Introduction
Healthcare workers (HCW) are at increased risk for traumatic experiences and subsequent development of traumatic stress symptoms (TSS; Skogstad et al., Reference Skogstad, Skorstad, Lie, Conradi, Heir and Weisæth2013). According to the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders or DSM-5 criteria (American Psychiatric Association, 2013), traumatic events are restricted to exposure to death, threatened death, actual or threatened serious injury or actual or threatened sexual violence. TSSs following traumatic events include intrusion (e.g., recurrent distressing memories of the traumatic event), negative mood (i.e., the persistent inability to experience positive emotions), dissociative symptoms (e.g., depersonalization and dissociative amnesia), avoidance (e.g., avoidance of memories, thoughts or feelings related to the traumatic event) and alterations in arousal (e.g., sleep disturbances, irritability and anger outbursts and hypervigilance) (Regier et al., Reference Regier, Kuhl and Kupfer2013). Symptoms typically begin immediately after the traumatic event, and persistence for at least 3 days is needed to meet the criteria for acute stress disorder (ASD). Once symptoms last longer than 1 month, the diagnosis of post-traumatic stress disorder can be considered. The pathophysiology of TSS is considered to be related to the failure to adapt to fear conditioning through extinction learning (Bryant, Reference Bryant2018), and neuroimaging studies have documented altered neural functioning in several brain regions (Cwik et al., Reference Cwik, Sartory, Nuyken, Schürholt and Seitz2017; Geuze et al., Reference Geuze, Vermetten, Ruf, de Kloet and Westenberg2008).
There is concern that the COVID-19 pandemic has increased the risk for TSS among HCW, as recent meta-analyses found high prevalence rates (13–22%) of post-traumatic stress disorder (PTSD) symptoms among HCWs active during coronavirus outbreaks (Carmassi et al., Reference Carmassi, Foghi, Dell’Oste, Cordone, Bertelloni, Bui and Dell’Osso2020; Salazar de Pablo et al., Reference Salazar de Pablo, Vaquerizo-Serrano, Catalan, Arango, Moreno, Ferre, Shin, Sullivan, Brondino, Solmi and Fusar-Poli2020; Salehi et al., Reference Salehi, Amanat, Mohammadi, Salmanian, Rezaei, Saghazadeh and Garakani2021; Serrano-Ripoll et al., Reference Serrano-Ripoll, Meneses-Echavez, Ricci-Cabello, Fraile-Navarro, Fiol-deroque, Pastor-Moreno, Castro, Ruiz-Pérez, Zamanillo Campos and Gonçalves-Bradley2020). The COVID-19 pandemic also questions prevailing PTSD models as these models only consider direct exposure to certain past life-threatening events as potentially traumatic, in line with strict DSM-5 diagnostic criteria (American Psychiatric Association, 2013; Husky et al., Reference Husky, Pietrzak, Marx and Mazure2021). Broader definitions of traumatic stressors also exist (World Health Organization, 2018), and in the context of this study, we assume that a prolonged exposure to intense stressors can also be experienced as traumatic and potentially yield TSS. This is in line with recent work (Bridgland et al., Reference Bridgland, Moeck, Green, Swain, Nayda, Matson, Hutchison and Takarangi2021) that suggests that TSS could also be caused by anticipating future events, indirect exposure to a potentially traumatic event, as well as a broader range of stressful experiences than those complying with DSM-5’s strict definition of trauma. This highlights the need for prospective studies that use large representative samples to examine the associations of various pandemic-related stressors with TSS among HCW (Husky et al., Reference Husky, Pietrzak, Marx and Mazure2021). Prospective studies are also needed to provide more insight into the complex patterns of TSS through time, including resilient, delayed onset, recovering and chronically distressed patterns (Bryant, Reference Bryant2018, Reference Bryant2019). In the context of the COVID-19 pandemic, it is therefore important to carefully differentiate between TSS directly following pandemic-related stressful experiences, on the one hand, and onset or persistence of TSS later in time, on the other hand.
We address this need by presenting prospective data from a large probabilistic sample of Spanish HCW, recruited as part of the MINDCOVID project (Alonso et al., Reference Alonso, Vilagut, Mortier, Ferrer, Alayo, Aragón-Peña, Aragonès, Campos, Cura-González, Emparanza, Espuga, Forjaz, González-Pinto, Haro, López-Fresneña, Salázar, Molina, Ortí-Lucas, Parellada and Pérez-Solà2021). Spain was among those countries hit particularly hard by the COVID-19 pandemic, especially during the first wave, which placed the Spanish healthcare system under extreme pressure (Pacchiarotti et al., Reference Pacchiarotti, Anmella, Fico, Verdolini and Vieta2020). We aim to (1) quantify prevalence, incidence and persistence of TSS related to the first wave of the COVID-19 pandemic (March–July 2020); (2) investigate associations of a wide range of pandemic-related stressful experiences with TSS and (3) obtain preliminary estimates of the proportions of TSS that are potentially preventable through interventions.
Materials and methods
Study design, population and sampling
As part of the MINDCOVID project (Alonso et al., Reference Alonso, Vilagut, Mortier, Ferrer, Alayo, Aragón-Peña, Aragonès, Campos, Cura-González, Emparanza, Espuga, Forjaz, González-Pinto, Haro, López-Fresneña, Salázar, Molina, Ortí-Lucas, Parellada and Pérez-Solà2021), a multicenter, observational, prospective cohort study of HCW was carried out, consisting of an initial assessment (T1) and a 4-month follow-up assessment (T2). For the initial assessment (May 5—September 7, 2020, i.e., just after the height of the first wave of the Spain COVID-19 pandemic; Supplementary Figure S1), HCWs were recruited from 18 healthcare institutions (six Spanish Autonomous Communities). Each centre contacted all employed workers using administrative email distribution lists (i.e., census sampling). Participants had to be currently employed and aged 18 years or older and were excluded if they were unable to understand the survey language or did not provide explicit consent. The invitation email included an anonymous link to the web‐based survey platform (Qualtrics.com). After opening the link, participants were provided with key information regarding the study and treatment of data and were asked if they had read all information and agreed to participate in the study. Two reminder emails were sent within a 2-to-4-week period after the initial invitation. A total of 8,996 HCWs participated in the T1 survey (response rate = 11.7%; May–September 7, 2020; Supplementary Figure S2). Of those, 4,809 (65.7% cooperation rate) participated in a follow-up survey (October 9–November 24, 2020), on average 4 months (120.1 days; SD = 22.2) after the initial assessment (Supplementary Figure S2). Sample characteristics slightly shifted between assessments, but the differences were very small (Supplementary Table S1). Inverse probability weighting (Seaman & White, Reference Seaman and White2011; Seaman et al., Reference Seaman, White, Copas and Li2012) was applied to account for these differences (see Statistical analysis section).
