Introduction
As is known, attention-deficit/hyperactivity disorder (ADHD) is a common neurodevelopmental disorder characterised by persistent attention deficit, hyperactivity or impulsivity across settings (Biederman and Faraone, Reference Biederman and Faraone2005). The onset typically occurs in childhood, and many symptoms often persist into young adulthood (Barkley et al., Reference Barkley, Fischer, Smallish and Fletcher2002). Symptoms of ADHD can cause a wide range of functional impairment in daily life, including executive function impairment (Holst and Thorell, Reference Holst and Thorell2019). It is demonstrated that people with ADHD are prone to injuries and accidents, such as burns (Yeh et al., Reference Yeh, Hou, Tseng, Chen, Yang, Kuo, Weng, Lee, Chen and Lee2020), fractures (Mangus et al., Reference Mangus, Bergman, Zieger and Coleman2004), concussions (Nelson et al., Reference Nelson, Guskiewicz, Marshall, Hammeke, Barr, Randolph and McCrea2016) and traumatic brain injuries (Rowe et al., Reference Rowe, Maughan and Goodman2004; Liao et al., Reference Liao, Yang, Kuo, Liang, Huang, Wang, Lee, McIntyre and Chen2018). A few studies have revealed the association between ADHD and traffic accidents (Jerome et al., Reference Jerome, Habinski and Segal2006; Barkley and Cox, Reference Barkley and Cox2007; Vaa, Reference Vaa2014). The association is possibly mediated by the core symptoms of ADHD, risky driving patterns, aggressiveness and comorbidities (e.g. substance use disorder and oppositional defiant disorder/conduct disorder [ODD/CD]) (Barkley and Cox, Reference Barkley and Cox2007; Vaa, Reference Vaa2014; Romo et al., Reference Romo, Julien Sweerts, Ordonneau, Blot and Gicquel2019). However, the above studies' results are limited because of lack of objective measures, small sample sizes, self- or parent-reported driving performance with long recall periods, referral bias and absence of adjustment for critical comorbid illnesses. One Swedish population-based cohort study by Chang et al. reported that patients with ADHD had an increased risk of severe traffic accidents compared to those without ADHD (males: adjusted hazard ratio [aHR] = 1.47 [95% confidence interval (CI) 1.32–1.63]; females: aHR = 1.45 [95% CI 1.24–1.71]) (Chang et al., Reference Chang, Lichtenstein, D'Onofrio, Sjolander and Larsson2014). However, their study group was restricted to adults. Adolescents tend to have more road safety problems, including risky driving behaviours, traffic violations and driving without a license (Dobhal et al., Reference Dobhal, Dobhal, Kashyap and Bhadoria2019), especially for individuals with ADHD (Curry et al., Reference Curry, Metzger, Pfeiffer, Elliott, Winston and Power2017; Curry et al., Reference Curry, Yerys, Metzger, Carey and Power2019). Therefore, it is reasonable to include the adolescent population in the long-term study to explore the association between ADHD and vehicle accidents.
