INTRODUCTION
Rapid notification of infectious diseases is the cornerstone of prompt public health action and provides essential information on local, regional and national disease epidemiology. Furthermore, the notification process provides an early warning system for the detection of outbreaks and for the surveillance of unusual clusters of infectious disease. However, under-reporting of notifiable diseases has been demonstrated in many countries for a variety of infectious diseases [Reference Harvey1–Reference Doyle, Glynn and Groseclose4]. Low clinician reporting has been associated with a large number of factors including excessive workload, lack of time, lack of motivation and lack of familiarity with the list of statutory notifiable diseases. Measures to tackle this problem focus on continuing medical education for clinicians to emphasize the importance of the notification process and the development of surveillance systems that are flexible and that can support the collection and management of infectious disease data.
The Hospital In-Patient Enquiry System (HIPE), which is maintained by the Healthcare Pricing Office (HPO), is the only source of standardized national morbidity data routinely collected for acute public hospitals in Ireland [5]. Each episode of patient care is coded according to the International Classification of Diseases 10th Revision – Australian Modification (ICD-10-AM) [6]. Between 2006 and 2011, the HIPE system captured data from 57 Irish acute public hospitals (>1·25 million discharges) representing a comprehensive coverage of discharges from acute public hospitals in Ireland. Therefore, the HIPE dataset offers an opportunity to identify and analyse notifiable infectious disease hospitalizations in Ireland.
In an Irish context, under-reporting has been well documented [7–Reference Kelly11]. In particular, a study conducted between 1997 and 2002 in a health board region in Ireland demonstrated that under-reporting by hospital clinicans was a significant issue [Reference Brabazon10]. A conservative estimate of 18% for under-reporting by hospital clinicians was estimated during this time-frame. Viral meningitis and viral encephalitis cases were significantly under-reported, so much so, that the trends over time for hospitalized cases were markedly different than the trends reported for corresponding notified cases. However, in 2004, major changes to the Irish infectious disease legislation and surveillance systems were introduced. These changes included an updated list of notifiable diseases [12], the introduction of laboratories as notifiers and the development of the Computerized Infectious Disease Reporting (CIDR) system [13] which facilitates rapid transfer and analysis of regional and national information on notifable diseases in a secure electronic environment.
The aim of this study was to compare hospitalizations due to notifiable diseases as identified through the HIPE system with national notification data reported to the Departments of Public Health and the Health Protection Surveillance Centre (HPSC) between 2006 and 2011. This analysis will assess if there has been under-reporting of hospitalized notifiable diseases by clinicians in recent years and explore the impact of changes to the infectious disease surveillance methods and regulations which were introduced in 2004.
METHODS
Data on notifiable infectious diseases as specified by the 1981 Irish Infectious Disease Regulations [14] are collected through a number of surveillance systems in Ireland. For the purposes of this study only those notifications that are collected through the CIDR system and the Tuberculosis (TB) Surveillance System maintained by the regional Departments of Public Health and the HPSC were included for analysis. Notification data from 2006 to 2011 on notifiable infectious diseases for the Republic of Ireland were extracted from CIDR on 13 November 2013. Aggregate data on national TB cases were kindly provided by HPSC.
Acute infectious gastroenteritis (AIG) was defined in the 2004 Irish case definitions for notifiable diseases as an acute onset of diarrhoea and vomiting with no known non-infectious cause or a laboratory-confirmed case of rotavirus. In practice, the majority of these AIG notifications at the time were rotavirus cases. On 4 May 2008, the AIG case definition was updated to include Clostridium difficile-associated disease. In late 2011, rotavirus and C. difficile became notifiable in their own right. Therefore, for the purposes of this study and to simplify the comparison of data between HIPE and CIDR, the term AIG was used to signify C. difficile and rotavirus notifications only.
