Introduction
Biologically controlled biomineralization is a fundamental process in the evolution of the animal kingdom. Though it was sporadically achieved already in the latest Precambrian, the so-called “Cambrian explosion,” ca. 540 Ma ago, marks the general advent of animals with hard parts in the fossil record. It is well known, that this event was not an instantaneous process, but that it was accomplished in a time span of ca. 20–25 Ma (Lowenstam and Margulis, Reference Lowenstam and Margulis1980; Chen et al., Reference Chen, Cheng and Iten1997; Thomas et al., Reference Thomas, Shearman and Stewart2000; Kouchinsky et al., Reference Kouchinsky, Bengtson, Runnegar, Skovstedt, Steiner and Vendrasco2012; Zhuravlev and Wood, Reference Zhuravlev and Wood2018). The acquisition and evolution of hard skeletons within the great majority of phyla and classes are the basic prerequisites for the reconstruction of animal form and function through time. Once hard parts were created in order to protect and stabilize soft tissues and organs, this evolutionary advantage indeed developed a great variety of morphologies, but in spite of this diversity and modification, solid skeletons were generally maintained as such until extinction of the equivalent groups.
A similar reduction of a hard skeleton into a mere soft-bodied stage seems achieved in some Coleoidea and Opisthobranchia (see below), but this accordance is based on notional paleontological evidence. An alleged late acquisition of a hard skeleton inherited from soft-bodied ancestors is suspected in Bryozoa and Scleractinia (see below), but in contrast to ascidians, once these groups had acquired calcified skeletons, they maintained this protective and stabilizing advantage until Recent. Thus, the very late acquirement of a solid calcareous skeleton among Tunicata and its later loss in favor of an almost soft-bodied stage is a very rare phenomenon in the geological record. This article describes and decrypts such an unusual case in the fossil rather poorly known class Ascidiacea of the subphylum Tunicata.
From their form and mode of life, Ascidiacea (sea squirts) appear as rather simple-structured organisms that superficially and functionally are evocative of Porifera. But the presence of a tubular dorsal nerve cord and a notochord in their larval stage and pharyngeal clefts allocates them a much higher position within the animal kingdom as a subphylum of the Chordata. Ascidians are an artificial, polyphyletic group that comprises three orders (Aplousobranchiata, Stolidobranchiata, Phlebobranchiata) with a total of ~2940 living species (Burda et al., Reference Burda, Hilken and Zrzavý2016). They generally lack hard parts, thus minimizing the possibility of preservation. Only a few genera of the orders Aplousobranchiata and Stolidobranchiata segregate tiny isolated spicules, embedded in the mantle (tunica) or other organs (Bronn, Reference Bronn1893–1911; Van Name, Reference Van Name1945; Monniot and Buge, Reference Monniot and Buge1971; Lambert et al., Reference Lambert, Lambert, Lowenstam and Carter1990), but their origin remains unknown (Lowenstam and Weiner, Reference Lowenstam and Weiner1989; Varol and Houghton, Reference Varol and Houghton1996). Tunica is the Latin word for mantle. Therefore, many zoologists regard both terms as synonymous (e.g., Lowenstam and Weiner, Reference Lowenstam and Weiner1989; Burda et al., Reference Burda, Hilken and Zrzavý2016). Others, however, distinguish an inner layer (tunic), in which the spicules are secreted, from an outer one (mantle; Brusca et al., Reference Brusca, Moore and Shuster2016). In order to guard against misunderstandings, in the present article both terms are used as synonyms because, with regard to fossils, the differential application of both terms would appear arbitrary.