Measures
Outcome variable
Thirty-day TSSs are defined as a positive screen on a four-item version (Zuromski et al., Reference Zuromski, Ustun, Hwang, Keane, Marx, Stein, Ursano and Kessler2019) of the PTSD checklist for DSM-5 or PCL-5 (Blevins et al., Reference Blevins, Weathers, Davis, Witte and Domino2015; Weathers et al., Reference Weathers, Litz, Keane, Palmieri, Marx and Schnurr2013). Several short forms of the Posttraumatic Stress Disorder Checklist or PCL exist (Bliese et al., Reference Bliese, Wright, Adler, Cabrera, Castro and Hoge2008; Lang & Stein, Reference Lang and Stein2005; Price et al., Reference Price, Szafranski, van Stolk-cooke and Gros2016). The PCL-5 four-item version we used generates outcomes that most closely parallel those of the full PCL‐5 and has been developed using different statistical techniques (exploratory factor analysis, stepwise logistic regression and machine learning methods) to identify the optimal integer-scored short-form scale (Zuromski et al., Reference Zuromski, Ustun, Hwang, Keane, Marx, Stein, Ursano and Kessler2019). Items selected for this optimal scale assess intrusion (“Suddenly feeling or acting as if the stressful experience were actually happening again (as if you were actually back there reliving it)?”), avoidance (“Avoiding external reminders of the stressful experience (for example, people, places, conversations, activities, objects, or situations)?”), negative alterations in cognition and mood (“Feeling distant or cut off from other people?”) and alterations in arousal and reactivity (“Irritable behaviour, angry outbursts, or acting aggressively?”). Each of the items is scored from 0 to 4 (“Not at all” to “Extremely”), which are then summed to obtain one single (unidimensional) short-form scale score. With a scale score cutoff point of seven, the short-form predicts PCL-5 ≥ 28 diagnostic thresholds with AUC = 0.916, sensitivity = 85.5%, and specificity = 97.8% (for additional details, see Zuromski et al., Reference Zuromski, Ustun, Hwang, Keane, Marx, Stein, Ursano and Kessler2019). The official Spanish translation of the PCL-5 scale was provided to us through email by the US National Center for PTSD (https://www.ptsd.va.gov/) and coincides with the translation available at the official website for Cognitive Processing Therapy for PTSD (https://cptforptsd.com/cpt-resources/). We use the more generic term ‘traumatic stress symptoms’ instead of ASD or PTSD because we did not explicitly determine the presence of TSSs for more than 30 days (criterion F for PTSD according to the DSM-5) and because a short screening instrument was used to assess the outcome.
In line with insights into the complex course of traumatic stress following traumatic events (Bryant, Reference Bryant2018, Reference Bryant2019), we used three operationalizations of TSS in time: (1) TSS prevalence at T1 in order to provide insight into the onset of traumatic stress most proximate to the pandemic-related stressful experiences under study; (2) TSS incidence, defined as the proportion of respondents with a positive four-item PCL-5 screen at T2 among those with a negative four-item PCL-5 screen at T1 in order to provide insight in the delayed onset of traumatic stress; and (3) TSS persistence, defined as the proportion of respondents with a positive four-item PCL-5 screen at T2 among those with a positive four-item PCL-5 screen at T1 in order to provide insight into the development of chronic patterns of TSS.
Distal (pre-pandemic) risk factors
We considered 11 distal (i.e., pre-pandemic) risk factors, assessed in the initial survey: age, gender, country of birth, marital status, pre-pandemic monthly income, having children in care, type of profession, type of workplace, number of pre-pandemic lifetime mental disorders (assessed using a checklist based on the Composite International Diagnostic Interview; Kessler & Üstün, Reference Kessler and Üstün2004), number of pre-pandemic physical health conditions (assessed using a seven-item checklist; Sangha et al., Reference Sangha, Stucki, Liang, Fossel and Katz2003) and 12-month physical or sexual assault.
Proximal risk factors (pandemic-related stressful experiences)
In line with factors risk factors found in previous literature (Annaloro et al., Reference Annaloro, Arrigoni, Ghizzardi, Dellafiore, Magon, Maga, Nania, Pittella, Villa and Caruso2021; Carmassi et al., Reference Carmassi, Foghi, Dell’Oste, Cordone, Bertelloni, Bui and Dell’Osso2020; X. Li et al., Reference Li, Zhou and Xu2021a; Serrano-Ripoll et al., Reference Serrano-Ripoll, Meneses-Echavez, Ricci-Cabello, Fraile-Navarro, Fiol-deroque, Pastor-Moreno, Castro, Ruiz-Pérez, Zamanillo Campos and Gonçalves-Bradley2020; Yuan et al., Reference Yuan, Gong, Liu, Sun, Tian, Wang, Zhong, Zhang, Su, Liu, Zhang, Lin, Shi, Yan, Fazel, Vitiello, Bryant, Zhou, Ran and Lu2021), four domains of pandemic-related stressful experiences were assessed in the initial survey. The recall period for all experiences was since the beginning of the Spain COVID-19 pandemic. A first domain considered three COVID-19 infection–related experiences: (1) personal COVID-19 infection status, that is, having been hospitalized for COVID-19 infection or having had a positive COVID-19 test or medical COVID-19 diagnosis not requiring hospitalization, (2) having loved ones infected with COVID‐19 and (3) having been in isolation or quarantine because of COVID‐19.
The second domain included eight work-related stressful experiences: (1) average weekly hours worked, (2) changes in assigned functions, team or working location; (3) perceived lack of training for assigned tasks (0–4 scale score), (4) the frequency of direct exposure to COVID-19 patients during professional activity (0–4 scale score), (5) the perceived lack of healthcare centre preparedness (0–4 scale score), (6) perceived frequency of lack of protective equipment (0–4 scale score), (7) having to make decisions regarding prioritizing care among COVID‐19 patients (assessed among medical doctors and nurses), and (8) having patient(s) in care who died from COVID‐19 (assessed among all HCW involved in patient care).
A third domain consisted of six variables that measured health-related stress using 0–4 scale scores: (1) feeling of little control over getting infected or not, (2) fear of infecting loved ones, (3) family and friends’ degree of worry of getting infected through the HCW, (4) degree to which people avoided the HCW’s family because of the HCW’s job, (5) personal health-related stress and (6) stress related to the health of loved ones.
