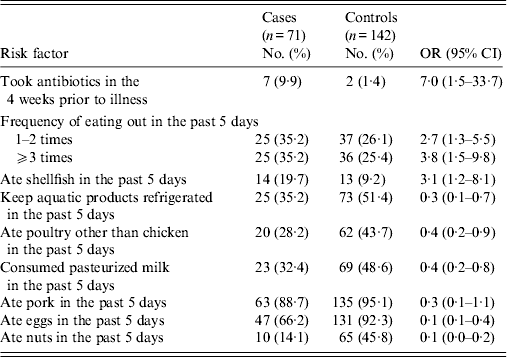
OR, Odds ratio; CI, confidence interval.
Infection was not statistically associated with having an underlying medical condition; prior antidiarrhoeal use; travel; animal contact; other family kitchen practices; consumption of fish, shrimp, crabs, chicken, beef, delicatessen meat, raw milk, dairy products, fruits, vegetables; or drinking-water source.
We found that cleaning kitchen counters with dishwashing liquid or detergent was more frequently reported by controls than by cases, although it did not reach statistical significance (64·8% vs. 56·3%; OR 0·6, 95% CI 0·3–1·1).
Assessment of the association between independent variables
When analysed separately, consumption of pasteurized milk and eggs were highly negative associated with consumption of shellfish, and consumption of nuts was highly associated with eggs. However, shellfish exposure is more biologically plausible than exposure to pasteurized milk, eggs, and nuts. Therefore, these variables were not included in the model.
Multivariable analysis
In the final multivariable model, antibiotics taken in the 4 weeks prior to illness (OR 8·1, 95% CI 1·2–56·4), eating out ⩾3 times (OR 3·3, 95% CI 1·1–10·1), and shellfish consumption (OR 3·2, 95% CI 1·0–9·9) were independent factors associated with an increased risk for illness (Table 4). Keeping the aquatic products refrigerated (OR 0·4, 95% CI 0·1–0·9) and pork consumption (OR 0·2, 95% CI 0·1–0·8) were independent factors associated with a reduced risk for infection. There was no association between eating poultry other than chicken in the 5 days before illness or interview and V. parahaemolyticus infection (OR 0·5, 95% CI 0·2–1·0) after adjusting for other risk factors. No significant first-order interactions were detected among the factors included in the analysis.
Table 4. Multivariable analysis of risk factors associated with V. parahaemolyticus gastroenteritis, China, July 2010–June 2011
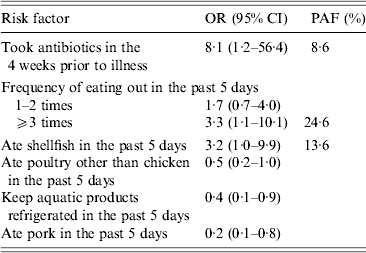
OR, Odds ratio; CI, confidence interval; PAF, population-attributable fraction.
PAF
Prior antibiotic use was reported by 9·9% of cases, frequent eating out by 35·2%, and shellfish consumption by 19·7%. The percentage of cases attributable to each of these risk factors was as follows: antibiotics taken in the 4 weeks prior to illness, 9%; frequent eating out, 25%; and shellfish consumption, 14% (Table 3).
DISCUSSION
This is the first reported study of the risk factors associated with sporadic V. parahaemolyticus gastroenteritis in China. Our study demonstrates that antibiotics taken in the 4 weeks prior to illness, frequent eating out and shellfish consumption were significant risk factors for sporadic V. parahaemolyticus gastroenteritis.
Our study shows that people infected with V. parahaemolyticus were eight times more likely to have been on antibiotics in the 4 weeks prior to becoming ill compared to controls for the same time period. Previous studies have identified prior antibiotic use as a risk factor for Salmonella and Campylobacter infections [Reference Delarocque-Astagneau19–Reference Glynn22]. Prior antibiotic use may increase susceptibility to ingested pathogens through several mechanisms, including prolonged alterations of the gut bacterial flora which would decrease individuals' resistance to infection when they became exposed. It is also possible that sub-clinical infection already exists and antibiotic use might be enough to induce a clinical case [Reference Cohen and Tauxe23].
In the present study, 53·5% of the case-patients were treated with an antimicrobial agent. However, gastroenteritis caused by V. parahaemolyticus is self-limiting in most patients; antimicrobial therapy does not decrease the duration of symptoms. Inappropriate usage of antimicrobial agents to treat humans is a major problem in China, as many Chinese take antibiotics before diagnostic tests are performed [Reference Ran24, Reference Zhou25]. It is reported that nearly half of acute gastroenteritis cases have taken antibiotics in China, which is much higher than in other countries [Reference Chen26].
It is unlikely that patients who reported antibiotic use prior to illness were actually reporting antibiotics taken after diagnosis. In the present study, only 8% patients took both antibiotics prior to illness and after diagnosis. If antibiotic use in the controls is underestimated, this may result in a false association of antibiotic use and V. parahaemolyticus infection. In this study, 1·4% of controls took antibiotics which is consistent with previous data on antibiotic use in the Chinese population (1·5%) [Reference Chen26].