Transport accident is a major public health issue and one of the leading causes of physical disability and death for young people worldwide (Toroyan et al., Reference Toroyan, Peden and Iaych2013). There is emerging evidence that medication used for ADHD not only alleviates core symptoms but also has a protective effect against trauma and accidental injuries (Tai et al., Reference Tai, Gau and Gau2013; Man et al., Reference Man, Chan, Coghill, Douglas, Ip, Leung, Tsui, Wong and Wong2015). Stimulants are considered the first choice for treating ADHD and are the most effective treatment (Cortese et al., Reference Cortese, Adamo, Del Giovane, Mohr-Jensen, Hayes, Carucci, Atkinson, Tessari, Banaschewski, Coghill, Hollis, Simonoff, Zuddas, Barbui, Purgato, Steinhausen, Shokraneh, Xia and Cipriani2018). In Taiwan, methylphenidate (MPH) is the only stimulant approved for ADHD treatment. Some studies reported that MPH could improve driving performance in ADHD patients (Cox et al., Reference Cox, Humphrey, Merkel, Penberthy and Kovatchev2004; Barkley and Cox, Reference Barkley and Cox2007). However, these studies' effect sizes were small, and most of them were tested in driving simulators or specific driving tests, not in the real world. Two population-based cohort studies, one in Sweden and then another in the United States, conducted by Chang et al., reported that medication treatment for ADHD, regardless of cumulative dose, significantly reduced the risk for severe traffic accidents in these patients (Chang et al., Reference Chang, Lichtenstein, D'Onofrio, Sjolander and Larsson2014; Chang et al., Reference Chang, Quinn, Hur, Gibbons, Sjolander, Larsson and D'Onofrio2017). In the real world, medication discontinuation or non-adherence is common in patients with ADHD (Jensen et al., Reference Jensen, Arnold, Swanson, Vitiello, Abikoff, Greenhill, Hechtman, Hinshaw, Pelham, Wells, Conners, Elliott, Epstein, Hoza, March, Molina, Newcorn, Severe, Wigal, Gibbons and Hur2007; Gau et al., Reference Gau, Chen, Chou, Cheng, Tang, Chang, Tzang, Wu, Huang, Chou, Liang, Hsu, Lu and Huang2008). Whether the duration of medication therapy plays a moderator role in the risk of injuries remains uncertain. One report showed protective effects within 1 month (Raman et al., Reference Raman, Marshall, Haynes, Gaynes, Naftel and Sturmer2013), but another reported at least 6 months (Chen et al., Reference Chen, Yang, Liao, Kuo, Liang, Huang, Huang, Lee, McIntyre and Lin2017). Hence, it is vital to examine the impact of different cumulative doses of MPH on transport accident risk.
In the current study, we used a nationwide population-based cohort study to investigate the association of psychiatric comorbidities with the risk of transport accidents in ADHD and MPH. We hypothesised that young people with ADHD are prone to transport accidents than those without, and using MPH in these patients reduces the risk. We also hypothesised that the risk reduction becomes more prominent as the cumulative dose of MPH increases.
Materials and methods
Data source
Taiwan established the National Health Insurance (NHI) programme, a single-payer compulsory insurance system, on 1 March 1995. Since its launch in 1995, the coverage of the NHI programme has steadily increased. Up to 2009, the coverage rose to 99.5% of the national population in Taiwan (Ho Chan, Reference Ho Chan2010). The Bureau of NHI derived all medical claims data from NHI beneficiaries to form the National Health Insurance Research Database (NHIRD), which comprised of the records of hospital inpatient and outpatient care, dental services, ambulatory care and medication prescription. NHIRD utilised a systematic random sampling to provide a representative research sample of the national population, named Longitudinal Health Insurance Database (LHID), which contains medical claims of 1 000 000 people from all NHI beneficiaries. There are no significant differences in sex, age or health care utilisation between this sample and all beneficiaries (NHR, 2013).
Ethics
The Ethics Institutional Review Board of Chang Gung Memorial Hospital had reviewed and approved this study.
Sample
This is a population-based retrospective cohort study. We had two research hypotheses: ADHD young patients have an elevated risk of transport accidents, and MPH can reduce the risk of transport accidents with different doses. To test the first hypothesis, we compared the transport accident incidence between ADHD cases and non-ADHD cases. For the ADHD group, we identified ADHD cases according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 314 from the NHIRD. In Taiwan, clinicians used the ICD-9-CM code 314 as an insurance claim code for ADHD in the DSM-IV because of the NHI registry system (Tai and Chiu, Reference Tai and Chiu2009). ADHD cases enrolled in this study required at least one inpatient diagnosis or more than two outpatient diagnoses within 1 year during the observation period from 1 January 1997 to 31 December 2012, and followed up to the end of 2013. The flow chart of participant selection was presented in Fig. 1. At first, we identified 114 486 diagnoses of ADHD within the observation period after excluding those who had unknown sex status, less than 12 years old, and older than 35 years old (age was calculated as the difference from their birthdate to the end of follow-up, 31 December 2013). On the other hand, we enrolled 338 261 non-ADHD participants aged between 12 and 35 years old from the LHID 2005 database from NHIRD, which composed of 1 million beneficiaries randomly sampled in the year 2005 (NHR, 2013). We followed up these participants from 1997 to 2013.