A fourth domain consisted of two financial factors: (1) having suffered a significant loss in personal or family income due to the COVID‐19 pandemic and (2) stress over one’s financial situation (0–4 scale score). For a detailed description of all measures, see Supplementary Methods.
Depression and anxiety
Depression and anxiety (included as covariates; see Statistical analysis section) were assessed using the Spanish version (Diez-Quevedo et al., Reference Diez-Quevedo, Rangil, Sanchez-Planell, Kroenke and Spitzer2001) of the Patient Health Questionnaire (PHQ-8; Kroenke et al., Reference Kroenke, Strine, Spitzer, Williams, Berry and Mokdad2009) and the Spanish version (García-Campayo et al., Reference García-Campayo, Zamorano, Ruiz, Pardo, Pérez-Páramo and López-Gómez2010) of the Generalized Anxiety Disorder scale (GAD-7; Spitzer et al., Reference Spitzer, Kroenke, Williams and Löwe2006).
Statistical analysis
Analyses were conducted with SAS System for Windows 9.4 (SAS Institute Inc., Cary, NC, USA) and R version 4.1.1 (R Core Team, 2021). Analyses were restricted to the 4,809 respondents who participated in both initial and follow-up surveys. Non-response and attrition bias were tackled by calculating sample weights through a raking and inverse probability weighting procedure that matches the final sample (n = 4,809) to (1) the target population of Spanish HCW in participating centrrs (n = 103,578) according to healthcare centre, gender, age and professional category (overall and within each healthcare centre); and (2) the full sample of T1 participants (n = 8,996) according to all T1 survey variables. Multivariable imputation by chained equations with 12 imputed datasets and 10 iterations per imputation was used to address the minimal problem of item-level missing data.
Differences in TSS prevalence, incidence and persistence across distal risk factors were assessed using the modified Rao-Scott Chi-squared test. Logistic regression was used to estimate the associations of distal risk factors and pandemic-related stressful experiences with TSS. Results are reported as odd ratios (ORs) with 95% confidence intervals (95% CI). Individual-level associations of distal risk factors with TSS were estimated using a multivariable model including all distal risk factors. Subsequently, individual- and population-level associations of each separate pandemic-related stressful experience with TSS were estimated, each time adjusting for all distal risk factors (considered covariates). All analyses were adjusted for time (i.e., week) of T1 survey participation in order to adjust for individual variations in follow-up time between the T1 and T2 assessments. Since causal relationships between the included pandemic-related stressful experiences are largely unknown, we refrained from constructing a fully adjusted multivariable model to avoid the risk of overadjustment bias (Schisterman et al., Reference Schisterman, Cole and Platf2009). Given concerns regarding the unknown discriminative validity of the PCL-5 versus general negative emotionality such as depression and anxiety (Bridgland et al., Reference Bridgland, Moeck, Green, Swain, Nayda, Matson, Hutchison and Takarangi2021) and given the high degree of co-morbidity between TSS/PTSD and other mental disorders (Bryant, Reference Bryant2018), we repeated the analyses investigating associations between pandemic-related stressful experiences and TSS, additionally adjusting for co-occurring depression and anxiety, and presented these results in Supplementary Tables S2–S3.
Population-level associations, that is, population attributable risk proportions (PARP) and their standard errors (SE) were calculated using simulation methods based on logistic regression equations. A PARP is the proportion of the cumulative predicted value of an outcome statistically explained by specific predictor variables (Centers for Disease Control and Prevention, 2012). PARPs can be interpreted as the expected proportional reduction in TSS if the risk factors or the causal factors accounting for the risk factors were eradicated in the population. It is important to note that PARPs can sum to more than 100% because some individuals with more than one risk factor can have TSS prevented in more than one way, and the prevented TSS cases of these individuals could be counted more than once (Rowe et al., Reference Rowe, Powell and Flanders2004).
Table 1. Associations of distal (pre-pandemic) risk factors with traumatic stress symptoms (n = 4,809)
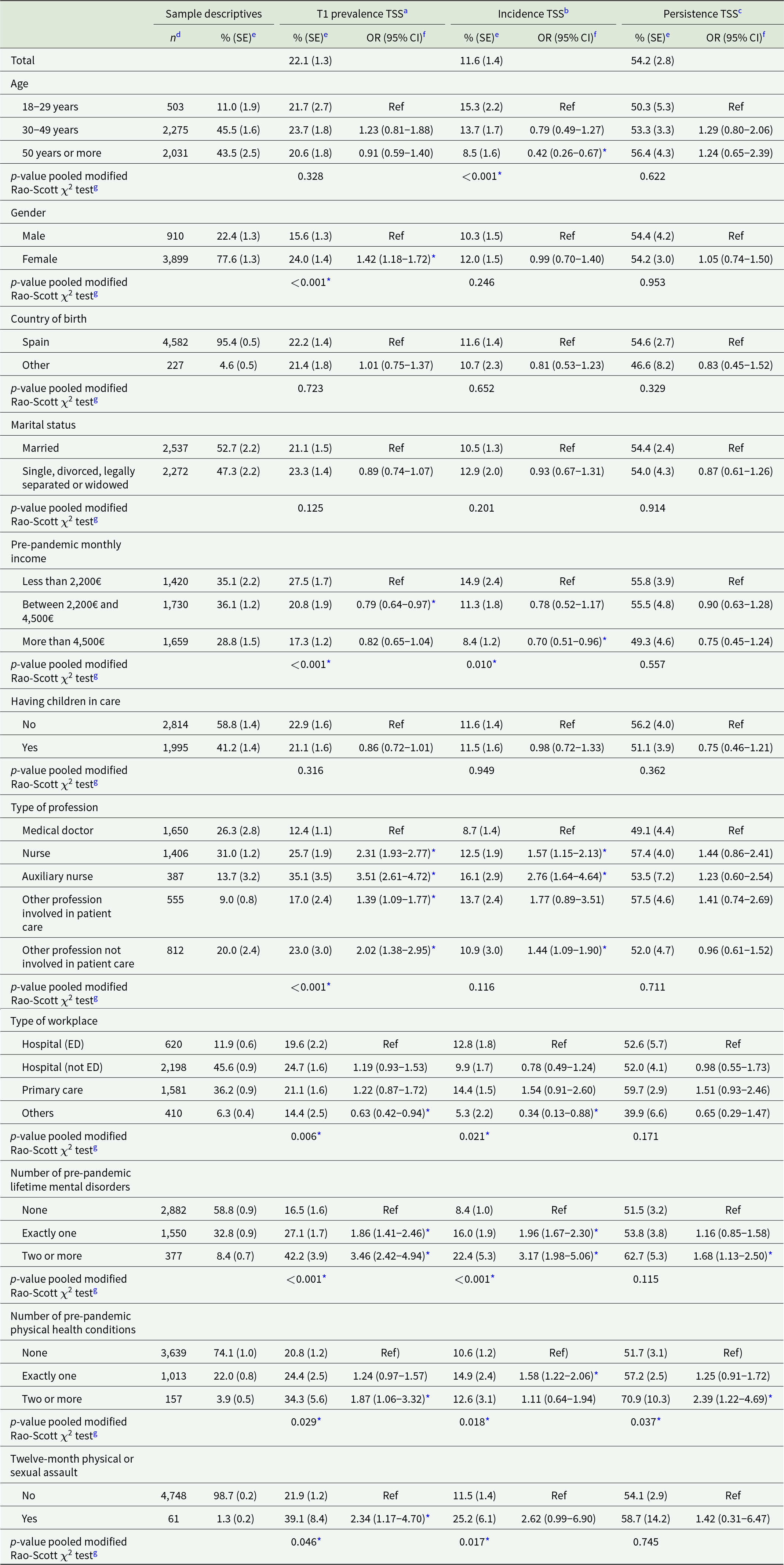
Abbreviations: CI = confidence interval; ED = emergency department; OR = odds ratio; SE = standard error; TSS = traumatic stress symptoms.