Spicules of congeneric predecessors among the above mentioned orders have been found in rocks as old as lower Liassic (Fig. 1; Bonet and Benviste-Velasquez, Reference Bonet and Benviste-Velasquez1971; Varol and Girgis, Reference Varol and Girgis1994). Generally, ascidian spicules consist of aragonite, more rarely of vaterite or other minerals (Lowenstam and Weiner, Reference Lowenstam and Weiner1989). Unlike some siliceous sponge spicules, they are never fused together and thus could not form solid skeletons. The discovery of complete soft-bodied ascidians (lacking spicules, however) in the lower Cambrian of China (Chen et al., Reference Chen, Huang, Peng, Chi, Wang and Feng2003) has revealed that ascidians existed already at an early stage of metazoan evolution, but this finding does not allow to reconstruct their relationships to Mesozoic to recent representatives of this class. A new evolutionary branch among ascidians is the recent discovery of calcareous tunicate exoskeletons in the Upper Triassic and their ancestors in the Permian (Wendt, Reference Wendt2018). In this article it has been demonstrated that these very unusual skeletons, which are composed of irregular aragonitic plates, can be assigned to no phylum other than to tunicates. This systematic position of these previously unknown organisms to the class Ascidiacea has been the fundamental clue for the systematic attribution of the new discoveries described in the present article. The fact, however, that they are endoskeletons (see below), opens an unusual insight into a new aspect of the evolution of this poorly known class which has no counterpart among their living representatives. Tunicate exoskeletons have long been known from Permian deposits in east Asia and Europe, but until recently they were erroneously attributed to rugose corals (Montanaro-Gallitelli, Reference Montanari-Gallitelli1956; Wendt, Reference Wendt2018). These skeletons are composed of a varying number (2 to ~35) of irregular plates that consist of acicular aragonite. This very unusual construction and mineralogy were crucial for their systematic attribution, though similar living representatives of this class are unknown. Comparably organized endoskeletons now add a new aspect to the fossil record of this largely ignored subphylum of the Chordata. The subsequent paragraphs are mainly devoted to these newly discovered endoskeletons.

Figure 1. Fossil record of tunicates. Black dot = soft-bodied; black triangles = calcareous exoskeletons; black square = calcareous endoskeletons; asterisks = spicules (modified from Wendt, Reference Wendt2018).
Endoskeletons are widespread in the animal kingdom and display a great variety of shape, function, and mineralogical composition. They are typical for the major phyla of Deuterostomia (Echinodermata and Vertebrata, including Conodontophorida) and many Protista (Foraminifera and Radiolaria), which exhibit a stunning variety of shapes and geometries. Endoskeletal spicules (sclerites) of different mineralogical composition evolved independently among several phyla and classes (Porifera, Octocorallia, Vermes, Holothuroidea, Tunicata). Apart from the calcareous octocoral Tubipora musica (see Spiro, Reference Spiro1971), only some Porifera (Hexactinellida and Lithistida), produced siliceous spicules, which are fused or articulated into compound endoskeletons forming cubic or irregular meshworks, thus giving more stability to the soft body. Among other phyla, compact solid calcareous endoskeletons occur only among the Mollusca (Coleoidea; for a different interpretation see Thomas and Reif, Reference Thomas, Reif, Schmidt-Kitttler and Vogel1991). Composite calcareous endoskeletons consisting of numerous plates with flexible boundaries are characteristic for Echinodermata and for the newly discovered fossil Tunicata.
Provenance
Only two incomplete specimens were available for the present study. They are derived from the same formation and locality as the tunicate exoskeletons mentioned above (Fig. 1). Extensive search for additional, either biostratigraphically older, contemporaneous, or younger specimens from European and North American reef specialists and collections remained unsuccessful. The two specimens were discovered among far over a million of skeletal remains in the Cassian Formation (lower Carnian) of the Dolomites (northern Italy; references in Wendt, Reference Wendt2018). The number of taxa and the diversity of skeletal remains collected from the Cassian Formation during almost two centuries are indeed impressive and assign this rock unit a singular rank among the “Fossil-Lagerstätten” in the geological record. According to Roden et al. (Reference Roden, Hausmann, Nützel, Seuss, Reich, Urlichs, Hagdorn and Kiessling2019), 1429 species have been described so far from this formation. A special feature of this unrivaled fauna is their often excellent state of preservation, exemplified by diagenetically almost unaltered aragonitic microstructures, which are among the oldest in earth history. In this state of perfection and considering its age, the Cassian fauna can even be regarded as unique.
