Published online by Cambridge University Press: 01 December 2011
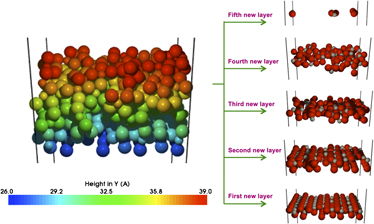
Results are presented for modeling the growth of TiO2 on the rutile (110) surface. We illustrate how long time scale dynamics techniques can be used to model thin film growth at realistic growth rates. The system evolution between deposition events is achieved through an on-the-fly Kinetic Monte Carlo method, which finds diffusion pathways and barriers without prior knowledge of transitions. We examine effects of various experimental parameters, such as substrate bias, plasma density, and stoichiometry of the deposited species. Growth of TiO2 via three deposition methods has been investigated: evaporation (thermal and electron beam), ion-beam assist, and reactive magnetron sputtering. We conclude that the evaporation process produces a void filled, incomplete structure even with the low-energy ion-beam assist, but that the sputtering process produces crystalline growth. The energy of the deposition method plays an important role in the film quality.