The HIPE datasets for the years from 2006 to 2011 were provided by the HPO through Health Atlas Ireland (HAI) [15]. Ireland updated to an ICD-10-AM-based clinical coding classification for all discharges from 1 January 2005. Therefore to eliminate any effect of this changeover, data were analysed for this study for a 6-year period from 2006 to 2011. All in-patient discharges from public hospitals in the Republic of Ireland participating in HIPE for the years 2006–2011, with a principal diagnosis relating to a notifiable infectious disease (Table 1) were extracted from HAI. This was achieved by identifying the relevant ICD-10-AM codes for all notifiable infectious diseases, based on the 1981 Infectious Disease Regulations (SI No. 390 of 1981 with subsequent amendments; see Table 1 for individual codes for each disease). These ICD-10-AM codes were inputted as ‘sub-selections’ into HAI to extract the relevant data. Only the principal diagnosis (the primary reason for admission) was selected in order to best represent new incident cases of disease and in order to eliminate co-existing or historical conditions from the analysis. In cases where patients had more than one hospitalization for the same condition over the 6-year period all duplicates were removed based on a first occurrence per patient basis using medical record numbers, gender and area of residence. This was done in order to assess the number of notifications that should have arisen due to hospitalization events.
Table 1. ICD-10-AM corresponding codes for notifiable diseases
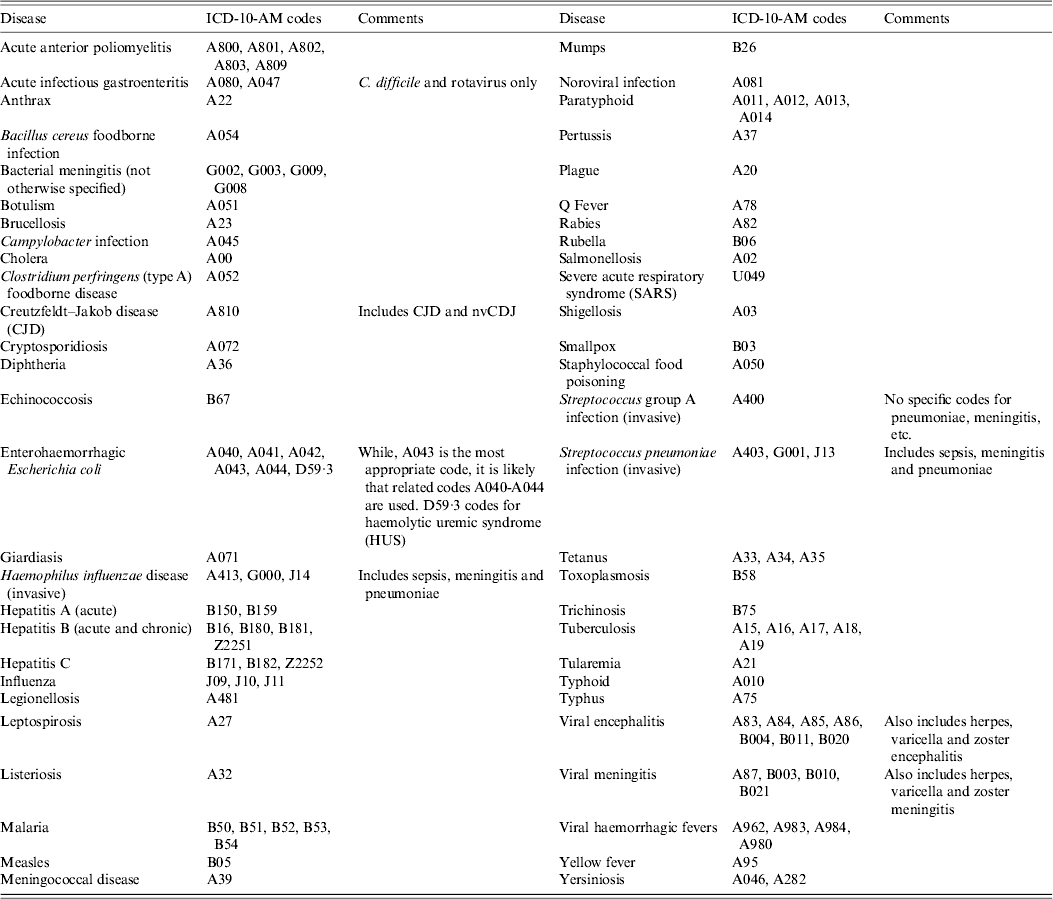
All data were analysed in the statistical package JMP (SAS institute Inc., USA). In order to protect the privacy of patients, where there were fewer than five cases, the data were aggregated into a category designated ‘Others’.
