INTRODUCTION
The zoonotic potential of Mycobacterium avium ssp. paratuberculosis has been debated for a century since it was first suggested that Crohn's disease (CD) is pathologically and clinically analogous to Johne's disease [Reference Behr and Collins1–Reference Grant3]. Research on this association has increased over the last 30 years, from the first isolation of M. paratuberculosis in a CD patient by Chiodini et al. and the subsequently published review of evidence supporting the potential association in 1989 [Reference Chiodini4, Reference Chiodini5]. A detailed history of this research can be found in a number of papers and books on this topic [Reference Behr and Collins1, Reference Naser6]. More recently M. paratuberculosis has been hypothesized to play a role in the development or progression of a number of other human diseases including HIV infection, sarcoidosis, diabetes mellitus type 1 (T1DM) and type 2 (T2DM), multiple sclerosis and Hashimoto's thyroiditis mainly on the basis of immune dysfunction hypotheses [Reference Nacy and Buckley7–Reference Sechi11].
These chronic diseases have no known cure and together they affect several hundred million people globally. CD is a chronic debilitating idiopathic gastroenteritis that affects 1–2 million people worldwide [Reference Naser6, Reference Molodecky12]. Similarly diabetes mellitus (T1DM, an autoimmune condition where insulin is not produced by the pancreas and T2DM, a condition where cells do not respond to normal levels of insulin) affects 382 million people worldwide [13]. Multiple sclerosis is a complex autoimmune disease of the central nervous system that affects an estimated 1·3 million people worldwide [Reference Cossu, Masala and Sechi8, 14]. Hashimoto's thyroiditis (chronic lymphocytic thyroiditis) is an autoimmune disease in which the thyroid gland is attacked by a variety of cell- and antibody-mediated immune processes and affects 7·2–36 million people worldwide [15]. Sarcoidosis is an unexplained abnormal collection of inflammatory cells in organs that usually resolves without medical intervention except in rare cases where it can become a chronic condition; it is estimated to affect 0·07–2·9 million people worldwide [Reference Erdal16].
Many of the hypotheses on the potential zoonotic role of M. paratuberculosis draw on pathological and clinical similarities (analogy) between Johne's disease in ruminants and CD in humans, and M. paratuberculosis’ ability to cause natural infection in non-ruminants, e.g. dogs, rabbits, non-human primates [Reference Behr and Collins1, Reference El-Zaatari, Osato and Graham17]. The evidence for M. paratuberculosis’ role in human disease to date points to multiple interacting factors such as dysregulation of the immune system (e.g. genetic predisposition, immune-mediated tissue injury) and environmental exposures, such as diet and exposure to pathogens or changes in the normal gut microflora that when combined may cause the disease [Reference Behr and Collins1, Reference Naser6]. Molecular mimicry has also been suggested to have a role in the pathogenesis of CD, T1DM and multiple sclerosis, proposing that an infectious agent like M. paratuberculosis has antigenic elements similar to the host that can induce autoimmunity [Reference Cossu, Masala and Sechi8].
This systematic review (SR) tabulates the global evidence and presents meta-analytical summaries of the current knowledge underpinning the public health question, ‘Is M. paratuberculosis zoonotic?’ [Reference Young18, Reference Sargeant19]. This SR updates a previous SR covering primary research until the end of 2005 [Reference Waddell20] and evaluates agreement or changes in research knowledge published over the last 9 years [Reference Naser6, Reference Feller21–Reference Eltholth23]. The scope of this SR is extended beyond potential association between M. paratuberculosis and CD, and includes all human diseases that have been linked with M. paratuberculosis. To the best of our knowledge no other SR has evaluated evidence on M. paratuberculosis as an infectious disease candidate for multiple human diseases.
The objectives of this SR/meta-analysis (MA) are: (1) to identify and characterize the research investigating an association between human diseases and M. paratuberculosis, (2) to estimate the strength of the association(s) and agreement across epidemiological studies using various detection methods and, (3) evaluate strengths, weaknesses, and gaps in current evidence. Knowledge gaps and future research priorities to address public health questions are highlighted in the Discussion.
METHODS
Protocol
An a-priori developed and pre-tested SR protocol (Supplementary material: Appendix 1) included the study question, sub-questions, definitions, procedure for literature search, study inclusion/exclusion criteria and checklists for conducting relevance screening, basic characterization, methodological assessment and data extraction on relevant primary research, following the general principles of SR methodology [Reference Young18, Reference Sargeant24, Reference Rajic, Young and McEwen25]. All primary research investigating the zoonotic potential of M. paratuberculosis and human disease, and published in English or French languages was analysed. This included case-control studies investigating associations between M. paratuberculosis and human disease, studies looking at the association between Johne's disease in ruminants and human disease and molecular epidemiology studies examining the relatedness of human and animal isolates of M. paratuberculosis. Relevant expertise within our SR research team included M. paratuberculosis, food safety, risk assessment, epidemiology, library, and synthesis research (e.g. SR and MA) methods.