Given the evidence implicating prior antibiotic use as a risk factor for human V. parahaemolyticus gastroenteritis, it is important to investigate the factors influencing the use of antibiotics, and to promote prudent usage of antibiotics by healthcare providers and the public.
In the multivariable analysis, there is an association between foodborne infections and frequent eating out. Outbreaks of V. parahaemolyticus are also frequently traced to food served commercially. During 2003–2008, a total of 248 (77%) of 322 outbreaks of V. parahaemolyticus reported to CDC occurred in restaurants or other commercial food settings [Reference Wu12]. This may represent cross-contamination of a variety of foods with V. parahaemolyticus pathogens present initially in seafood. Such cross-contamination is difficult to assess by case-control methodology because it is not obvious to the consumers. Other studies also highlight the association between sporadic foodborne bacterial infections and eating out [Reference Sobel27, Reference Kimura28].
In the present study, consumption of shellfish increased the odds for disease by more than threefold. We estimated that almost 14% of V. parahaemolyticus cases occurring in the population are attributable to shellfish consumption. Although shellfish have been suspected as a potential vehicle for V. parahaemolyticus infections, this is the first time that eating shellfish was demonstrated to be associated with sporadic V. parahaemolyticus infections in China. Infections of V. parahaemolyticus due to the consumption of raw oysters are more common during the warmer months [Reference Hlady and Klontz29]. High levels of V. parahaemolyticus have been detected in retail oysters collected during spring and summer months in southeast China [Reference Chen30]. V. parahaemolyticus would be of little public health importance if shellfish were always consumed after they were cooked. This study suggests that greater public health education should be focused on the proper handling of cooked shellfish products.
Keeping the aquatic products refrigerated was independently associated with a decreased risk of infection. The minimum temperatures required for the growth of V. parahaemolyticus are 8·3°C [Reference Miles31]. Poor temperature control of storage favours bacterial growth. The shellfish were generally stored at ambient temperatures without cooling devices at the markets in China, which would lead to significant increases in the V. parahaemolyticus level. Continued prevention efforts to educate the food handler and consumer about the risks from keeping the aquatic products unrefrigerated are needed.
Our study identified that consumption of pork and poultry other than chicken might protect against infection, nevertheless we believe the observed effect was probably due to controls being more likely to eat pork and poultry other than chicken than cases, as their frequency of shellfish consumption was lower, thereby creating spurious protective effects.
Outbreak investigations of V. parahaemolyticus infections in China have implicated meat and meat products as the major food with cross-contamination as the major contributing factor [Reference Wu12]. The natural habitat of V. parahaemolyticus is estuarine and marine environments. If cross-contamination is the main cause of V. parahaemolyticus infections in China, and the source of the V. parahaemolyticus contamination is expected to be seafood, it would be expected that serving seafood in the same place would be linked to outbreaks as well. The infectious dose of V. parahaemolyticus is generally believed to be very high. To confirm V. parahaemolyticus outbreaks, isolating 105 Kanagawa-positive organisms/g from epidemiologically implicated food is a sufficient condition [Reference Todd32]. Food poisoning of V. parahaemolyticus infection in China is defined as V. parahaemolyticus strains with the same serotype or other biological properties isolated from the implicated food, equipment or utensils used, and faecal samples or vomitus from patients [33]. There is no requirement regarding the level of V. parahaemolyticus in the implicated food, which may lead to misjudgement of the implicated food. Further investigation and research on this issue is required.
Due to the limitations inherent in the case-control study design, bias cannot be excluded as an alternative explanation for our findings. We did not enrol every diagnosed patient during the study period, and differences in the enrolment procedure could have introduced bias. Because of the design limitation, we did not collect the detailed information for the excluded cases, so we cannot compare the difference in demographics between excluded cases and enrolled cases. In our study, we attempted to reduce recall bias by limiting the recall period to 5 days prior to illness onset (cases) and interview (controls). To minimize potential information bias, all interviewers were trained thoroughly and a standardized questionnaire and protocol for case and control interviews were used.
In summary, our study illustrates that prior antibiotic use, frequent eating out, and shellfish consumption are risk factors for V. parahaemolyticus gastroenteritis in east China. Therefore, efforts to prevent V. parahaemolyticus gastroenteritis should be focused on implementation of farm-to-table measures aimed at shellfish. Additionally, public health, physicians and consumers should promote activities associated with these risk factors that decrease the opportunity for disease. These findings are based on relatively limited data and should be considered preliminary. Further studies are needed to determine more fully the risk factors for V. parahaemolyticus infection, such as prior antibiotic use and shellfish consumption, in order to develop and implement appropriate intervention strategies.
ACKNOWLEDGEMENTS
We thank all local CDCs and hospitals for their considerable efforts. We also thank the study participants, parents, and guardians for their cooperation. The authors also gratefully thank Dr Yukiko Yamada, Japan, for her advice on the manuscript. This project was financially supported by the National Health and Family Planning Commission of the People's Republic of China (no. 201302005) and the Universities Innovation Ability Promotion Plan – Collaborative Innovation Centre for Green Processing and Safety Control of Food in Beijing and Tianjin Area.
DECLARATION OF INTEREST
None.