RESULTS
During the 6-year period between 2006 and 2011 there were 22 079 hospitalizations with a principal diagnosis of a notifiable disease (Table 1). These notifiable disease hospitalizations represented 0·62% of all hospitalizations for this 6-year period. When duplicates were removed, there were 20 826 hospitalizations. The most common notifiable disease hospitalization was for AIG: C. difficile and rotavirus only (29·6%, n = 6153); followed by influenza (10·1%, n = 2095) and TB (10·1%, n = 2094). Unsurprisingly, the majority of these hospitalizations were recorded as emergency admissions (90·5%, n = 18 844).
A total of 182 456 bed-days were taken up by this cohort of patients with a notifiable disease as principal diagnosis. Overall, TB and AIG (C. difficile and rotavirus) hospitalizations took up the highest number of bed-days (42 007 and 40 585 days, respectively). However, Creutzfeldt–Jakob disease and listerosis hospitalizations had the highest average length of stay (58 and 25·9 days, respectively). The total number of days spent by these notifiable infectious disease patients in an intensive care unit (ICU) was 13 384 days or 7·3% of the total hospital stay for this cohort of patients. Invasive pneumonococcal disease (IPD) hospitalizations took up the highest number of ICU days (24·5%, n = 3282), followed by influenza (22·2%, n = 2975) and meningococcal disease (10·1%, n = 1347). The total and average ICU length of stay for IPD and meningococcal disease remained relatively stable over the 6-year period; however, there was a large increase in total and average ICU length of stay for influenza during 2009, 2010 and 2011 (Fig. 1). Overall, botulism and Legionella hospitalizations had the highest average length of stay in ICUs (9 and 4·3 days, respectively).

Fig. 1. Intensive care unit average length of stay (ICU ALOS) over time for influenza, meningococcal disease and invasive pneumococcal disease (IPD).
There were 477 deaths recorded among these hospitalizations due to notifiable diseases. The highest number of deaths occurred in AIG cases (34%, n = 162), almost all of which were recorded as C. difficile infections. The next most common diseases for which deaths were recorded was IPD (20·3%, n = 97) and TB (11·7%, n = 56).
Between 2006 and 2011, there were 86 571 cases of notifiable diseases reported in Ireland (Table 2). When hospitalization and notification data were compared, there were four diseases for which substantial surplus hospitalizations were evident: viral meningitis, viral encephalitis, bacterial meningitis (not otherwise specified) and malaria. Viral meningitis and viral encephalitis demonstrated the largest discrepancies between the hospitalized and notified data with a difference of 912 and 416 incidences, respectively. These two diseases accounted for a large proportion of the overall length of stay (20839 bed-days, 11·4%) for all notifiable diseases, took up 1439 days in ICUs (10·8% of total) and had 31 deaths recorded over the 6-year period. For the third disease, bacterial meningitis not otherwise specified, for which there were more hospitalizations than notifications (difference = 147), an overall length of stay of 4935 bed-days was recorded, of which 558 days were spent in ICUs. There were smaller numbers of overall and ICU days taken up by malaria hospitalizations.