Search strategy
The original search strategy by Waddell et al. [Reference Waddell20] was simplified and updated based on recent improvements in electronic bibliographic databases and the number of databases was reduced based on the results of unpublished research on search sensitivity and specificity [Reference Harris26]. The following search algorithms were implemented on 27 September 2013 in three databases, Pubmed, Scopus and Current Contents:
((johne disease) OR (johne's disease) OR (johne* disease) OR paratuberculosis OR (mycobacterium paratuberculosis) OR (mycobacteria AND crohn*)) AND ((crohn* disease) OR (crohn disease) OR (crohn's disease) OR (inflammatory bowel disease) OR (inflammatory bowel diseases) OR diabetes OR sarcoidosis OR lymphadenitis OR immunocompromis* OR (AIDS) OR (Blau syndrome) OR (multiple sclerosis) OR thyroiditis)
For research on CD, publication date was set from 1 January 2005 onward without any other restrictions or filters as this aspect was already investigated in the previous SR covering research to 2005 [Reference Waddell20]. For all other diseases we did not impose any restrictions. Citations retrieved from all databases were imported into reference management software Procite v. 5.0.3 (ISI Researchsoft, 1995) and de-duplicated. Search verification was twofold; first, reference lists of review articles [Reference Naser6, Reference Sohal27–Reference Kaevska and Hruska31] and three SRs published on M. paratuberculosis [Reference Feller21–Reference Eltholth23] were searched by hand. Second we identified all citations relevant to this SR during relevance screening for a scoping review on sources of M. paratuberculosis being conducted at the same time to identify any potentially missed citations. Any potentially relevant unique citation identified by search verification was added to the SR at the relevance screening stage and processed through all SR tools as appropriate.
Abstract and article-level relevance screening and study characterization
Through initial abstract-based screening (Fig. 1) by two reviewers working independently, all potentially relevant primary research in English or French investigating the zoonotic potential of M. paratuberculosis was identified. Non-primary research studies (e.g. narrative reviews) or primary research studies outside of the scope were intentionally excluded. All citations deemed relevant at the abstract-based screening level were procured as full articles. Abstract screening was conducted by two independent reviewers using standardized and pre-tested checklists. Reviewer agreement (κ ⩾ 0·8) was evaluated using 30 abstracts. Conflicts were resolved through consensus between respective reviewers and if this was not possible, by a third team member.
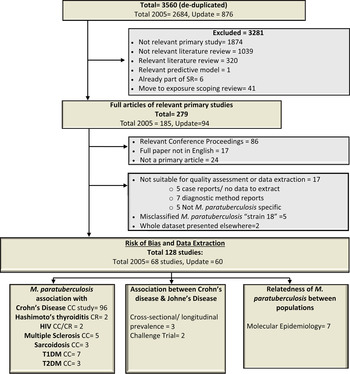
Fig. 1. Flow chart describing the movement of 3560 citations, the exclusion of 3432 and the inclusion of 128 studies through the systematic review process. HIV, Human immunodeficiency virus; T1DM/T2DM, diabetes mellitus type 1/type 2; CC, case-control study; CR case report or case series.
Risk of bias, GRADE and data extraction
Full paper assessment using a direct modification of the risk of bias and Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria endorsed by the Cochrane collaboration was undertaken on each study and GRADE criteria were summarized for groups of studies contributing to each MA [Reference Guyatt32–35]. The risk-of-bias assessment aims to assess the internal validity of the study which informs of the GRADE criteria [Reference Higgins, Altman, Higgins and Green33, 34]. GRADE criteria are summarized across groups of similar studies to indicate the level of confidence in the current evidence [35]. The 1- to 4-star grading system indicates: high confidence that the effect estimate is close to the true effect (****); moderate confidence in the effect estimate, but future studies may be substantially different (***); limited confidence in the estimate of effect, the true effect may be substantially different (**); and very little confidence in the estimate of effect, the true effect is likely to be substantially different (*) [Reference Balshem36–Reference Schunemann38]. The data extraction tool (Supplementary material, Appendix 1) aimed to efficiently capture the pertinent information and results needed for MA from each study. Both steps were conducted by two reviewers working independently and conflicts were resolved by consensus.
Study management and data analysis
All SR stages, i.e. relevance screening, risk-of-bias assessment and data extraction, were conducted in Microsoft Excel 2010 (Microsoft Corp., USA). The data were analysed descriptively [Stata v. 13 (StataCorp., USA)], followed by random-effects MA of selected subgroups of data in Comprehensive Meta-analysis Software v. 2 (Biostat, USA), which employs the method of moments weighting procedure [Reference DerSimonian and Laird39]. Heterogeneity, or across study variation, was quantified by Higgins’ I 2 [Reference Higgins40]. Meta-regression was conducted in Stata v. 13 using the random-effects maximum likelihood (REML) weighting procedure and changes in T 2, an estimate of τ 2 or true standard variance across studies was used to quantify the amount of heterogeneity that was explained by covariates in the model [Reference Borenstein41]. Where appropriate, homogenous MAs with more than 10 lines of data were evaluated for publication bias by Begg's and Egger's [Reference Egger42, Reference Begg and Mazumdar43] tests. If publication bias was detected, Duval & Tweedie's trim-and-fill method was used to estimate the potential impact on the estimate and conclusions of the MA [Reference Duval and Tweedie44]. Results with high heterogeneity are still reported in this review as we believe that they are more informative than reporting a range of results. However, we caution readers to use heterogeneous results carefully as the source of heterogeneity is unknown and future research may significantly change the results of the MA.