Morphology and growth
Both specimens are incomplete colonies that were probably attached to a firm- or hardground on the sea-floor. Similar growth forms also are common among living ascidians (e.g., Amarovicium, Synocium [Bronn, Reference Bronn1893–1911], and Polycarpa [Monniot, Reference Monniot2002]). In Toscanisoma multipartitum the base of the colony is not preserved (Fig. 2.4), but Toscanisoma triplicatum (for diagnosis see below, paragraph Systematic Paleontology) shows an undamaged flattened base (Fig. 3.3, 3.4). The most remarkable feature is the fact that the outer surface of both species is completely smooth and that growth lines are totally lacking. Also, by staining of thin sections with Mutvei solution (Schöne et al., Reference Schöne, Dunca, Fiebig and Pfeiffer2005), which often reveals concealed growth increments, no traces of marginal accretion within the plates could be detected. The irregular wrinkles in the basal portion of Toscanisoma triplicatum n. gen. n. sp. (Fig. 3.3, 3.4) display an irregular upgrowth, which must not be mistaken for growth lines. The skeleton is composed of polygonal or rounded plates of very contrasting size and outline. The smallest plates (2 × 2 mm across) are observed at the top of the only complete zooid of Toscanisoma multipartitum (Fig. 2.1, 2.3), the largest ones (7 × 10 mm) in the more basal portions of both species described below. The plates fit closely together and show very faint boundaries on the outer side (Figs. 2.1, 2.2, 3.1). At a first glance they appear as post-mortem cracks, but the corresponding inner surfaces show that the plates are separated by distinct