Table 2. Hospitalizations vs. notifications (2006–2011) Ireland
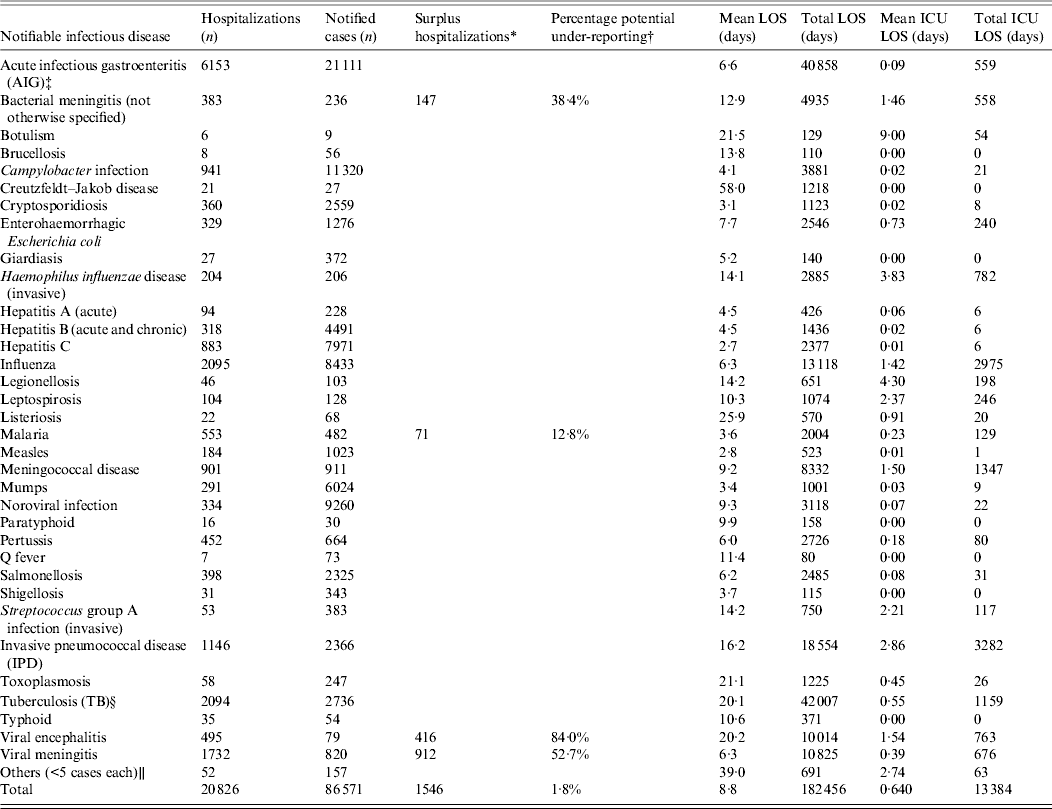
LOS, Length of stay; ICU, intensive care unit.
See Table 1 for explanation of hospitalization codes corresponding to each notifiable infectious disease.
* The surplus hospitalizations are calculated by subtracting the number of notified cases from the number of hospitalizations.
† The percentage of hospitalizations potentially under-reported is calculated by dividing the number of surplus hospitalizations by the number of notified cases plus the number of surplus hospitalizations.
‡ For the purposes of this study AIG only includes C. difficile and rotavirus cases. C. difficile only became notifiable on 4 May 2008; however, hospitalization data includes C. difficile infection from 2006 to 2011.
§ TB notification data obtained from annual reports published by the Health Protection Surveillance Centre.
∥ Further breakdown of figures could not be given due to data protection reasons.
Comparison of the notification data with hospitalization data over time (Fig. 2) demonstrates that for viral encephalitis, in particular, the distribution over time is quite different for the hospitalization and notification data. In the cases of viral meningitis, bacterial meningitis and malaria, the distribution over time follows a broadly similar pattern in both datasets. However, in the case of viral meningitis and malaria in 2010 and 2011, the gap between the CIDR and HIPE datasets narrows suggesting that reporting for these diseases may be improving over time.

Fig. 2. Hospitalizations and notifications for (a) viral meningitis, (b) bacterial meningitis, not otherwise specified, (c) viral encephalitis and (d) malaria between 2006 and 2011.
In total, these viral meningitis, viral encephalitis, bacterial meningitis (not otherwise specified) and malaria incidents accounted for 1546 surplus hosptalizations and if all were potential extra notifications would bring the total number of notifications for this period to 88 117 or add an extra 1·8% to the notification data.
DISCUSSION
To the best of our knowledge, this is the first time that the list of notifiable diseases for Ireland has been coded according to the ICD-10-AM international classification of diseases as a comprehensive set and been compared to the national HIPE (hospitalization) dataset.
During the 6-year period between 2006 and 2011, this study has estimated that only a potential 1·8% of extra notifications could have arisen due to hospitalized cases. A similar study over a 6-year period (1997–2002) in a health board region in Ireland [Reference Brabazon10] produced a conservative estimate of under-reporting of hospitalized notifiable infectious disease cases of 18%. If we assume that this estimate was also indicative of under-reporting at the national level at the time then, there has been a substantial reduction (>tenfold) in under-reporting in recent years in Ireland. This reduction can mainly be accounted for by the introduction of laboratories as notifiers in 2004 and also the use of the CIDR system [13] which facilities laboratories and public health personnel to process cases of notifiable diseases. Our data supports the view that these changes have substantially improved under-reporting rates in Ireland and therefore had a significant impact on the quality and completeness of the Irish notification data overall.