RESULTS
Description of relevant studies
The updated electronic search yielded 876 citations after de-duplication and Figure 1 shows the progression of papers through the SR process. A total of 128 articles, 60 added in this update 2005–2013, are included and summarized in this SR (Supplementary Appendix 2, list of included studies). All studies were published in English and originated from the USA/Canada (30%), Europe (52·6%), Asia (9·8%), Australia and New Zealand (6·0%) and the Middle East (0·8%). The top three publishing countries were: USA (25·6%, n = 34), Italy (18·8%, n = 25, 16 from a single research group) and UK (12%, n = 16). The first relevant study was published in 1980; however, the majority (65%) of the studies summarized in this SR were published since 2000. Studies were mainly observational (95%) and case control was the most common study design (87%). Patients with CD were the focus of 80% of studies, with human immunodeficiency virus (HIV) (1·6%), sarcoidosis (2·4%), T1DM (5·5%), T2DM (2·4%), multiple sclerosis (5·5%), Hashimoto's thyroiditis (1·6%) and human (disease not reported in a few prevalence surveys and molecular epidemiology studies) (3·9%) also captured in this SR.
Studies were categorized in three main groups; (1) those that evaluated the association of M. paratuberculosis with human disease, (2) the association between Johne's disease in ruminants and human disease (mainly CD), and (3) molecular epidemiology studies investigating the relatedness of M. paratuberculosis isolates from different species to evaluate evidence for strain sharing and zoonotic potential. There were 116 studies that contributed 163 lines of data summarized in Tables 1–4 on the association of M. paratuberculosis with CD (96 studies), HIV (2), sarcoidosis (3), T1DM (7), T2DM (3), multiple sclerosis (5), Hashimoto's thyroiditis (2). Five studies with varying study designs and objectives looked at evidence for an association between Johne's disease and CD including two cross-sectional studies examining high exposure occupations and CD [Reference Jones45, Reference Qual46], one case series that tested newly diagnosed CD patients over 5 years for M. paratuberculosis [Reference Wagner47] and two challenge trials that investigated whether M. paratuberculosis isolates from humans could produce Johne's disease in goats [Reference Van Kruiningen48, Reference Gitnick49]. Seven molecular epidemiology studies looked at the relatedness of M. paratuberculosis isolates from a variety of species including humans [Reference Singh50–Reference Francois, Krishnamoorthy and Elion56].
Table 1. Summary of findings: association of direct detection of Mycobacterium paratuberculosis and Crohn's disease in humans
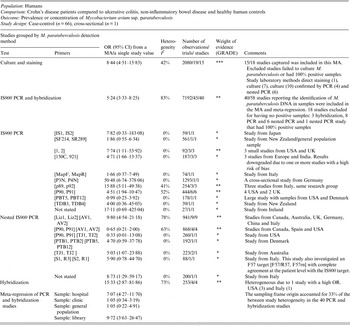
OR Odds ratio; CI, Confidence interval; MA, meta-analysis; PCR, polymerase chain reaction; I 2, measure of heterogeneity.
GRADE: GRADE Working Group grades of evidence (see explanations): **** high quality: very confident the true effect lies close the estimate of effect;
*** moderate quality: moderate confidence the true effect is likely close to the estimate of effect, but is possibly substantially different; ** low quality: limited confidence in the estimate of effect, the true effect may be substantially different; * very low quality: very little confidence in the estimate of effect, the true effect is likely substantially different from the estimate of effect.
The potential association of M. paratuberculosis with Crohn's disease was investigated in 67 studies by culture, PCR or hybridization to identify M. paratuberculosis in samples (tissue, blood, faeces). Forty-nine out of 67 studies provided usable data and are included in the meta-analyses presented in this table. The 18 excluded either had no M. paratuberculosis isolated or 100% of samples were positive. The methodological soundness of included studies was poor [case-control (66) and cross-sectional (1)] and there was a lot of unexplained heterogeneity in the PCR and hybridization studies. Many studies did not detect a significant association between M. paratuberculosis and Crohn's disease; however, the MA results were significant for both the culture and PCR results. Due to the heterogeneity and low methodological quality of the included studies, future research will likely alter the estimates from this summary-of-findings table.
Table 2. Summary of findings: association of Mycobacterium paratuberculosis and Crohn's disease in humans by ELISA
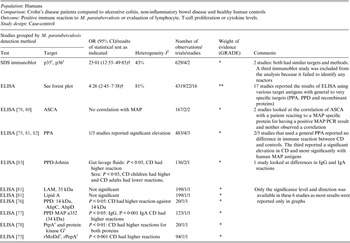
OR, Odds ratio; CI, Confidence interval; SDS, sodium dodecyl sulfate; r recombinant protein; † results of a random effect meta-analysis;ELISA, enzyme-linked immunosorbent assay; PPA, protoplasmic antigen; PPD, purified protein derivative; I 2, measure of heterogeneity; ASCA, anti-Saccharomyces cerevisiae antibody; MAP, Mycobacterium avium ssp. paratuberculosis; PCR, polymerase chain reaction; CD, Crohn's disease; LAM, lipoarabinomannan; AhpC, alkyl hydroperoxide reductase C; AhpD, alkyl hydroperoxide reductase D; PtpA, protein tyrosine phosphatase A.