a Prevalence of TSS is defined as a positive screen on the four-item PCL-5 at T1 (n = 4,809).
b Incidence of TSS is defined as the proportion of respondents with a positive four-item PCL-5 screen at 4-month follow-up (n = 412) among those with a negative four-item PCL-5 screen at T1 (n = 3,796).
c Persistence of TSS is defined as the proportion of respondents with a positive four-item PCL-5 screen at 4-month follow-up (n = 536) among those with a positive four-item PCL-5 screen at T1 (n = 1,013).
d Number of observations (n) are unweighted.
e Proportions (%, SE) are weighted.
f Results represent one logistic regression model including all distal risk factors, additionally adjusting for time (i.e., week) of T1 survey participation.
g p-value based on a F test to evaluate statistical significance based on multiple imputations, using the procedure by Li et al. (Reference Li, Raghunathan and Rubin1991)
* Indicate statistically significant results (α = 0.05).
Results
Individual-level associations of pre-pandemic (distal) risk factors with TSS
Thirty-day prevalence of TSS at T1 was estimated at 22.1%; 4-month incidence and persistence of TSS were estimated at 11.6% and 54.2%, respectively. TSS estimates stratified by distal risk factors and adjusted associations of distal risk factors with TSS are shown in Table 1. Compared to medical doctors, all other professional categories had higher odds for both TSS prevalence and incidence, especially auxiliary nurses and nurses. A history of pre-pandemic mental disorders was also strongly associated with prevalence and incidence of TSS, but persistent TSS was only significantly associated with having two or more lifetime mental disorders. A similar, although less consistent pattern was found for pre-pandemic physical health conditions. No clear associations were found for age, gender, and income, although results suggest higher TSS incidence among younger HCW, higher TSS prevalence among females, and higher odds for TSS onset among those with lower pre-pandemic monthly income levels. No associations were found for the country of birth, marital status, and having children in care with TSS.
Table 2. Associations of pandemic-related stressful experiences with traumatic stress symptoms (n = 4,809)
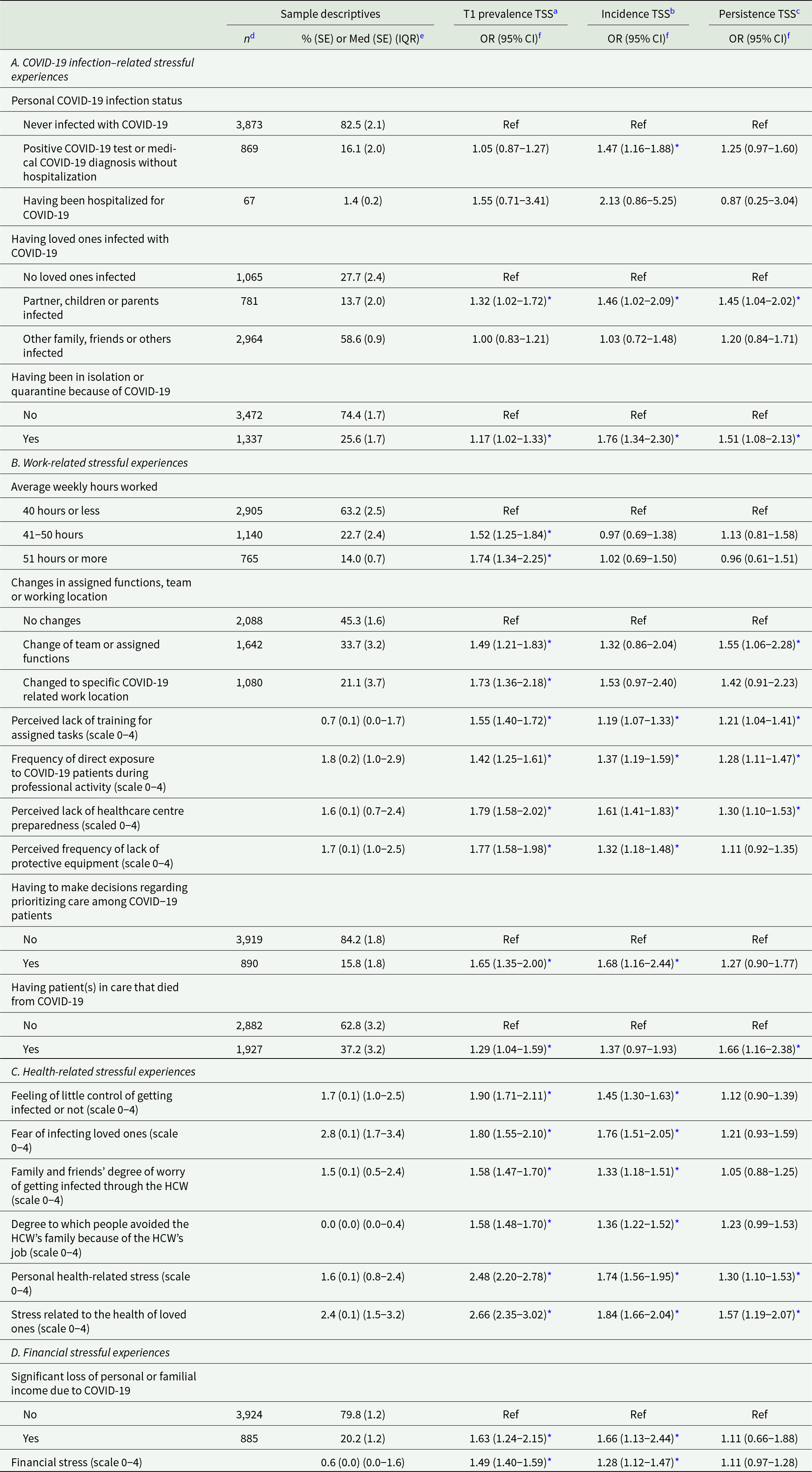
Abbreviations: CI = confidence interval; ED = emergency department; IQR = interquartile range; Med = median; OR = odds ratio; SE = standard error; TSS = traumatic stress symptoms.