Fig. 1. Flow chart for participants enrolled in this cohort study. ADHD, attention-deficit/hyperactivity disorder; LHID, longitudinal national health insurance research database; NHIRD, National Health Insurance Research Database.
To test the second hypothesis, we further used the 114 186 identified ADHD cases and classified them into MPH users (n = 89 826) and non-users (n = 24 660). Furthermore, to eliminate possible indication bias (i.e. patients with ADHD at higher risk of transport accidents may be more likely to be prescribed MPH), we performed within-person comparisons using the self-controlled case series (SCCS) model. In this model, participants with ADHD who had ever taken MPH and had a history of transport accidents were enrolled, and each participant served as his or her own control. We compared the rate of events during medication and non-medication periods.
Medication
In Taiwan, MPH is the only stimulant approved for ADHD. MPH included in our study was N06BA04 according to the Anatomical Therapeutic Chemical (ATC) code. Atomoxetine, which launched in Taiwan in 2007, is a non-stimulant approved for ADHD treatment. Before 2017, MPH had remained the first-line treatment according to the stipulation of the Bureau of NHI. Only 4% of all ADHD patients in the database were prescribed with ATX (Lee et al., Reference Lee, Yang, Shyu, Yuan, Yang, Lee, Lee and Wang2016). Hence, we confined our analysis to those with MPH prescriptions.
Transport accidents
The main outcome of our study was a transport accident. We identified the transport accidents according to the ICD-9-CM, code E810–E825. We further categorised the transport accidents into traffic accidents (ICD-9 codes: E810–E819) and non-traffic accidents (ICD-9 codes: E820–E825). Patients with ADHD were followed up for transport accidents as an outcome to the end of 2013.
Covariates
Several covariates were selected, including sex, age, and psychiatric comorbidity. Psychiatric comorbidity included autism spectrum disorder (ICD-9 code: 299), tic disorders (ICD-9 code: 307.2), epilepsy (ICD-9 code: 345), conduct disorder (ICD-9 code: 312), opposition defiant disorder (ICD-9-CM code: 313.81), anxiety disorders (ICD-9 code: 300), major depressive disorders (ICD-9 codes: 296.2, 296.3, 300.4 and 311), bipolar disorders (ICD-9 codes: 296.0, 296.1, 296.4–296.8 and 301.13) and schizophrenia (ICD-9 code: 295).
Statistical analysis
The statistical analyses were performed by using SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA) and R 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria). We used a chi-square (χ 2) test to compare the characteristics between the ADHD and non-ADHD groups. In response to two research hypotheses: ADHD young patients have an elevated risk of transport accidents, and MPH can reduce the risk of transport accidents with different doses, we performed two different analyses: between-subjects comparison and within-subjects comparison. There were two parts of the between-subjects comparison. First, by using a Cox proportional hazards regression model with adjustment for demographics and psychiatric comorbidities, we compared the risk of transport accidents between ADHD groups with and without each specific comorbidity and non-ADHD group. Analyses were stratified by sex and age category to examine the sex- and age-specific associations of transport accidents. Based on Korn and colleagues' suggestion, age was used as the time-scale in the survival analysis (Korn et al., Reference Korn, Graubard and Midthune1997). The results were further presented as aHRs with 95% CIs. Second, among individuals with ADHD, we compared the risk of transport accidents between MPH users with and without each specific comorbidity and non-users. We categorised these MPH users into three subgroups based on their cumulative defined daily dose (DDD): (1) 0, (2) >0 to <180 (0–180) and (3) ⩾180, and the group of 0 of DDD served as the control group in the analysis. The WHO proposed the DDD as a unit to measure drug exposure and recommended 30 mg per day to be one DDD of MPH. In clinical practice, if the patient takes medication continuously every day, 180 DDD stands for 180 days of refill prescription. As previous studies showed, the protective effect of MPH on the risk of suicide or fractures can be found in patients with prescriptions of 180 days or longer (Chen et al., Reference Chen, Yang, Liao, Kuo, Liang, Huang, Huang, Lee, McIntyre and Lin2017; Liang et al., Reference Liang, Yang, Kuo, Liao, Lin, Lee, McIntyre, Kelsen, Wang and Chen2018). Also, most ADHD patients received medication for less than 180 days (Chen et al., Reference Chen, Yeh, Chen, Chang, Wu and Lin2011; Garbe et al., Reference Garbe, Mikolajczyk, Banaschewski, Petermann, Petermann, Kraut and Langner2012). In order to determine the effect of dose on risk more precisely, we chose 180 DDD as the cut-point while categorising. We also estimated the preventable fraction: the proportion of transport accidents in patients with ADHD not taking MPH that could be prevented by MPH medication, calculated as follows:1 − adjusted hazard ratio
For within-patient comparisons, we compared the risk of transport accidents between the periods of medication and non-medication within MPH users by using the SCCS model (Whitaker et al., Reference Whitaker, Farrington, Spiessens and Musonda2006). Personal MPH records, including prescription date and prescribed days, were extracted throughout the entire study period (by the end of 2013). The results were presented as relative incidences (RRs) with 95% CIs. The RR estimated in the SCCS model was obtained by comparing the rate of transport accidents during the time periods of individuals having MPH with that during all other non-medication periods. The SCCS model automatically adjusts for all time-invariant factors (e.g. sex and genetic factors) and possible indication bias for the same patient before and during the follow-up. We defined the effective period of MPH exposure as 0–90 days after the prescription of the drug (Man et al., Reference Man, Coghill, Chan, Lau, Hollis, Liddle, Banaschewski, McCarthy, Neubert, Sayal, Ip, Schuemie, Sturkenboom, Sonuga-Barke, Buitelaar, Carucci, Zuddas, Kovshoff, Garas, Nagy, Inglis, Konrad, Hage, Rosenthal and Wong2017; Hollis et al., Reference Hollis, Chen, Chang, Quinn, Viktorin, Lichtenstein, D'Onofrio, Landen and Larsson2019). We further divided the effective period into three 1-month effective periods: 0–30, 31–60 and 61–90 days after MPH initiation (Shin et al., Reference Shin, Roughead, Park and Pratt2016). Finally, we merged the three effect periods into one period to evaluate the average pooled estimate of MPH use for the transport accidents risk. If patients had drug exposure continuously, we would record consecutive exposure effective periods. For example, if an individual was taking two 30-day supply of prescription successively, the effective periods will be 0–30, 0–30, 31–60 and 61–90 days.
Outcomes
Characteristics of subjects with or without ADHD
The study sample comprised of 114 486 ADHD patients and 338 261 non-ADHD participants. Table 1 lists the characteristics of ADHD patients and non-ADHD participants. Compared to the non-ADHD group, the ADHD group was more likely to be male, younger and comorbid with other psychiatric disorders (p < 0.001). Although the risk of transport accidents was 0.6% for the ADHD group and 1.0% for the non-ADHD, the ADHD group was at a younger age at the time of transport accidents (p < 0.001). Regardless of the ADHD group or the non-ADHD group, most transport accidents were traffic accidents (97.9%, 662/676 for ADHD and 98.8%, 3213/3252 for non-ADHD, respectively).