GRADE: GRADE Working Group grades of evidence (see explanations): **** high quality: very confident the true effect lies close the estimate of effect; *** moderate quality: moderate confidence the true effect is likely close to the estimate of effect, but is possibly substantially different; ** low quality: limited confidence in the estimate of effect, the true effect may be substantially different; * very low quality: very little confidence in the estimate of effect, the true effect is likely substantially different from the estimate of effect.
The potential association of M. paratuberculosis with CD was investigated in 30 studies that used ELISA to determine if the immune response to a M. paratuberculosis antigen was different in CD compared to controls. All studies except one used blood samples as well as gut lavage samples. Target antigens ranged from non-specific PPA or LAM preparations to targeted pure recombinant proteins. Nineteen of the 30 studies provided usable data and are included in the meta-analyses presented in this table and Figure 2. The three studies excluded from this table had no reaction [Reference Kallinowski63], analysed inflammatory bowel disease patients as one group [Reference Juste85] or did not test the control group [Reference McFadden and Houdayer84]. The methodological soundness of included studies was poor (case-control), there was a lot of heterogeneity in the methods and the target antigens. Many studies individually did not detect a significant association between M. paratuberculosis and CD; however, overall the meta-analyses detected a significant association and the ELISA results were similar to those from culture and PCR studies. Due to the heterogeneity and low methodological quality of the included studies, future research will likely alter the estimates from this summary-of-findings table.
Table 3. Summary of findings: association of Mycobacterium paratuberculosis and Crohn's disease in humans using lymphocyte/cytokine assays
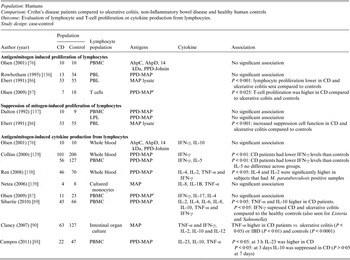
CD, Crohn's disease; PBMC, peripheral blood macrophage cells; AhpC, alkyl hydroperoxide reductase C; AhpD, alkyl hydroperoxide reductase D; PPD, purified protein derivative; PBL, peripheral blood lymphocytes; MAP, Mycobacterium avium ssp. paratuberculosis; LPL, lamina propria lymphocytes; IL, interleukin.
GRADE: GRADE Working Group grades of evidence (see explanations): **** high quality: very confident the true effect lies close the estimate of effect; *** moderate quality: moderate confidence the true effect is likely close to the estimate of effect, but is possibly substantially different; ** low quality: limited confidence in the estimate of effect, the true effect may be substantially different; * very low quality: very little confidence in the estimate of effect, the true effect is likely substantially different from the estimate of effect.
The potential association of M. paratuberculosis with CD was investigated in 11 studies that examined lymphocyte proliferation or cytokine production in response to M. paratuberculosis. Various cytokines were evaluated, most studies did not have extractable data, and data was displayed graphically in the papers. The methodological soundness of included studies was poor (case-control), there was a lot of heterogeneity in the methods and most are not directly comparable to each other. Many studies individually did not detect a significant difference in the immune reaction of CD samples vs. controls. Due to the heterogeneity and low methodological quality of the included studies, future research will likely alter the estimates and conclusions in this table.
Table 4. Summary of findings: association of Mycobacterium paratuberculosis and diseases other than Crohn's disease in humans
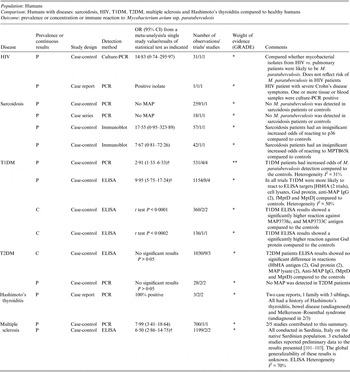
OR, Odds ratio; CI, Confidence interval; P, Prevalence results (+/-); C, results on a continuous scale; † results of a random effect meta-analysis; PCR, polymerase chain reaction; MAP Mycobacterium avium ssp. paratuberculosis.
GRADE: GRADE Working Group grades of evidence (see explanations): **** high quality: very confident the true effect lies close the estimate of effect; *** moderate quality: moderate confidence the true effect is likely close to the estimate of effect, but is possibly substantially different; ** low quality: limited confidence in the estimate of effect, the true effect may be substantially different; * very low quality: very little confidence in the estimate of effect, the true effect is likely substantially different from the estimate of effect.