a Prevalence of TSS is defined as a positive screen on the 4-item PCL-5 at T1 (n = 4,809).
b Incidence of TSS is defined as the proportion of respondents with a positive four-item PCL-5 screen at 4-month follow-up (n = 412) among those with a negative four-item PCL-5 screen at T1 (n = 3,796).
c Persistence of TSS is defined as the proportion of respondents with a positive four-item PCL-5 screen at 4-month follow-up (n = 536) among those with a positive four-item PCL-5 screen at T1 (n = 1,013).
d Number of observations (n) are unweighted.
e Proportions (%, SE) and Medians (SE) (IQR) are weighted.
f Each row represents a separate logistic regression model, each time adjusting for all distal risk factors and time (i.e., week) of T1 survey participation.
* Indicate statistically significant results (α = 0.05).
Individual-level associations of pandemic-related stressful experiences with TSS
Adjusted associations of pandemic-related stressful experiences with TSS are shown in Table 2. Higher odds for all TSS outcomes under study were found among those with a COVID-19 infection of their partner, child, or parent, and among those who had been in isolation or quarantine for COVID-19. Those who tested positive for COVID-19 or ever had an established diagnosis of COVID-19 had significantly higher odds for TSS incidence only. All the eight work-related stressful experiences under study were significantly associated with TSS prevalence at T1. Five out of eight were also associated with TSS incidence, especially having to make decisions regarding prioritization of care and perceived lack of healthcare centre preparedness. Odds for persistent TSS were significantly higher among those who had a patient in care who died of COVID, those who had to change teams or functions, followed by perceived lack of healthcare centre preparedness, perceived lack of training for assigned tasks, and frequency of direct exposure to COVID-19 patients. All six health-related stressful experiences were found to be associated with both TSS prevalence and incidence, while personal health-related stress and stress related to the health of loved ones were also associated with persistence of TSS. Finally, loss of personal or familial income due to the pandemic and financial stress were associated with both TSS prevalence and incidence but not persistence of TSS.
Population-level associations between pandemic-related stressful experiences and TSS
Adjusted PARP estimates for the associations of pandemic-related stressful experiences with TSS are shown in Table 3. Two stressful experience domains were consistently associated with all three TSS outcomes, most strongly with TSS prevalence and incidence: health-related and work-related stressful experiences. Within these domains, consistently high PARP was found for frequency of direct exposure to COVID-19 patients, perceived lack of healthcare centre preparedness, personal health-related stress, stress related to the health of loved ones and fear of infecting loved ones, with PARP in the range of 48.5–93.4% for TSS prevalence and incidence and in the range of 28.3–60.3% for TSS persistence. Financial factors were associated with both TSS prevalence and incidence, while infection-related experiences were only weakly associated with TSS incidence.
Table 3. Population attributable risk proportions for the associations of pandemic-related stressful experiences with TSS (n = 4,809)
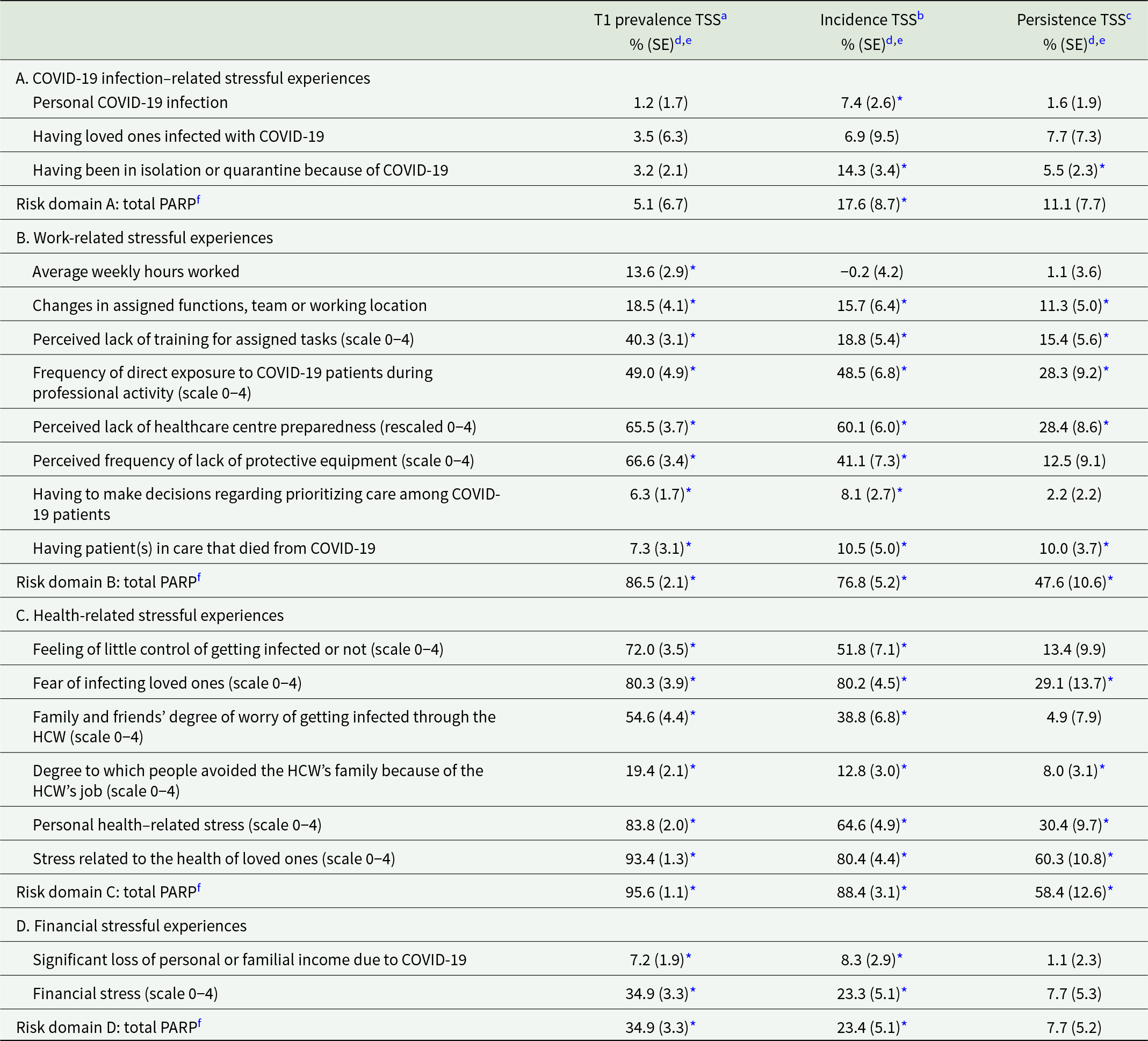
Abbreviations: COVID-19 = coronavirus disease 2019; SE = standard error; TSS = traumatic stress symptoms.