Table 1. Demographic characteristics of ADHD and non-ADHD youths and young adults, aged between 12 and 35 years
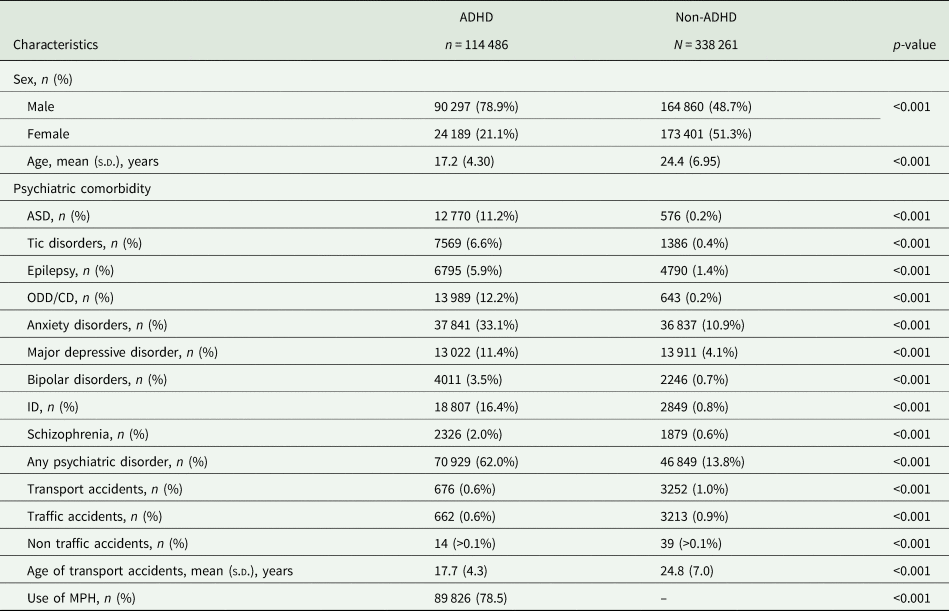
ADHD, attention-deficit/hyperactivity disorder; ASD, autism spectrum disorder; CD, conduct disorder; ID, intellectual disability; MPH, methylphenidate; ODD, oppositional defiant disorder.
Comparisons of the transport accidents risk between ADHD and non-ADHD
The risk of transport accidents between the ADHD group with and without each specific comorbidity and the non-ADHD group was presented in Table 2. We found that male ADHD cases had a higher risk of transport accidents than the non-ADHD group, with an aHR of 1.24 and 95% CI 1.10–1.39. The risk was noticeable when male ADHD patients were comorbid with epilepsy, ODD/CD and intellectual disability (ID). In the stratification analysis, we found the risks of transport accidents were less profound in female or adult individuals with ADHD. Female individuals with ADHD were only found to have higher risks of transport accidents when comorbid with autism spectrum disorder (ASD) or ID. In addition, adult individuals with ADHD were not associated with a higher risk of transport accidents regardless of psychiatric comorbidity.
Table 2. Cox proportional hazard regression model analysis for risk of transport accidents and psychiatric comorbid disorders between ADHD and non-ADHD, stratified by sex and age
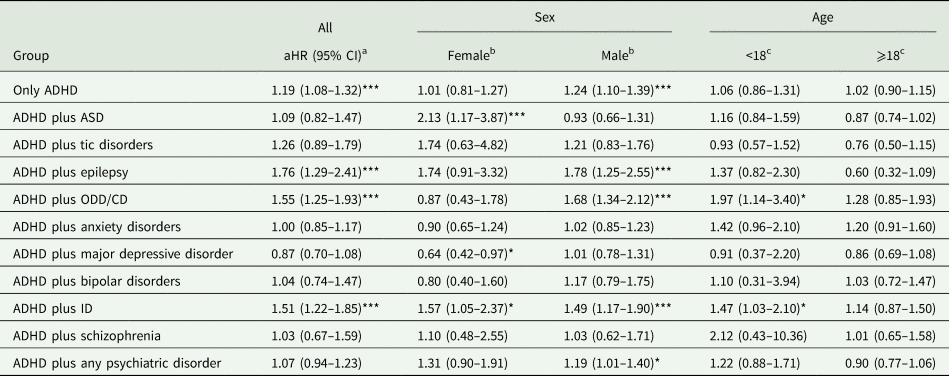
ADHD, attention-deficit/hyperactivity disorder; ASD, autism spectrum disorder; CD, conduct disorder; ID, intellectual disability; ODD, oppositional defiant disorder.
a Analysis was adjusted for age, sex and psychiatric comorbidity.
b Analysis was adjusted for age and psychiatric comorbidity.
c Analysis was adjusted for sex and psychiatric comorbidity.
