INTRODUCTION
Dengue, a mosquito-borne viral disease transmitted principally by Aedes aegypti and Aedes albopictus mosquito vectors is emerging as one of the world's most rapidly spreading and important infectious diseases [1]. About half of the world's population lives in dengue-endemic countries, with estimations that over 50 million infections occur annually [Reference Gubler2]. Most clinical cases are non-fatal illnesses with fever, arthralgia and rash, although fatal haemorrhagic disease occurs in a proportion. Dengue incidence, especially in South-East Asian countries including Malaysia, Cambodia, Vietnam and Singapore numbers in the tens of thousands annually with an overall increasing trend in case numbers reported through time [1]. In Malaysia, dengue incidence has been increasing, with a rise in reported cases from 7103 in 2000 to >46000 cases in 2010 [Reference Mohd-Zaki3]. In more recent times a 98% increase in dengue incidence has been reported, from 21900 cases in 2012 to 43346 cases in 2013. Similarly, deaths due to dengue increased 163%, with 92 and 35 deaths in 2013 and 2012, respectively [4].
Uncontrolled urbanization, population increase and movement, the circulation of multiple dengue serotypes, ineffective public health infrastructure, increased international trade and travel have all been implicated in the spread of dengue around the world [Reference Gubler and Clark5]. Control programmes principally reliant on vector control strategies have had some success in reducing dengue incidence, but have been largely ineffective in controlling the continued global spread of the disease [Reference Nam6–Reference Eisen10]. There remains a need to develop new strategies to control the spread of this disease.
In Malaysia the established dengue surveillance programme functions basically as a passive system with little predictive capacity [Reference Azil, Li and Williams11]. However, data generated by the surveillance system has an enormous potential for disease prediction [Reference Gubler12]. Computer-based mathematical models can potentially be used to increase understanding of local dengue epidemiology, and then inform the development of novel targeted control measures. Mathematical models have been used in the prediction of dengue outbreaks [Reference Helfenstein13].
The development of computer-based models that can be used to describe dengue activity can improve understanding of dengue epidemiology, and can be used to help inform the development of new control strategies. Dengue models should incorporate weather data to predict incidence in future climates. This has been done previously using both statistical modelling [Reference Hales14] and mechanistic process-based models [Reference Bannister-Tyrrell15]. Models that include information on population immunity rates can be used to plan future vaccination strategies (should vaccines become available). Models incorporating mosquito ecology allow in silico testing of vector control strategies before implementation.
Statistical models, comprising single equations linking dengue incidence with inputs such as meteorological data, have been created to describe dengue activity [Reference Hales14], and mathematical models have also been successfully developed [Reference Pongsumpun16]. However, these incorporate implicit assumptions about dengue ecology. The models typically contain very few input variables, and are therefore heavily limited in their usefulness for exploring dengue ecology and testing new management scenarios, particularly at a fine spatial scale.
Conversely, mechanistic, process-based models explicitly describe disease ecology. For instance, by modelling links between rainfall, breeding habitat, temperature and food input with mosquito larval development rates and using the foregoing independent variables to calculate dengue incidence. Such modelling enables researchers to test a number of hypotheses regarding determinants of dengue incidence, provided that the model can be validated initially against observed incidence. Stochastic mathematical models have been developed to investigate dengue ecology [Reference Pongsumpun16-Reference de Castro Medeiros18], which have incorporated greater amounts of environmental inputs and enable sophisticated hypotheses concerning dengue ecology to be tested.
We decided to use an existing two-component system incorporating an entomological model, CIMSiM [Reference Focks19] and a disease model, DENSiM [Reference Focks20]. Both CIMSiM and DENSiM have been validated for mosquito productivity [Reference Williams21] and dengue incidence [Reference Bannister-Tyrrell15].
We reparameterized the model and tested its validity to provide a practical tool for Malaysian dengue researchers, public health practitioners and policy-makers. Our goal was to develop a reparameterized version of the CIMSiM-DENSiM model for exploring dengue ecology in Malaysia and elsewhere. We sought to demonstrate utility by testing the impact of changes in climate and dengue importation rates on incidence.