zigzag sutures similar to Zardinisoma (Ascidiacea, order Khmeriamorpha, Wendt, Reference Wendt2018) (Figs. 2.1, 2.2, 3.2). Careful preparing of the interior of individual zooids did not reveal any skeletal structures such as tabulae, septa or dissepiments. Budding in Toscanisoma multipartitum starts at the base, but in T. triplicatum only ~20 mm higher up, just showing the incipient division of the basal cup into three zooids, the distal parts of which are not preserved (Fig. 3.5).
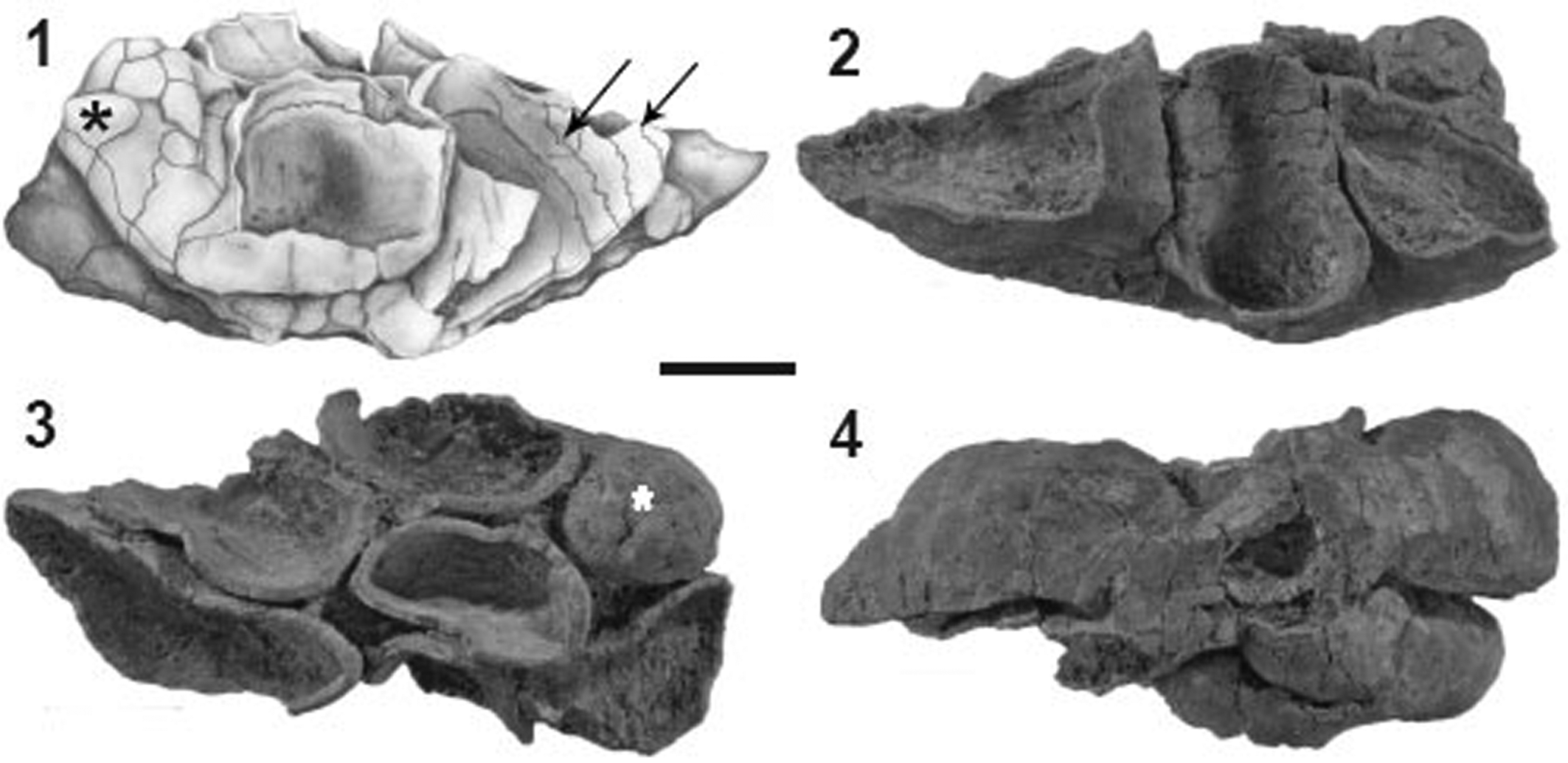
Figure 2. Toscanisoma multipartitum holotype (GPIT/TU 82). (1) Lateral view (drawing); (2) opposite side; (3) view from top; (4) base. Asterisks mark complete zooids, arrows indicate zigzag sutures on inner surfaces. Scale bar = 10 mm.
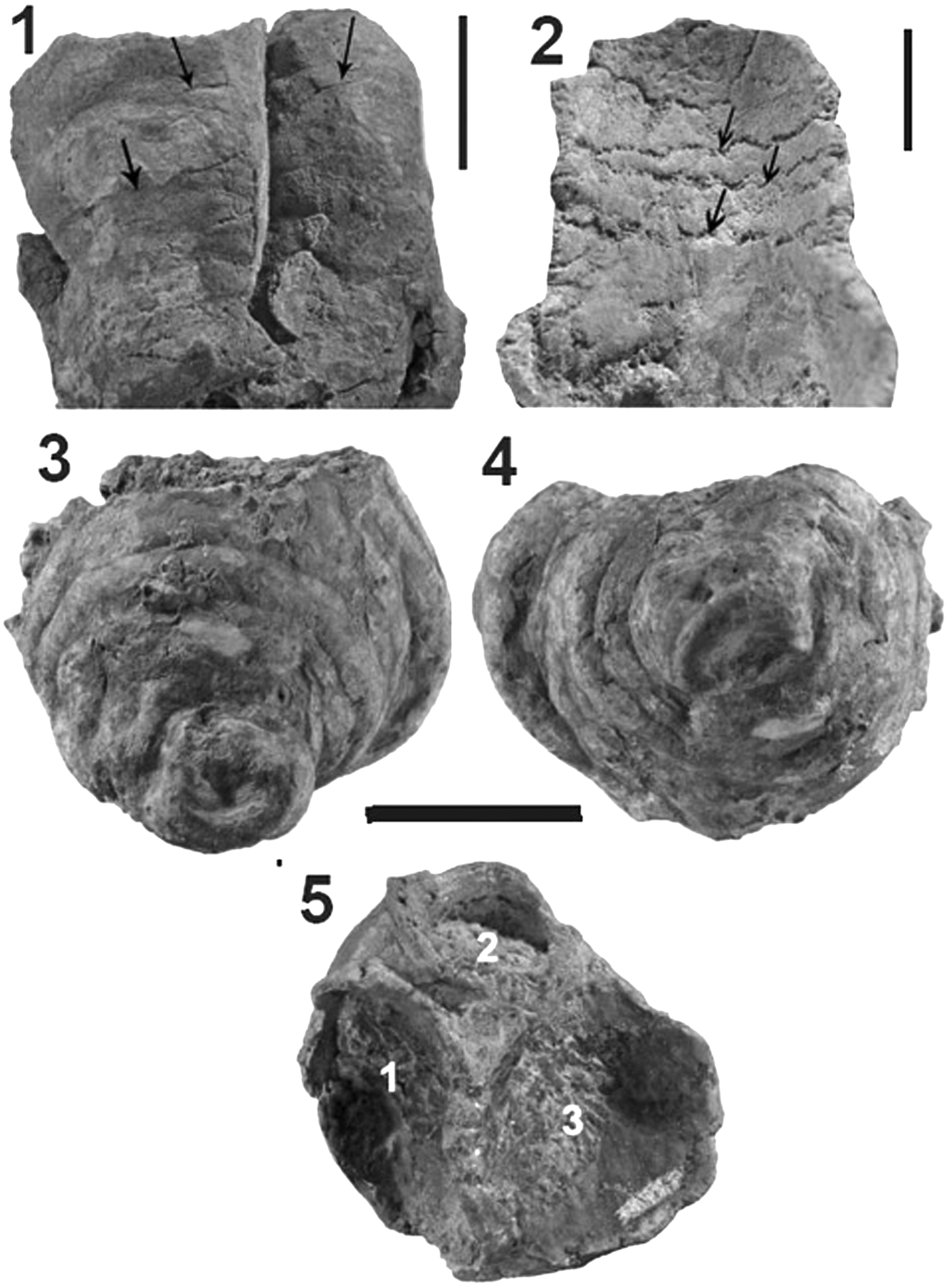
Figure 3. (1, 2) Toscanisoma multipartitum holotype (GPIT/TU 82). (1) Lateral view of 2 zooids, arrows indicate outer plate boundaries; (2) close-up of Figure 2.2; note zigzag boundaries of half ring-like plates (arrows). (3–5) Toscanisoma triplicatum holotype (GPIT/TU 83). (3) Lateral view; (4) view from base; (5) view from top; 1, 2, 3 mark cavities of three incomplete zooids. Scale bars for (1, 2) = 5 mm; (3–5) = 15 mm.
Mineralogy and microstructure
Staining of thin sections with Feigl-solution and x-ray diffraction analysis reveal that the skeletons consist of aragonite. The presence of minor amounts of calcite must be attributed to traces of early cements and/or post-depositional diagenetic alterations. The latter have also affected the majority of the original aragonite crystals, which are generally micritized (Fig. 5.3, 5.4) or