The non-ADHD served as the reference group.
Comparisons of the effect of MPH on transport accidents risk in ADHD
Table 3 presents the dose-effect of MPH medication on transport accidents risk in patients with ADHD. Based on the Cox proportional hazard regression, MPH was associated with a reduced risk for transport accidents, and a significant dose–response relationship was observed. Compared to those without MPH use, the ADHD group with 0–180 DDDs had aHR of 0.23 (95% CI 0.19–0.26), and the group with more than 180 DDDs had aHR of 0.07 (95% CI 0.01–0.52). The preventable fractions of MPH medication were 0.77 and 0.93, indicating that 77 or 93% of transport accidents could be prevented if they took an equal dose of MPH medication (i.e. 0–180 DDD and ⩾180 DDD, respectively). Furthermore, the effect of MPH was also significant in ADHD patients comorbid with different neurodevelopment and psychiatric disorders (Table 4).
Table 3. Dose–response analysis of use of MPH of on transport accidents in ADHD youths using the Cox proportional hazard regression

ADHD, attention-deficit/hyperactivity disorder.
a Analysis was adjusted for age, sex and psychiatric comorbidity.
Preventable fraction is calculated as 1 minus relative risk.
Table 4. Cox proportional hazard regression model analysis of use of MPH of on transport accidents and psychiatric comorbid disorders in ADHD youths

ADHD, attention-deficit/hyperactivity disorder; ASD, autism spectrum disorder; CD, conduct disorder; ID, intellectual disability; ODD, oppositional defiant disorder.
Analysis was adjusted for sex and psychiatric comorbidity.
aIndividuals with ADHD did not take MPH medication or take less than 90 DDD; served as the reference group when the effect of MPH medication was examined in ADHD plus psychiatric comorbidities.
All analyses were adjusted for age, sex and psychiatric comorbidity.
For within-comparison, we conducted a conditional Poisson regression model for the SCCS cohort study (Table 5). Although the average risk during the first 3 months after using MPH was reduced (RR = 0.85), the effect did not reach significance level (95% CI 0.57–1.25). We found a potential beneficial effect of MPH on transport accidents, which seemed to be higher during the second to third month after using MPH (RR = 0.35, 95% CI 0.11–1.11), although not statistically significant.
Table 5. Conditional Poisson regression model, within comparison, for SCCS study design of transport accidents in ADHD youths with use of MPH

This analysis was conducted in ADHD youths who had a history of MPH use and transport accidents visit.
RR was calculated by conditional Poisson regression, adjusted for all time-invariant covariates that are constant within each individual during the follow-up.
SCCS analysis for ADHD was conducted using effect period for MPH medication set at 0–90 days.
Discussion
Our study found that male patients with ADHD had an increased risk of transport accidents, especially for those comorbid with epilepsy, ODD/CD or ID. Female ADHD patients comorbid with ASD and ID were also at risk of accidents. For ADHD patients with or without other psychiatric disorders, MPH prescription reduced the risk of transport accidents, and the protective effect was dose-related. At doses <180 DDD and ⩾180 DDD, MPH showed a significantly reduced risk for transport accidents by 77 and 93%, respectively.