METHODS
Mechanistic models for A. aegypti and dengue in Malaysia
We used two models, which are linked together in a single software package (Dengue Models v. 3.27, University of California, USA). Within that package reside two sub-models. The entomology model CIMSiM (container inhabiting mosquito simulation) has been previously validated for its ability to simulate mosquito productivity in Australia [Reference Williams21] and has been used to assess A. aegypti population dynamics and survival [Reference Williams22] along with population size [Reference Williams23]. The virus model DENSiM (dengue simulation) was used recently to investigate the impact of inter-annual weather variation on dengue activity in Australia [Reference Bannister-Tyrrell15]. CIMSiM must be run initially to simulate mosquito population, then DENSiM is run to estimate dengue incidence based on vector population from CIMSiM.
Creation of a location file for Kuala Lumpur
CIMSiM/DENSiM require so-called ‘location files' to operate. Such files can be reparameterized with unique information about the weather, human demographics and virus activity at a particular location. No changes to the mathematical relationships linking environmental variables, mosquito productivity and infection were altered during this reparameterization. The district of Petaling was selected in this study, which is an urban district in the state of Selangor with an area of 549·3 km2 and a population of 1·88 million in 2012 (population density of 3423/km2).
An initial location file was created as follows:
Human population
A virtual village/community of 10000 people was created. Birth- and death-rate data were obtained from the International database of the United States Census Bureau (http://www.census.gov/population/international/data/idb/informationGateway.php) and from published records [Reference Bujang24]. While urban populations can be much larger than this, a group of 10000 people is representative of a village or urban enclave of a size typical for a socioeconomically homogeneous Malaysian community.
Meteorological data
Daily weather observations were sourced for the period 2007–2012 (http://www.tutiempo.net/) for Subang meteorological station in Petaling district, Malaysia. These were then imported into CIMSiM following calculation of saturation deficit as an atmospheric moisture measure.
Serological data
We modelled a single dengue serotype assuming a starting seroprevalence of 50% (0·5) for all age groups other than those aged <1 year based on published DENV seroprevalence data for Malaysia. In 2008, 91·6% of 1000 subjects were found to have DENV-specific IgG [Reference Mohd-Zaki3, Reference Azami25]. But the study did not differentiate the four serotypes, all of which circulate in Malaysia. Therefore, we considered 50% seropositivity for a single serotype a reasonable estimate.
Simulations
We conducted a number of simulations of a single dengue serotype into our study population ‘village’ of 10000 individuals. These were all performed for the period 2007–2012. However, 2007 was used as an equilibration year [Reference Focks19], so only data from 2008 onwards are presented.
Simulation 1: validation
In order to validate the model for Malaysian conditions, we introduced 10 viraemic people per week into our population for the period 2008–2010 (the time period for which we had available dengue case data). We then used a cross-correlation procedure (Stata v. 11, USA) to compare total monthly simulated dengue prevalence with monthly dengue case data from Petaling district, Malaysia (data provided by Disease Control Division, Ministry of Health).
Simulation 2: testing importance of importation rate
To test the potential importance of importation rate we ran simulations for rates at 1, 5, 10, 15, 20 and 25 people per week, and assessed monthly average dengue infections in our model village.
Simulation 3: testing importance of climate change
To determine the utility of CIMSiM/DENSiM to study future dengue transmission under a climate change scenario, 1 °C was added to the daily 2010, 2011 and 2012 temperature observations. Specifically, each daily temperature value was increased by 1 °C, thereby maintaining the same level of temperature variation. This value was chosen as it represents a likely temperature increase under a range of carbon emission scenarios by 2100 [Reference Field, Barros, Dokken, Mach and Mastrandrea26].
A simulation was then run to compare monthly dengue activity in 2010–2012 and in a scenario in which each day of that year was 1 °C warmer. Our simulation did not account for the likely spatial heterogeneity of future climate change, nor predictions for greater variation in temperatures. In this way our simulation is very simplistic, but nevertheless demonstrative of possible temperature change effects on dengue epidemiology.
Cross-correlation (Stata v. 12) was then performed to see whether slightly elevated temperatures changed seasonality. Average monthly simulated dengue cases were compared with a Student's t test.