The potential association of M. paratuberculosis with diseases other than Crohn's disease was investigated in 20 studies (2 HIV, 2 sarcoidosis, 7 T1DM, 3 T2DM, 5 multiple sclerosis and 2 Hashimoto's thyroiditis). Methodological soundness of included studies was poor (case-control, case report and case series), and most were examining immune reactions, thus examining the association indirectly. T1DM and multiple sclerosis show consistent, statistically significant results across studies and outcomes. The hypothesis of M. paratuberculosis involvement in Hashimoto's thyroiditis requires investigation. No statistically significant association was reported for T2DM, sarcoidosis or HIV. Thus, additional studies on any of these diseases could change the conclusions and would likely alter the estimates from this summary-of-findings table.
Excluded: Reid & Chiodini (1993) [Reference Reid and Chiodini140] on sarcoidosis; excluded because the antigens were not specific to M. paratuberculosis.
M. paratuberculosis association with CD
One hundred and eight studies examined the association between M. paratuberculosis bacteria or antigen with CD, and of these 96 were case-control studies. Sixty-seven case-control studies directly detected the presence of M. paratuberculosis from human samples; faeces, blood, or intestinal tissue using culture [confirmed by staining, histology and polymerase chain reaction (PCR)], PCR (single and nested) or hybridization. Of these 67 studies, 18 were excluded from summarization and analysis because they did not have any M. paratuberculosis-positive results (17 studies) or they had 100% positive samples in both groups (1) [Reference Sasikala57–Reference Cellier74]. There were 15 studies (19 lines) that reported detection of live M. paratuberculosis by culture with an overall odds ratio (OR) of 8·44 [95% confidence interval (CI) 4·51–15·83, I 2 = 42%; Table 1]. Similarly, across 40 studies (45 lines) that detected M. paratuberculosis DNA (44) or RNA (1) in samples by PCR and hybridization the overall OR of 5·24 (95% CI 3·33–8·25, I 2 = 83%) showed a heterogeneous positive association. Investigating reasons for this heterogeneity by subgroup analysis of PCR primers and using meta-regression for selected explanatory variables indicated that the origin of the sampling frame (hospital, clinic, general population or isolate library) accounted for a significant proportion (33%) of heterogeneity. Substantial variation in OR by different detection methods was observed across studies, particularly in PCR primers used (many developed in-house); the results are synthesized by primer in Table 1. Several primers yielded non-significant results across several studies. No other a-priori selected explanatory variable accounted for the heterogeneity, including choice of control group (healthy, other intestinal disease or ulcerative colitis), M. paratuberculosis detection methods (direct PCR, nested PCR, hybridization), sample type (faeces, blood or intestinal tissue: biopsy vs. resected), sample state (fresh, frozen, paraffin embedded), primer size, GRADE (down grade or no grade change) or risk of bias (low, unclear, high) designation. In direct detection studies, 27 reported a significant positive association, 15 a positive non-significant association, five a negative non-significant association, and two a significant negative association. A further 17 did not isolate any M. paratuberculosis and one reported 100% samples positive; these studies could not assess association. Since most studies were secondary-based case-control designs, confidence in this evidence is moderate to weak (GRADE ** to ***).
Thirty-eight studies (54 lines of data) used evidence of immune response by ELISA or immunoblot and/or examined differing results in lymphocyte or cytokine assays in CD patients compared to controls. Similar to the results for detection of M. paratuberculosis, 16 ELISA studies (22 lines) used a number of antigens, demonstrated high heterogeneity between studies and had an overall OR of 4·26 (95% CI 2·45–7·38, I 2 = 81%; Fig. 2). The two studies (four lines) that reported results from sodium dodecyl sulfate (SDS) immunoblot had an overall OR of 25·01 (95% CI 12·55–49·85, I 2 = 43%; Table 2). Four other studies reported significant associations with reactivity to M. paratuberculosis [Reference Shin75–Reference Bach78], four reported no significant association [Reference Lee79–Reference Walmsley82] and one reported contradictory associations between children and adults with CD [Reference Verdier83] (Table 2). Three studies were excluded from this summary because no reaction to M. paratuberculosis occurred [Reference Kallinowski63], only CD patients were tested [Reference McFadden and Houdayer84] and results were not reported separately for CD and ulcerative colitis patients [Reference Juste85]. Eleven studies (14 lines) examined differences in lymphocyte proliferation or cytokine production in response to M. paratuberculosis between CD patients and controls (Table 3). A significant association between CD patients and controls was reported in two of four studies using lymphocyte proliferation where peripheral blood lymphocytes (PBL) were suppressed and there was increased suppression cell function in one study [Reference Ebert86] and higher T-cell proliferation in the other for CD patients compared to controls [Reference Olsen87]. Eight studies examined cytokine production, three found no association and the remaining five reported lower IFN-γ and higher IL-4, IL-2, TNF-α in CD patients compared to controls. Only IL-10, examined in four studies, had conflicting results in two studies [Reference Campos88, Reference Sibartie89] and non-significant results in two studies [Reference Olsen76, Reference Clancy90], Table 3. Overall studies examining immune reactions to M. paratuberculosis in CD patients and controls reported significant positive association in 22 studies and non-significant results in 15 studies (14 positive and one negative).