Previous studies had reported that patients with ADHD tend to be reckless or inattentive during driving, which could attribute to traffic accidents (Barkley and Cox, Reference Barkley and Cox2007). A population-based cohort study in Sweden conducted by Chang et al. showed that, regardless of gender, ADHD patients had a 42 to 47% increase in transport accident risk compared to people without ADHD (Chang et al., Reference Chang, Lichtenstein, D'Onofrio, Sjolander and Larsson2014). The total follow-up time was 4 years (Chang et al., Reference Chang, Lichtenstein, D'Onofrio, Sjolander and Larsson2014). As in previous studies, our results supported that patients with ADHD had a higher risk of transport accidents after adjusting sex and psychiatric comorbidities (aHR = 1.19 [95% CI 1.08–1.32]). In our study, we not only extended the follow-up time to 17 years but also enrolled cases under the age of 18, the legal minimum driving age in Taiwan. Based on Curry et al.'s retrospective cohort study in North America, ADHD adolescents drivers had 1.36 times (95% CI 1.25–1.48) higher risk of a car accident (Curry et al., Reference Curry, Metzger, Pfeiffer, Elliott, Winston and Power2017). Also, as in many Asian countries, motorcycles are one of the primary means of vehicles in Taiwanese daily life (World Health Organization, 2015). Because of the ease of use of motorcycles and road traffic space limitations, unlicensed teenage motorcyclists and related severe traffic crashes are not uncommon in Taiwan (Chen et al., Reference Chen, Wang, Linkov and Pai2018; World Health Organization, 2015). As in our stratification analyses, ADHD patients who were less than 18-year-old and concurrent with ODD/CD or ID were at major risk for transport accidents, which may due to their immature impulse control or impaired cognitive function.
ADHD is highly concurrent comorbid with other mental illnesses (Jensen and Steinhausen, Reference Jensen and Steinhausen2015). In our study, male ADHD patients and specifically in those with epilepsy, ODD/CD or ID had increased risk of transport accidents, increased by 78, 68 and 49%, respectively. In Taiwan, the proportion and severity of traffic accidents for men are much higher than for women (Chang et al., Reference Chang, Li, Lu, Artanti and Hou2020). This may partially explain why only male patients were significantly associated with accidents in our study. In our study, female ADHD patients were only found to have higher risks of accidents when being comorbid with severe neurodevelopment disorders (i.e. ASD or ID). As past studies have shown, youths with destructive behaviour disorders or mental retardation often exhibit excessive impulsive or risky behaviours. Thompson et al. reported that ADHD concurrent with conduct problems was prone to risky driving in a case-control study (Thompson et al., Reference Thompson, Molina, Pelham and Gnagy2007). Savage et al. performed secondary analysis from the National Longitudinal Transition Study-2 (NLTS2) and reported that adolescents with mild ID were at greater risk for engagement in risky behaviours (Savage and Bouck, Reference Savage and Bouck2017). In addition, some studies have reported poor driving performance of autistic patients in virtual settings, some of which may be due to impaired executive function in complex situations (Chee et al., Reference Chee, Lee, Patomella and Falkmer2019; Patrick et al., Reference Patrick, Schultheis, Agate, McCurdy, Daly, Tarazi, Chute and Hurewitz2020). Epilepsy alone can also contribute to traffic accidents as the results of one Swedish nationwide cohort study (Sundelin et al., Reference Sundelin, Chang, Larsson, Lichtenstein, Almqvist, Tomson and Ludvigsson2018). Therefore, our results highlight the importance of paying attention to transport problems in patients with ADHD, especially among those who also have the above diseases.
For comorbidities between ADHD and ASD, despite the growing body of research pointing at the frequent co-occurrence of ADHD and ASD, the previous DSM-IV-TR has not allowed a dual diagnosis of ADHD and ASD. In 2013, the DSM-5 has modified the ADHD diagnostic criteria, allowing a co-morbid diagnosis of ADHD with ASD (Leitner, Reference Leitner2014). To address the co-morbid diagnosis of ADHD with ASD, we did not specify that an ASD diagnosis is an exclusion criterion for ADHD as recommended in the DSM-IV. In this study, individuals with ADHD can be identified as having ASD if they have received the diagnosis of ASD, and 11.2% of individuals with ADHD also had ASD, which was slightly lower than that in a recent meta-analytical review (21%) (Hollingdale et al., Reference Hollingdale, Woodhouse, Young, Fridman and Mandy2020), but close to that in a Danish nationwide study (12.4%) (Jensen and Steinhausen, Reference Jensen and Steinhausen2015). We found that a higher risk of transport accidents was only found in female individuals with ADHD who were comorbid with ASD, but not in males. It is possible that females referred for clinical evaluation and diagnosed with ASD might show more obvious impairments in emotional and behavioural problems than males with ASD (Frazier et al., Reference Frazier, Georgiades, Bishop and Hardan2014).