transformed into larger ones (Fig. 5.6) whose mineralogical composition (aragonite or calcite) cannot be determined with the SEM. Several portions of the skeleton, however, retain the original microstructure, which consists of up to 30 μm long and 2–4 μm wide acicular crystals. Their three-dimensional arrangement is similar to that of coralline sponges (Wendt, Reference Wendt1984). Sun et al. (Reference Sun, Marcus, Frazier, Giuffre, Mass and Gilbert2017) have shown that acicular aragonite crystals in scleractinian corals grow 10 times faster along the c-axis than along the a-axis. From their similar dimensions, this differential growth rate can also be assumed for the crystals of the fossil ascidians. They are mostly arranged irregularly (Fig. 5.1, 5.2, 5.5), but locally also fan-shaped (clinogonally) (Fig. 5.3, 5.4). Towards the inner and outer sides of the plates, the crystals are generally arranged parallel (orthogonal) and perpendicular to the surfaces (Fig. 4.4). The patchy arrangement of the isolated or bundled crystals shows that biomineralization started more or less simultaneously at several nucleation points within the entire mantle (tunica), but it cannot be decided if this has been an endo- or extracellular process. Growth of the crystals was inhibited by contact with adjacent ones, thus resulting in a dense irregular porous meshwork that was probably infused with soft tissue. This growth pattern is totally different from the endoskeletons of echinoids and belemnites (which both show evident growth lines), but it resembles that of spherical spicules of some living ascidians (Monniot, Reference Monniot1970).