a Prevalence of TSS is defined as a positive screen on the four-item PCL-5 at T1 (n = 4,809).
b Incidence of TSS is defined as the proportion of respondents with a positive four-item PCL-5 screen at 4-month follow-up (n = 412) among those with a negative four-item PCL-5 screen at T1 (n = 3,796).
c Persistence of TSS is defined as the proportion of respondents with a positive four-item PCL-5 screen at 4-month follow-up (n = 536) among those with a positive four-item PCL-5 screen at T1 (n = 1,013).
d Proportions (%, SE) are weighted.
e Each row represents a separate logistic regression model, each time adjusting for all distal risk factors and time (i.e., week) of T1 survey participation.
f Risk domain total PARPs are based on four separate logistic regression models, one for each of the four proximal risk factor domains (A–D). Each model includes the proximal risk factors from the corresponding proximal risk factor domain (but not the other domains), adjusting for all distal risk factors and time (i.e., week) of T1 survey participation.
* Statistically significant results (α = 0.05).
Analyses additionally adjusting for co-occurring depression and anxiety
We re-estimated all individual-level and population-level associations between pandemic-related stressful experiences and TSS, additionally adjusting for co-occurring depression (PHQ-8 total score) and anxiety (GAD-7 total score; see Supplementary Tables S2–3). Main findings from these analyses were as follows: (1) none of the associations between COVID-19 infection–related experiences and TSS remained significant, (2) having a patient in care die of COVID-19 was the only experience that remained significantly associated with TSS persistence (OR = 1.86; PARP = 8.0%), (3) all of the other stressful experiences remained significantly associated either with TSS prevalence or TSS incidence, except having to make decisions regarding prioritizing care among COVID-19 patients. While PARP estimates for the associations of the domains of health-related and work-related stressful experiences with TSS prevalence and incidence decreased considerably in strength (i.e., with a factor 1.5–1.9), these PARP were still in the range of 45.5–59.1%.
Discussion
Prevalence of TSSs among HCWs active during the first wave of the Spanish COVID-19 pandemic is estimated at 22.1%, and incidence and persistence of TSS 4 months later into the pandemic is estimated at 11.6% and 54.2%, respectively. While almost all of the pandemic-related stressors under study were associated with TSS onset (i.e., either prevalence or incidence of TSS) to some extent, considerably less were associated with persistence of TSS.
Our T1 TSS prevalence estimate of 22.1% is in line with pooled estimates found in meta-analyses of post-TSSs among HCWs active during coronavirus outbreaks (13–22%; Y. Li et al., Reference Li, Scherer, Felix and Kuper2021b; Salazar de Pablo et al., Reference Salazar de Pablo, Vaquerizo-Serrano, Catalan, Arango, Moreno, Ferre, Shin, Sullivan, Brondino, Solmi and Fusar-Poli2020; Salehi et al., Reference Salehi, Amanat, Mohammadi, Salmanian, Rezaei, Saghazadeh and Garakani2021; Serrano-Ripoll et al., Reference Serrano-Ripoll, Meneses-Echavez, Ricci-Cabello, Fraile-Navarro, Fiol-deroque, Pastor-Moreno, Castro, Ruiz-Pérez, Zamanillo Campos and Gonçalves-Bradley2020) and higher than the prevalence of 15.8% estimated in the Spanish general population during the pandemic (González-Sanguino et al., Reference González-Sanguino, Ausín, Castellanos, Saiz, López-Gómez, Ugidos and Muñoz2020). Nurses, especially auxiliary nurses, were found to be the most vulnerable medical profession for developing TSS, with 2.3–3.5 times higher odds for TSS prevalence and 1.6–2.8 times higher odds for TSS incidence, compared to medical doctors, highlighting the negative impact of the COVID-19 pandemic on nurses’ mental well-being (Maben et al., Reference Maben, Conolly, Abrams, Rowland, Harris, Kelly, Kent and Couper2022). It is not possible to compare estimates of our prospective TSS outcomes with other HCW populations since very few previous studies present longitudinal data (Baumann et al., Reference Baumann, Kaschel and Kuhl2007; Cai et al., Reference Cai, Cui, Liu, Li, Gong, Liu, Wan, Yuan, Li, Chen and Wang2020; Canal-Rivero et al., Reference Canal-Rivero, Armesto-Luque, Rubio-García, Rodriguez-Menéndez, Garrido-Torres, Capitán, Luque, Crespo-Facorro and Ruiz-Veguilla2022; Jordan et al., Reference Jordan, Shannon, Browne, Carroll, Maguire, Kerrigan, Hannan, McCarthy, Tully, Mulholland and Dyer2021; Yamane et al., Reference Yamane, Zarabian, Devine, Benjenk, Farrar, Park, Kim, Davison and Heinz2022) and none calculated incidence or persistence rates. An important finding from our study is therefore that just over half of HCW with significant TSS (i.e., 12% in total) continue to experience TSS 4 months later. This highlights the fact that PTSD estimates from previous studies among HCW may be inflated by transient stress reactions and ASD due to the exclusive use of cross-sectional surveys without follow-up assessments. Nevertheless, our estimate of 12% of HCW with persistent TSS is still considerably higher than reliable lifetime PTSD diagnosis estimates in the pre-pandemic general Spanish population (2.2–4.5%; Koenen et al., Reference Koenen, Ratanatharathorn, Ng, McLaughlin, Bromet, Stein, Karam, Meron Ruscio, Benjet, Scott, Atwoli, Petukhova, Lim, Aguilar-Gaxiola, Al-Hamzawi, Alonso, Bunting, Ciutan, de Girolamo and Kessler2017). Further monitoring of our HCW cohort is therefore warranted in order to detect the onset of PTSD symptoms, especially since many cases of PTSD do not present ASD symptoms directly following the traumatic experience and symptoms of PTSD may fluctuate over time (Bryant, Reference Bryant2018).