However, for some diseases, particularly viral meningitis and viral encephalitis, there are still discrepancies between numbers hospitalized and numbers notified. This gap in clinician reporting has been highlighted in a number of studies since 1997 [Reference Brabazon9–Reference Kelly11]. The fact that these particular diseases are mainly diagnosed clinically, means that the numbers notified nationally will continue to underestimate the true burden of disease unless significant efforts are made to engage with clinicians. The underestimation of these diseases and of bacterial meningitis (not otherwise specified) is concerning in light of the fact that viral meningitis cases take up a large number of bed-days, that there are high numbers of deaths among viral encephalitis cases, and the severity of bacterial meningitis cases. For these three diseases in particular, the notification data underestimates the true burden of disease and for viral encephalitis the pattern of hospitalized cases over time is also not reflected in the notification data. This has implications for understanding the epidemiology and transmission of these infections in an Irish context. Interestingly, during 2010 and 2011 some improvements in under-reporting for viral meningitis and malaria were demonstrated. These improvements may in part be due to the increased numbers of laboratories utilizing CIDR to discharge their notification obligation and/or an increase in samples referred to laboratories for testing. Overall, this study has highlighted four main diseases that account for the majority of all under-reported hospitalized cases and provided a set of target diseases for which education sessions or other interventions might aid in encouraging clinician reporting.
The ICU length of stay for influenza was shown to increase significantly between 2009, 2010 and 2011. This increase is not surprising as it coincides with the circulation of the H1N1 pandemic strain of influenza which saw a large increase in the requirement for intensive care in influenza patients. Clearly, analysis of this sort of data could provide a foundation for planning how the acute service responds to the changes in the epidemiology of infectious diseases.
Review of hospital discharge data has been shown to be a useful tool for evaluating notifiable disease surveillance systems and under-reporting rates in a number of countries, including the USA [Reference Mor, DeMaria and Naumova16, Reference Boehmer17], Greece [Reference Mellou18, Reference Jelastopulu, Merekoulias and Alexopoulos3], Spain [Reference Fernandez-Cano19], England [Reference Davidson2] and Ireland [Reference Brabazon9, Reference Brabazon10, Reference Kelly11]. Rates of under-reporting for specific diseases vary widely both between countries (e.g. a 75% under-reporting rate for viral encephalitis in England [Reference Davidson2] compared to >80% underreporting rate for Ireland [Reference Brabazon10, Reference Kelly11]), within countries (8% under-reporting rate for salmonellosis in western Greece [Reference Jelastopulu, Merekoulias and Alexopoulos3] compared to a national Greek under-reporting rate of 52·8% [Reference Mellou18]) and over time (3·8–22·8% under-reporting for pertussis in Spain depending on year of study [Reference Fernandez-Cano19]). This variation in under-reporting levels suggests that a number of factors influence under-reporting rates including differing surveillance systems, case definitions and reporting procedures between countries. In fact, Gibbons et al. [Reference Gibbons20] suggest that multiplication factors could be used to adjust for underestimation to provide a more accurate estimate of incidence and these should be disease, country, age and sex specific. Therefore, it is essential that studies on disease under-reporting to surveillance systems are performed regularly so that the true epidemiological burden of disease is understood within a population.
The main limitation of this study was the inability to remove hospital transfers from the HIPE dataset which is, in the main, due to discrepancies between hospitals in coding for such transfers. Therefore, it is possible that some cases may have more than one episode of hospital in-patient care recorded. However, the discrepancies in numbers between the hospitalized and notified cases are so large for some diseases that they cannot solely be attributed to over-representation by hospital transfers and clearly represent a large cohort of under-notified patients. The introduction of a unique patient identifier for the Irish health system would eliminate many such issues in the analysis of this and other health data.
The HIPE dataset is a very useful research tool; however, due to the volume of records to be processed and validated, it will never be a routinely used data source for current information on notifiable diseases. Therefore the notification process is an important system for the collection of real-time clinical and laboratory infectious disease data on an ongoing basis. The CIDR environment provides a flexible way for laboratories to discharge their notification obligations and these developments have made a huge impact on the quality of surveillance data in Ireland.
Any support that can be given to hospital clinicians to improve their understanding of the notification process and to encourage their participation will be beneficial in the long term.
ACKNOWLEDGEMENTS
This report would not have been possible without the HIPE system provided by the Healthcare Pricing Office and the web-based facilities of Health Atlas Ireland. We are also grateful for notification data supplied through CIDR and the TB surveillance system by the Health Protection Surveillance Centre
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
DECLARATION OF INTEREST
None.