Figure 4. Toscanisoma triplicatum holotype (GPIT/TU 83). (1–3) Spicules; (4) orthogonal microstructure.
A very surprising discovery in SEM observations is the presence of rare calcareous monaxon spicules, up to 140 μm long and 2–10 μm thick, embedded in the crystalline skeleton of Toscanisoma triplicatum. They are the oldest unquestionable spicules of ascidians ever reported. One spicule has a rounded globular end and an acute top resembling monaxon spicules (tylostyles) of siliceous sponges (Fig. 4.1). The smooth surface and the obliquely fractured end suggest that this spicule may be a single crystal, a feature that is not known from other fossil or living tunicates. Another monaxon spicule is slightly arcuate and has tapering ends (Fig. 4.2). These simple spicules are obviously the most primitive ones, but are also known from fossil and living genera such as Herdmania and Pyura, which belong to the order Stolidobanchiata (Van Name, Reference Van Name1945; Monniot, Reference Monniot2002). The third type of spicule, which is the longest one (140 μm), has regular longitudinal ridges and grooves (Fig. 4.3). This type has never been observed in fossil or recent ascidians, but resembles the individual rays of spherical spicules (Varol and Houghton, Reference Varol and Houghton1996). The scarcity of these spicules shows that they had no essential importance for the stability of the skeleton, but they document the presence of specialized cells (scleroblasts) within the mantle (tunica) and/or the adjacent inner epithelium. The intimate intergrowth of the spicules with the crystals of the skeleton and the total absence of growth increments in the latter clearly indicate that the entire skeleton was formed within the tunica or mantle and that it is consequently an endoskeleton. In living ascidians spicules may migrate into blood vessels and internal organs (Lowenstam and Weiner, Reference Lowenstam and Weiner1989), but it is impossible to imagine that they moved into a solid skeleton.
Comparisons
Only few examples within the fossil record have been reported in which an endo- or exoskeleton has successfully been constructed not only very late in the evolution of a higher ranking taxon, but was later again abandoned in favor of a return to an almost soft-bodied stage. In gastropods, a secondary shell loss seems to have occurred several times, in particular in the subclasses Pulmonata and Opisthobranchia, in which relics of larval shells may be still present but were subsequently resorbed (Brusca et al., Reference Brusca, Moore and Shuster2016). This feature is found in the suborder Nudibranchia, but due to its lacking fossil record, their phylogeny and position within the subclass Opisthobranchia is unknown (Wollscheid-Lengelin et al., Reference Wollscheid-Lengelin, Boore, Brown and Wägele2001; Hallas et al., Reference Hallas, Simison and Gosliner2016) and their evolution from hard- to soft-bodied organisms remains speculative. Another comparable example is typified by some living soft-bodied Octopoda of the subclass Coleoidea (Cephalopoda) whose skeleton (gladius)-bearing living representatives (e.g., Vampyroteuthis) may have evolved by decalcification from Cretaceous phragmocone-bearing ancestors (Fuchs and Iba, Reference Fuchs and Iba2015; Sutton et al., Reference Sutton, Perales-Raya and Gilbert2016). Unfortunately, intermediate forms from the Tertiary, which would corroborate this assumption, are still lacking (personal communication, D. Fuchs, 2019). One may also regard the relatively late appearance of hard skeletons of Bryozoa in the Ordovician as a faintly comparable example, but their presumed origin from soft-bodied late Cambrian progenitors (Pywackia [see Landing et al., Reference Landing, Jonathan, Antcliffe, Brasier and English2015]) is very contentious. Also the late arrival of scleractinian corals in the early Middle Triassic might represent a comparable evolutionary step from soft-bodied to