An important contribution from our study is that we investigated associations of a wide range of pandemic-related stressful experiences with TSS. Of all domains under study, we found that health-related stressful experiences were consistently the ones with the strongest association with all three operationalizations of TSS in time (i.e., prevalence, incidence and persistence). This is in line with previous studies using cross-sectional study designs that found that traumatic stress was associated with fear of infecting others (Bayazit et al., Reference Bayazit, Ozel, Arac, Dulgeroglu-Bayazit and Joshi2022; Billings et al., Reference Billings, Ching, Gkofa, Greene and Bloomfield2021; Greene et al., Reference Greene, Harju-Seppänen, Adeniji, Steel, Grey, Brewin, Bloomfield and Billings2020; Norful et al., Reference Norful, Rosenfeld, Schroeder, Travers and Aliyu2021), having family members infected (Al Falasi et al., Reference Al Falasi, Al Mazrouei, Al Ali, Al Dham Ani, Al Ali, Al Kindi, Dalkilinc, Al Qubaisi, Campos, Al Tunaiji and Baltatu2021; Blanco-Daza et al., Reference Blanco-Daza, de la Vieja-soriano, Macip-Belmonte and Tercero-Cano2022), stress over one’s own health and feeling little control over getting infected (Annaloro et al., Reference Annaloro, Arrigoni, Ghizzardi, Dellafiore, Magon, Maga, Nania, Pittella, Villa and Caruso2021; Blanco-Daza et al., Reference Blanco-Daza, de la Vieja-soriano, Macip-Belmonte and Tercero-Cano2022; Johnson et al., Reference Johnson, Ebrahimi and Hoffart2020; Luceño-Moreno et al., Reference Luceño-Moreno, Talavera-Velasco, García-Albuerne and Martín-García2020; Ouyang et al., Reference Ouyang, Geng, Zhou, Wang, Zhan, Shang, Jia, Yan, Zhang, Li and Liu2022; Si et al., Reference Si, Su, Jiang, Wang, Gu, Ma, Li, Zhang, Ren, Ren, Liu and Qiao2020). We confirm these previous findings, and our study now suggests that health-related stressful experiences were also associated with delayed onset as well as persistence of TSS among HCW following the first COVID-19 outbreak in Spain. Work-related stressful experiences were also strongly associated with TSS, especially perceived lack of centre preparedness and lack of protective equipment, both of which have been extensively described in the literature as important risk factors (Annaloro et al., Reference Annaloro, Arrigoni, Ghizzardi, Dellafiore, Magon, Maga, Nania, Pittella, Villa and Caruso2021; D’Ettorre et al., Reference D’Ettorre, Ceccarelli, Santinelli, Vassalini, Innocenti, Alessandri, Koukopoulos, Russo, D’ettorre and Tarsitani2021; Norful et al., Reference Norful, Rosenfeld, Schroeder, Travers and Aliyu2021; Serrano-Ripoll et al., Reference Serrano-Ripoll, Meneses-Echavez, Ricci-Cabello, Fraile-Navarro, Fiol-deroque, Pastor-Moreno, Castro, Ruiz-Pérez, Zamanillo Campos and Gonçalves-Bradley2020). Of note, we also found that having been in isolation or quarantine due to COVID had a significant association with TSS incidence, in line with findings from previous cross-sectional studies (Carmassi et al., Reference Carmassi, Foghi, Dell’Oste, Cordone, Bertelloni, Bui and Dell’Osso2020; Pan et al., Reference Pan, Xu, Kuang, Zhang, Fang, Gui, Li, Tefsen, Zha and Liu2021; Serrano-Ripoll et al., Reference Serrano-Ripoll, Meneses-Echavez, Ricci-Cabello, Fraile-Navarro, Fiol-deroque, Pastor-Moreno, Castro, Ruiz-Pérez, Zamanillo Campos and Gonçalves-Bradley2020; Yuan et al., Reference Yuan, Gong, Liu, Sun, Tian, Wang, Zhong, Zhang, Su, Liu, Zhang, Lin, Shi, Yan, Fazel, Vitiello, Bryant, Zhou, Ran and Lu2021).
While our findings suggest that almost all of the pandemic-related stressful experiences under study are able to provoke traumatic stress to some extent, only a few were predictive of TSS persistence. The risk factor with the highest association with persisting TSS in our study was having patient(s) who died from COVID, in line with previous cross-sectional findings (Bayazit et al., Reference Bayazit, Ozel, Arac, Dulgeroglu-Bayazit and Joshi2022; Leng et al., Reference Leng, Wei, Shi, Cao, Wei, Xu, Zhang, Zhang, Xing and Wei2021; Lockett et al., Reference Lockett, Fergerson, Pyszczynski and Greenberg2022). Interestingly, this was also the only stressful experience under study considered consistent with DSM-5 criterion A of PTSD and the only one consistently associated with TSS persistence in all analyses (i.e., even after additionally adjusting for co-occurring depression and anxiety). This highlights the need for further empirical evidence in order to settle the ongoing debate on which traumatic experiences could be considered, triggering events for a PTSD diagnosis in the context of the COVID-19 pandemic (Bridgland et al., Reference Bridgland, Moeck, Green, Swain, Nayda, Matson, Hutchison and Takarangi2021; Husky et al., Reference Husky, Pietrzak, Marx and Mazure2021).