RESULTS
CIMSiM/DENSiM validation
Simulation 1 demonstrated very good concordance between predicted and observed case numbers in Petaling district (Fig. 1) with correlations between simulated and observed dengue up to 0·72 (Table 1). Because predicted and observed incidence data were standardized to an overall mean of zero (Fig. 1), we were only seeking to compare seasonality and relative dengue activity, not a direct simulation of the number of expected cases. Inter-annual variation in dengue activity was simulated (and broadly matched that observed in Petaling district). Such variation in the model is related to the unique weather conditions in each year.

Fig. 1. Results from simulation 1 showing simulated and actual dengue cases in Petaling district, standardized to a mean of zero to demonstrate concordance of seasonality.
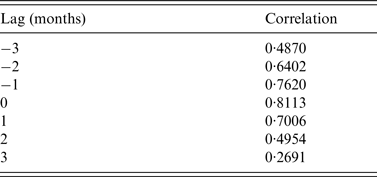
Table 1. Cross-correlation result for monthly standardized simulated vs. actual dengue case prevalence in Petaling district (2008–2010), for matched and lagged months

* Not significant at P < 0·05.
Effect of importation rate
Dengue transmission was sensitive to importation rate, with higher rates of importation each week leading to increased monthly incidence (Fig. 2); seasonality was little changed.

Fig. 2. Results from simulation 2 showing simulated dengue activity in Petaling district for variable rates per week of viraemic human importation.
Repeated simulations with importation rates varying from 1 to 25 per week demonstrated a positive linear relationship of importations and incidence (Fig. 3). However, halving and doubling importation rates did not equate with a halving and doubling of dengue activity in simulations. Furthermore, higher simulated importation rates did not necessarily lead to the largest individual outbreaks. For instance, the largest outbreak observed in simulation was in 2010 at the lowest importation rate (one viraemic person per week).

Fig. 3. The relationship between simulated dengue activity and variable rates of viraemic human importation for Petaling district.
Modelling the potential impact of climatic change
Overall dengue activity in a slightly warmer climate [monthly average (±s.e.) in our simulated population: 638·7 ± 57·7] was less than that in the current climate (774·3 ± 43·4), a result that was statistically significant (t = 4·0, P = 0·0003, d.f. = 35).
There was no discernible alteration in simulated dengue seasonality as a result of increased temperature (Fig. 4). Cross-correlation showed the strongest correlation was at 0 months lag (Table 2).

Fig. 4. Results from simulation 3 showing simulated dengue activity in Petaling district for 2010–2012 and with a 1 °C daily temperature increase to demonstrate the potential for the impact of climate change.
Table 2. Cross-correlation result for simulated dengue case prevalence in Petaling district (2010–2012) compared to 2010–2012 (+1 °C)
In order to explain the slightly decreased dengue activity simulated when temperatures increased by 1 °C, human seroprevalence (a marker of immunity in the simulated population) and the abundance of infective mosquitoes were also plotted for the same period (Fig. 4). In a warmer climate, the infective mosquito abundance increases to a higher peak earlier in the simulation compared to the current climate. This is reflected in a concurrent higher level of human seroprevalence earlier in the simulation. These simulations indicate large outbreaks with consequently increased seroprevalence. Because of high seroprevalence, incidence is lower due to a smaller population at risk later in the simulation.
DISCUSSION
Seasonal variation is evidence for the association between local weather and dengue incidence. Inter-annual variation in weather creates changes in dengue incidence consistent with observed incidence in the district of Petaling, Malaysia. Our results show that the seasonality and relative magnitude of dengue in Malaysia can be modelled using DENSiM. The results also validate our reparameterization.
Despite the success of the model's reparameterization (evidenced by the strong cross-correlation result), DENSiM is not capable of accurately predicting future dengue case numbers. Rather, the model should be used to project seasonality and transmission intensity, and can aid in predictions of future trends in dengue cases (rather than actual case numbers). This limitation is due in part to the fact that DENSiM models dengue infection, not clinical cases, which may or may not develop as a result of infection. Furthermore, great discrepancies typically exist between the number of clinical cases and those recorded by health authorities, owing to the highly variable nature of patients attending healthcare facilities when infected, and of the performance of serological diagnostics [Reference Horstick and Morrison27]. Nonetheless, a number of scenarios can be explored for their potential impact on dengue ecology and management using DENSiM. For example, importation rates and changes to climate (as described here) can be assessed for their relative impact on dengue dynamics. Utilizing functions in the entomological component in this model (CIMSiM), the effect of various mosquito control scenarios on dengue transmission can be specifically tested. Such tests can be used to design optimal mosquito control strategies and plan expenditure.