In our study, the prescription of MPH was associated with a decreased risk of transport accidents. Our results also revealed that as MPH dosage increasing, there was a more apparent protective effect of MPH against transport accident risk. The protective effect of MPH against injuries was shown in previous studies (Tai et al., Reference Tai, Gau and Gau2013; Chen et al., Reference Chen, Yang, Liao, Kuo, Liang, Huang, Huang, Lee, McIntyre and Lin2017). This effect may be possibly mediated by the alleviation of core symptoms and enhancement of executive function. One Swedish nationwide cohort study conducted by Chang et al. reported that ADHD medication reduced the risk of serious traffic accidents in adult male patients by 58% (HR = 0.42 [95% CI 0.23–0.75]), but not in female patients (Chang et al., Reference Chang, Lichtenstein, D'Onofrio, Sjolander and Larsson2014). Another similar United States population cohort by Chang et al. found similar risk reduction in traffic accidents in both male and female patients by 38 and 42%, respectively. However, these studies did not investigate the dose–response effect. We also compared the effect of MPH on the events when ADHD was concurrent with other psychiatric comorbidities. Our results supported the prominent protective effect of MPH in the above situations. On the other hand, we used within-patient comparisons to control the unmeasured confounders consistent over time. The results showed that MPH had a potential protective trend in transport accidents within 3 months (RR = 0.85 [95% CI 0.57–1.25]) and the second to third month, although not significant (RR = 0.35 [95% CI 0.11–1.11]). This non-significant result could be partially explained by the fact that although this is a large-scale claim data, which contains over one hundred thousand patients with ADHD, the extremely low incidence of transport accidence (i.e. 0.6%) enlarged the confidence interval.
Overall, our study used a nationwide population database cohort to investigate the association of psychiatric comorbidities with the transport accidents in ADHD young people and MPH, with particular attention to the dose–response effects of MPH. We performed two parts of the analysis: between-subjects comparison and within-subject comparisons. All registration medical claim data came from the nationally representative sample of NHI, minimising the selection and recall bias. By excluding transport accidents before ADHD diagnosis, we have precluded the reverse association between ADHD and road traffic accidents as much as possible. The advantage of between-subjects comparison was that we were able to examine the MPH effect in different dose groups. However, confounding by indication cannot be eliminated. For example, those with a severe degree of ADHD symptoms, an exhibition of risky behaviours, or comorbid with other psychiatric illnesses were more likely to be prescribed medication. Hence, we also performed within-subject comparisons to adjust for time-invariant factors.
Some limitations affect the interpretation of our findings. First, there is a lack of several potential confounding factors such as adherence, lifestyle, alcohol use and subject bodyweight in our database. Also, we cannot attribute the cause of transport accidents. ADHD patients might also be at risk of transport accidents due to poor executive function or judgement. As to the within-subject comparison, there were still some unmeasured time-varying confounding factors (e.g. parental and school supervision) that interfere with the results.
Conclusions
Our study found that male ADHD patients had a higher risk of transport accidents, especially when comorbid with epilepsy, ODD/CD or ID. Female ADHD patients exhibited elevated transport accident risk when comorbid with ASD or ID. Treatment with MPH lowered the risk of accidents in a dose–response relationship. These results highlight the safety road issues in the ADHD young population and the importance of treating ADHD.
Data
The current study was based on the National Health Insurance Research Database, managed by the National Health Research Institutes. This database was owned by the National Health Research Institutes.
Acknowledgements
None.
Financial support
This study was supported by grants from the Chang Gung Memorial Hospital (CLRPG6G0042 and CLRPG6G0043).
Conflict of interest
None.
Ethical standards
The authors declared that all procedures contributing to this study meet the ethical standards of the pertinent national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000.