Limitations
Some limitations of our study need to be considered. First, TSS were measured using a self-reported four-item version of the PCL-5 and not through face-to-face clinical diagnosis. In addition, the time frame of TSS assessment only spans the past 30 days; hence, it is possible that persistent cases did not experience TSS during all 4 months between T1 and T2 assessment, and incident cases may include reactivation of TSS experienced earlier. The latter was partly addressed by adjusting all analyses for pre-pandemic mental disorders. Second, although we established temporality in our study, we did not ask respondents which specific stressful experience(s) provoked TSS, and we cannot exclude that the stressful experiences identified as significant in the analyses are mere markers of co-occurring truly traumatic events (assessed or not assessed in our study). Third, although we included a wide range of pandemic-related stressful experiences in our study, important stressful experiences may have been missed. Fourth, T1 participation in our study was low, however, in line with the pooled response rate of 13.0% among HCW web-based surveys worldwide (Cho et al., Reference Cho, Johnson and VanGeest2013). We improved representativeness of our data by including census sampling and state-of-the-art missing data handling techniques to minimize selection bias. Finally, HCW who were sick during assignments may not have participated in the study, potentially causing selection bias.
Conclusions
Onset and persistence of TSS among Spanish HCWs active during the first wave of the COVID-19 pandemic was high. TSS onset was especially high among nurses and auxiliary nurses and associated with a wide range of pandemic-related stressful experiences, including direct COVID-19 infection–related events, as well as health-related, work-related and financial factors. It remains unclear to what extent these experiences have intrinsic traumatic stress-provoking potential if they represent risk factors for subsequent PTSD onset or if they simply represent different markers for a prolonged period of time associated with traumatic stress. Future research should delineate etiological causal frameworks for the onset of TSS and PTSD. Such frameworks could subsequently guide prevention interventions and hereby address the absolute lack of research on effective interventions for mental disorders among healthcare personnel, both on the individual and on the organizational level (Petrie et al., Reference Petrie, Crawford, Baker, Dean, Robinson, Veness, Randall, McGorry, Christensen and Harvey2019).
Our study suggests that interventions tackling the onset of traumatic stress during viral outbreaks should primarily increase healthcare centre preparedness for viral outbreaks in order to prevent unexpected work-related changes, moral distress and health-related stress. Furthermore, due to the variability of TSS across time, there needs to be a clear assessment of trauma-related emotional distress at different timepoints of a crisis. This will allow to identify individuals who need help, as well as prevent development of persistent TSS (Bryant, Reference Bryant2018; Fanai & Khan, Reference Fanai and Khan2022). Healthcare centres’ preparedness should be done through bettering equipment, human resources, training and protocols. It is therefore encouraging that the International Labor Organization and the World Health Organization have recently published a guide on developing and implementing stronger occupational health and safety programs for health workers, focusing on various occupational hazards, including infectious and psycho-social hazards (World Health Organization, & International Labour Organization, 2022). The COVID‐19 pandemic has highlighted the importance of a well‐functioning healthcare system. Improving future mental health and promoting fair financial and working conditions among HCW should therefore be an absolute priority.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S2045796023000628.
Availability of Data and Materials
The de-identified participant data as well as the study protocol and statistical analysis plan used for this study are available upon reasonable request from the corresponding authors (P.M. and J.A.) as long as the main objective of the data sharing request is replicating the analysis and findings as reported in this paper.
Acknowledgements
The authors would like to thank all healthcare workers who participated in the study in the midst of very stressful and busy times, as well as Puri Barbas for the management of the project.
Author contributors
A.P.-V.D. and P.M. reviewed the literature. P.M., G.V., M.F., J.A., E.A., V.P.-S., J.M.H., R.C.K. and R.B. conceived and designed the study. E.A., C.S., J.D.M., N.L.-F., T.P., J.I.P., J.I.E., J.M.P.-T., M.E., N.P.F., A.G.-P., C.R., I.D.C.-G., A.A.-P., M.C., A.P.-Z., E.V. and V.P.S. acquired the data. A.P.V.D., P.M., I.A. and G.V. cleaned and analysed the data. A.P.V.D., P.M., G.V. and J.A. drafted the initial version of the manuscript. All authors reviewed the initial draft and made critical contribution to the interpretation of the data and approved the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. Jordi Alonso, IMIM, CIBERESP and UPF can also be contacted for correspondence jalonso@imim.es.
Financial support
B.L.A. acknowledges a mobility grant (BA21/00002) from the Instituto de Salud Carlos III-Subdirección General de Evaluación y Fomento de la Investigación, Plan Nacional 2008–2011 and 2013–2016. This work was supported by the Instituto de Salud Carlos III (ISCIII)/Ministerio de Ciencia e Innovación/FEDER (J.A., COV20/00711); ISCIII-FSE Post-doctoral contract Sara Borrell (P.M., CD18/00049); Departament de Recerca i Universitats/Generalitat de Catalunya (J.A., AGAUR 2021 SGR 00624); PERIS, Departament de Salut (I.A., SLT017/20/000009) and ISCIII-FSE Miguel Servet (P.M., CP21/00078). Additional partial funding was received from the Special Research Fund KU Leuven (R.B., EDC-D9624-DOA/ 2020/007). Partial funding was also received from the Castilla y Leon GRS Special COVID-19 Research Funding (J.M.P.-T., GRS COVID 32/A/20).
Competing interests
E.A. has received personal fees from Lundbeck and Esteve. E.V. reports personal fees from Abbott, Allergan, Angelini, Lundbeck, Sage and Sanofi, grants from Novartis and Ferrer and grants and personal fees from Janssen, outside the submitted work. J.M.P.-T. reports personal fees from Angelini, Janssen and Lunbeck and grants from Janssen, outside the submitted work. In the past 3 years, R.C.K. was a consultant for Cambridge Health Alliance, Canandaigua VA Medical Center, Holmusk, Partners Healthcare, Inc., RallyPoint Networks, Inc. and Sage Therapeutics. R.C.K. has stock options in Cerebral Inc., Mirah, PYM, Roga Sciences and Verisense Health. A.G.-P. has received grants and served as consultant, advisor or CME speaker for the following entities: Janssen-Cilag, Lundbeck, Otsuka, Pfizer, Sanofi-Aventis, Alter, Angelini, Exeltis, Novartis, Rovi, Takeda, the Spanish Ministry of Science and Innovation (CIBERSAM), the Ministry of Science (Carlos III Institute), the Basque Government and the European Framework Program of Research. All other authors reported no conflicts of interest.
Ethical standards
The study complies with the principles established by national and international regulations, including the Declaration of Helsinki and the Code of Ethics. The study was approved by the Research Integrity and Good Scientific Practices Committee of IMIM‐Parc de Salut Mar, Barcelona, Spain (2020/9203/I) and by all participating centres’ institutional review boards (IRBs).