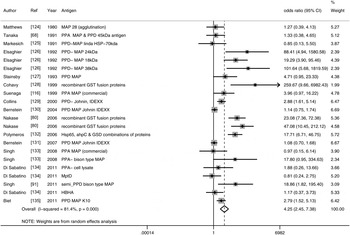
Fig. 2. Random-effects meta-analysis of 16 case-control studies (I 2 = 81%) that examined the association of immune reactions measured by ELISA to M. paratuberculosis in Crohn's disease patients compared to controls. MAP, M. paratuberculosis; PPD, purified protein derivative; PPA, protoplasmic antigen, HSP, heat shock protein; AHP, alkyl hydroperoxide reductase; GSD, glycosyl transferase; HBHA, heparin-binding haemagglutinin.
A small number of non-case control observational studies and experiments relevant to the evaluation of the association between M. paratuberculosis and CD were identified. As shown in Table 5, two cross-sectional surveys did not find an association between CD and high-risk occupations such as dairy farming and practising veterinary medicine on large animals [Reference Jones45, Reference Qual46]. The third study was a case series of newly diagnosed CD patients that were followed and periodically tested for M. paratuberculosis over 5 years. In this study of 13 patients, three always tested positive, three were always negative and the other seven tested both positive and negative over time [Reference Wagner47]. Two studies were challenge trials where M. paratuberculosis strains isolated from humans were used to experimentally challenge young goats; however, neither trial was of long enough duration to produce clinical Johne's disease in the goats and only one trial described Johne's disease-like lesions [Reference Van Kruiningen48, Reference Gitnick49].
Table 5. Summary of findings: association of Mycobacterium paratuberculosis and Crohn's disease in humans by non-case-control study design

JD, Johne's disease; CD, Crohn's disease.
GRADE: GRADE Working Group grades of evidence (see explanations): **** high quality: very confident the true effect lies close the estimate of effect; *** moderate quality: moderate confidence the true effect is likely close to the estimate of effect, but is possibly substantially different; ** low quality: limited confidence in the estimate of effect, the true effect may be substantially different; * very low quality: very little confidence in the estimate of effect, the true effect is likely substantially different from the estimate of effect.
There were a limited number of observational studies that looked at an association with JD, and results from prevalence and longitudinal prevalence surveys.
Molecular epidemiology studies (n=7) make up the last type of relevant evidence by comparing isolates from different species and humans, some linked by geography and time, while others are from diverse sources around the world (Table 6). Globally, human isolates were considered most likely to be of bovine origin [Reference Wynne51–Reference Singh55], although the human isolates from two studies were also closely related to ovine type M. paratuberculosis [Reference Ghadiali52, Reference Motiwala53]. In addition, two studies from India reported that a high proportion of human isolates were bison type, results so far unique to India [Reference Francois, Krishnamoorthy and Elion56, Reference Singh91]. Wynne et al. [Reference Wynne51] described human isolates that matched the bovine isolate from samples taken at the same time in the same region, lending evidence for potential sharing of M. paratuberculosis between species.
Table 6. Summary of findings: molecular epidemiology evidence for the relatedness of Mycobacterium paratuberculosis from various animal species and humans

PCR-REA, Polymerase chain reaction-restriction enzyme analysis; CD, Crohn's disease; nIBD, non-inflammatory bowel disease; CGH, comparative genome hybridization; UC, ulcerative colitis; MPIL, multiplex PCR of IS900 integration loci; AFLP, amplified fragment length polymorphism; RFLP, restriction fragment length polymorphism.
GRADE: GRADE Working Group grades of evidence (see explanations): **** high quality, very confident the true effect lies close the estimate of effect; *** moderate quality: moderate confidence the true effect is likely close to the estimate of effect, but is possibly substantially different; ** low quality: limited confidence in the estimate of effect, the true effect may be substantially different; * very low quality: very little confidence in the estimate of effect, the true effect is likely substantially different from the estimate of effect.
Only 7 studies have examined the relatedness of M. paratuberculosis isolated from humans and animals. Many studies used an isolate library for their sampling frame, thus, are representative of a geographic area or point in time. Most human isolates were reported to cluster with bovine isolates, however some from Ghadaili [Reference Ghadiali52] and Motiwala [Reference Motiwala53] also clustered with ovine isolates and those from India were most likely to be bison type (Singh [Reference Singh50, Reference Francois, Krishnamoorthy and Elion56]). Overall a close relationship between human and animal strains was reported, but it is still unclear if there is limit strain diversity in human M. paratuberculosis. Further research should focus on representative sampling and further evaluation of strain diversity from human isolates. Due to heterogeneity in the molecular methods and limited extrapolation of the samples evaluated future studies may completely change the conclusions of this summary-of-findings table.
M. paratuberculosis association with human disease other than CD
The majority of research on M. paratuberculosis’ association with human disease has focused on CD described in the previous section; however, there are 20 studies (16 case-controls, three case reports and one case series) that investigated the potential association of M. paratuberculosis with other human diseases (Table 4). The evidence within these studies was deemed very weak (GRADE *), meaning future research may completely change the conclusions of current research. The exception was T1DM that was recently investigated in seven studies (from the USA and Italy) all of which agreed an association may exist, providing weak but consistent evidence (GRADE * to **) for an association (Table 4).