Influence of importation rate on dengue activity
The impact of importation rate on dengue activity can be estimated (Fig. 3). If viraemic importations to a district were halved, this would not necessarily halve dengue activity. A reduction from 20 importations per week to 10 would only lead to a 23% reduction in dengue (from 927 to 715 cases per month on average; Fig. 3). Changes to industry and economic conditions may alter migration patterns and our DENSiM simulations have utility for predicting consequent changes in dengue epidemiology, permitting appropriate public health responses.
Extreme reductions in viraemic traveller importation rate (to as low as one person per week here) do not lead to reduced risk of large outbreaks (Fig. 3). Indeed, the largest outbreak observed in simulations occurred at the lowest importation rate. This is probably related to low simulated seroprevalence with the initial low importation rate.
These results challenge the presumed effectiveness of control programmes with moderate impacts on dengue transmission. Reduced immunity in a population as a result of reduced transmission can create later explosive outbreaks. It has been demonstrated that reductions in dengue activity associated with vector control in Thailand potentially exacerbate the frequency of dengue haemorrhagic disease [Reference Nagao and Koelle28]. The importance of seroprevalence in modulating dengue epidemiology (as in Fig. 5), and the relative ineffectiveness that halving the importation rate has on the size of dengue outbreaks (as in Fig. 2), again highlights the importance of vaccine development for dengue. The results presented here indicate that widespread use of a dengue vaccine to raise seroprevalence is likely to be the most effective way to control dengue transmission.

Fig. 5. Simulated dengue infective mosquitoes and human seroprevalence for dengue in Petaling district for 2010–2012 and with a 1 °C daily temperature increase (from simulation 3) to demonstrate the potential for the impact of climate change.
Climate change
DENSiM could be used to examine changes to transmission under climate change. Increases in temperature (for instance) could be programmed into weather files and the simulations re-run. Our 3-year simulation with a 1 °C rise in daily temperature altered simulated transmission. However, dengue activity was reduced, most likely due to a higher initial intensity of transmission. Transmission intensity was increased because of higher abundance of infective mosquitoes, leading to a more precipitous increase in human seroprevalence, and hence immunity to dengue, with reduced incidence later in the simulation. Future work that incorporates a range of climate change projections and captures predictions for changes in weather variability could be conducted using the DENSiM model as long as future climate predictions downscaled to daily increments were available.
This work demonstrates that temperature elevations have the capacity to change dengue transmission dynamics in South East Asia, albeit using only a single serotype for simulation. It is simplistic to assume that increased temperature will increase dengue incidence. Our results suggest temperature increase is likely to cause changes to the intensity of outbreaks, perhaps with reduced dengue transmission in some circumstances.
The reparameterization of dengue models (CIMSiM and DENSiM) for Malaysian conditions provides users with a sophisticated tool to enable a number of hypotheses concerning dengue management to be addressed. This allows anticipation of changes in transmission and consequently targeting of control measures. These models are a useful tool for predicting future dengue trends. Augmenting the existing passive dengue surveillance programme in Malaysia with a weather-based predictive capacity using these models can establish an effective early warning system for the disease.
Climate change is likely to impact transmission intensity and consequently human immunity. Human importation rate is likely to be an important factor for dengue transmission management in the future. In low- and middle-income countries in South East Asia the efficient allocation of scarce health resources is crucial. The modelling approach presented here, which we have demonstrated to be feasible, has great potential to increase cost-efficiency of resource allocation to dengue surveillance and control.
ACKNOWLEDGEMENTS
The authors thank the Director General of Health Malaysia for permission to publish. We acknowledge the financial assistance of the Regional World Health Organization Representative Office to Malaysia, Brunei Darussalam and Singapore. Professor Tom Scott and Andy Garcia from the University of California Davis are thanked for their collaboration and provision of the latest updated dengue models, and for permission to extend the use of these to the Malaysian context. Dana Focks and Tessa Knox provided guidance on the initial use of CIMSiM and DENSiM. This project was partially funded through the Australian National Health and Medical Research Council (project 1003371).
DECLARATION OF INTEREST
None.