Random-effects MA of the association between T1DM and direct detection of M. paratuberculosis by PCR yielded an OR of 2·91 (95% CI 1·33–6·33) [Reference Paccagnini10, Reference Rosu92–Reference Naser94] and for the association between T1DM and detection of an immune reaction to M. paratuberculosis by ELISA yielded an OR of 9·95 (95% CI 5·75–17·25) [Reference Rosu92–Reference Masala95] two additional ELISA studies not included in the MA reported significantly higher reactions to M. paratuberbulosis in T1DM patients compared to controls [Reference Cossu96, Reference Cossu97]. Since our SR search, a study from India has reported finding no association with T1DM [Reference Rani98].
Recently, very weak evidence for a significant association with multiple sclerosis and M. paratuberculosis has been investigated in Sardinia, Italy, which is a unique isolated population that has very high levels of autoimmune disease [Reference Cossu99, Reference Frau100], three studies [Reference Cossu101–Reference Cossu103] were removed from this summary as they are preliminary data to Frau et al. [Reference Cossu100] to avoid double-counting results (Dr Sechi, personal communication). The generalizability of this association needs to be investigated. A hypothesis was presented for an association between M. paratuberculosis infection and Hashimoto's thyroiditis based on two case reports involving one family [Reference Sisto9, Reference D'Amore104]. This has been further supported by a study published after our SR search, also from Italy, that provides evidence for an association with M. paratuberculosis [Reference Rosu105]; however, future research on other populations is needed to provide further evidence for this hypothesis. By contrast, there was no observed association between M. paratuberculosis infection with T2DM [Reference Rosu92, Reference Naser94, Reference Rosu106] and non-significant results from HIV [Reference Richter107, Reference Naser108] and sarcoidosis studies [Reference El-Zaatari109–Reference Lisby, Milman and Jacobsen111]. The latter two diseases were examined over a decade ago and no further studies examining this association have been published.
DISCUSSION
This SR is an in-depth analysis of the current global evidence underpinning the zoonotic potential of M. paratuberculosis. The 60 studies added to this review since the original search in 2005 [Reference Waddell20], indicate that research on the potential association with human disease is still very active and not limited to CD, but now includes a possible association with T1DM, multiple sclerosis and Hashimoto's thyroiditis that will require further research to confirm or refute the existence of a true association. The MAs of case-control studies on CD conducted on different test categories demonstrate agreement in the direction and relative strength of the association. However, there is a general lack of evidence that explains the role of M. paratuberculosis in CD and the wide variation in control or disease group M. paratuberculosis prevalence across studies, indications that the evidence required to understand the role, if any, of M. paratuberculosis in the initiation or progression of CD has not been realized. Thus, we re-confirm our previous conclusions [Reference Waddell20] that while the zoonotic potential of M. paratuberculosis cannot be ignored, due to important knowledge gaps in understanding its role and importance in the development or progression of human disease, its impact on public health cannot yet be quantified or described.
The strongest evidence for an association between M. paratuberculosis and human disease (mainly from studies on CD) arises from the studies that identify culturable organisms or DNA in appropriate samples; these studies have been conducted in increasing numbers since the original SR [Reference Waddell20]. Moreover, these studies were based on CD populations around the world and included faecal and blood samples in addition to tissue samples (intestinal biopsy and resection). This group of 18 studies was homogenous, demonstrated culturable M. paratuberculosis in the samples and thus yielded the highest confidence in the MA conclusions. However, there were a number of methodological issues with this group of studies that ranked the risk of bias in eight of them (five published since 2005) as high or unclear due to inappropriate sampling frames and poor reporting of study design. There are many widely accepted guidelines for study design and reporting (e.g. STROBE statement for reporting observational studies) that if adhered to, should improve the utility and transparency of future studies [Reference Ebrahim and Clarke112–Reference Vandenbroucke114].
Studies that used PCR and hybridization to detect M. paratuberculosis demonstrate the existence of M. paratuberculosis DNA (or RNA) in the sample. From this information we cannot know if the detected DNA is from a culturable organism, but we can compare the frequency of occurrence between disease and control groups. There were 57 PCR and hybridization studies, of which 22 were published since 2005. There was a lot of heterogeneity in the results of these studies from sample preparation to the vast numbers of primers used across studies, including many that have been developed ‘in house’ and never validated. It should also be noted that some more widely used primers have produced only insignificant results for an association with CD, thus we encourage future researchers to consider using primers that have been validated to improve the interpretation and comparability across studies.
In the meta-regression significant heterogeneity was explained by accounting for the origin of the sampling frame (i.e. hospital vs. general population). The association between M. paratuberculosis and CD was only significant for case-control studies that used samples from hospitalized patients and tissue library samples, whereas samples taken from outpatient clinics and the general population showed no relationship. There are several possible reasons for this, including selection bias and different sampling strategies or the type of sample, which was not significant in the univariate meta-regression; however, outpatient studies were more likely to use faecal and blood samples and have less control over the medical history of the controls which could lead to misclassification. Alternatively, the outpatient findings may reflect a more realistic association than the secondary-based case-control studies. The potential discrepancy in results between primary- and secondary-based studies will require further appropriately designed research.
Across case-control studies there was considerable variability in the prevalence of M. paratuberculosis prevalence in both the control and diseased groups ranging from 0% to 100%. This inconsistency has not been explained in the literature, but may be due to true differences in prevalence, differences in test sensitivity and detection limits or even variation in sampling strategy. To complicate this further, a small cohort study by Wagner et al. [Reference Wagner47] demonstrated that some CD patients intermittently test positive for M. paratuberculosis by PCR over time and infection may persist despite antimicrobial treatment. Research has not adequately addressed the effects of stage of disease and various treatments on the outcome of M. paratuberculosis testing and there is conflicting results on the relationship between M. paratuberculosis test results and CD activity status (measure of disease symptoms occurring at the time of sample collection) [Reference Ebert86, Reference Olsen87, Reference Del Prete115–Reference Kobayashi, Blaser and Brown117].
Studies that have shown an immune reaction to M. paratuberculosis provide evidence of prior exposure to the organism, and there is some evidence that there is a different immune response to M. paratuberculosis in CD patients compared to controls. Overall, these studies provided evidence for an association at a similar magnitude to the direct detection studies, but individually, there were many studies that did not detect a significant association between M. paratuberculosis and human disease. Many of the lymphocyte and cytokine assay studies were working on the hypothesis that there is immune dysfunction in CD patients and they were attempting to demonstrate up-regulation or down-regulation of the inflammatory pathways. This type of work is also being done for Johne's disease with some recent successes in the identification of M. paratuberculosis activated immune pathways that lead to the clinical signs [Reference Bannantine and Bermudez118]. Across these studies there was little consistency in their approach, lymphocyte populations, antigens or cytokines, thus limiting the overall conclusions that can be drawn.
Evidence for the ecological association between Johne's disease and CD is limited and not strong. The two cross-sectional studies failed to find an association between high M. paratuberculosis exposure populations and CD [Reference Jones45, Reference Qual46]. A recent molecular epidemiology study demonstrated evidence of M. paratuberculosis strain sharing between human and bovine isolates from the same geographical range [Reference Wynne51]. This is in agreement with other studies that showed human isolates were more likely to be bovine type than ovine type M. paratuberculosis, including a recently published genome sequence from human breast milk [Reference Wynne51, Reference Motiwala53–Reference Singh55, Reference Bannantine119]. Exceptions to these results are studies from India, where high proportion of an indigenous bison-type M. paratuberculosis, classified as newly evolved, have been reported [Reference Singh50, Reference Francois, Krishnamoorthy and Elion56, Reference Singh120–Reference Sohal122]. The potential public health implications of high prevalence of M. paratuberculosis in humans from India remain unclear [Reference Singh50]. Future molecular research should seek to confirm the extent that different M. paratuberculosis strains may be shared between different species.
There are several limitations to the retrospective case-control studies, case series and case reports which make up 90% of the research captured in this SR. These include an inability to evaluate temporal relationships and M. paratuberculosis exposure [Reference Dohoo, Martin and Stryn123]. Selection bias can easily occur in case and control selection and the studies lack generalizability to the whole population [Reference Dohoo, Martin and Stryn123]. However, due to the long incubation period of mycobacterial infections and therefore long latency between likely exposure and disease, and the comparative low incidence of some of the studied diseases (particularly CD), case-control is often the only feasible study design [Reference Dohoo, Martin and Stryn123]. While we have gained more evidence over the past seven years, there is still a lack of longitudinal prospective cohort or case-controls studies in high exposure or low disease populations, respectively, that may help us understand the potential relationship between exposure to M. paratuberculosis and disease outcomes. Ultimately, we are still lacking an understanding of M. paratuberculosis’ role in CD or other human diseases.
This updated SR summarizes the largely epidemiological research on the association between M. paratuberculosis and human disease (mainly CD). It highlights important knowledge gaps such as understanding M. paratuberculosis's role in disease and the relationship between exposure and disease development in humans. Although we presented some strong and consistent MA results of the association between M. paratuberculosis and human disease; the variability in M. paratuberculosis prevalence in controls and diseased groups and a lack of understanding whether some or all CD is caused or propagated by M. paratuberculosis are important knowledge gaps. These are the reasons for the lack of support for recommending further interventions against human exposure to M. paratuberculosis beyond what the dairy and ruminant industries have already developed for animal health and economic reasons. Thus, M. paratuberculosis may have a role in human disease; however the evidence is not strong enough to inform the potential public health impact of M. paratuberculosis exposure.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S095026881500076.
ACKNOWLEDGEMENTS
The authors thank the reviewers, and Zee Leung, Vi Nguyen, Shannon Harding, Miranda Galati, Judy Greig, and Ian Young for their participation in this project. Thanks are due to the Public Health Agency of Canada for their support in conducting this research and the library staff for obtaining articles.
DECLARATION OF INTEREST
None.