skeleton-bearing organisms, if one accepts that their possible ancestors must be searched among Paleozoic soft-bodied sea anemones (Stanley, Reference Stanley2003; Stolarski et al., Reference Stolarski, Kitahara, Miller, Cairns, Mazur and Meibom2011; Janiszewska et al., Reference Janiszewska, Stolarski, Kitahara, Neuser and Mazur.2015). In fact, this hypothesis is widely accepted among specialists of scleractinian evolution. But their direct relationship with rugosan corals is still a matter of debate, although the missing links in the early Triassic remain to be discovered (Cuif, Reference Cuif1977; Stolarski, Reference Stolarski1996). In this respect, it is worth noting that a late Paleozoic rugose coral (Numidiaphyllum) from the upper Permian of Tunisia not only possessed an originally aragonitic (recrystallized) skeleton, but also displayed a scleractinian pattern of cyclic septal insertion (Wendt, Reference Wendt1990). This unusual case might support the assumption that Rugosa were possible ancestors of Scleractinia. Scleractinian-like Rugosa were also reported from the Ordovician, suggesting that this “Bauplan” was already established in the early Paleozoic (Ezaki, Reference Ezaki1998). But in contrast to these still rather speculative similarities and assumed evolutionary trends, the discovery of tunicate spicules in the Late Triassic reveals a direct relationship to their soft-bodied descendants in the Early Jurassic. These arguments indeed allocate endo- and exoskeleton-bearing Ascidiacea a singular position in the fossil record.
Material and methods
Methods of study included manual preparation, thin sectioning, staining with Feigl-solution, SEM, and x-ray diffraction. SEM observations were performed on fresh fracture surfaces (thus avoiding contamination), on untreated outer and inner plate surfaces and on polished thin sections, which were etched with Mutvei-solution or EDTA (Marin et al., Reference Marin, Pokroy, Luquet, Layrolle and De Groot2007).
Repository and institutional abbreviation
Types, figures, and other specimens examined in this study are deposited in the collections of the Geological-Paleontological Institute of the University of Tübingen under the numbers GPIT/TU 1–83.
Systematic paleontology
Subphylum Tunicata Lamarck, Reference Lamarck1816
Class Ascidiacea Blainville, Reference Blainville and Cuvier1824
Order Cassianomorpha new order
Diagnosis
Colonial sessile Ascidiacea composed of 3–7 (eventually more) diverging hollow tubes (zooids) forming a compound solid aragonitic skeleton. The latter has a smooth outer surface without any traces of external and/or internal growth lines and is therefore interpreted as an endoskeleton that was secreted within the soft-bodied mantle (tunica). Individual zooids are composed of >10 irregular plates each, which have smooth, barely visible, straight or slightly curved margins on the outer and zigzag ones on the inner surface, suggesting a certain flexibility. The microstructure consists of acicular crystals of aragonite, up to 30 μm long and 2–4 μm wide. Internal skeletal elements, such as septa, tabulae, or dissepiments, have not been observed. For dimensions, see Table 1.
Table 1. Measurements of species of Toscanisoma.

Etymology
After the Cassian Formation (Upper Triassic, northern Italy), morph (Greek) = shape.
Remarks
The apical plates could presumably be unfolded in order to outstretch the atrial and branchial siphons. Movement of the plates was probably achieved by muscles, which were fixed in the porous marginal portions of the plates. The lack of growth lines, the local presence of spicules in one species, and the colonial growth form clearly distinguish the new order from Khmeriamorpha (Wendt, Reference Wendt2018).
Family Cassianosomidae new family
Diagnosis
As for order.
Etymology
After the Cassian Formation. Soma (Greek) = body.
Genus Toscanisoma new genus
Type species
Toscanisoma multipartitum n. sp.
Diagnosis
A colonial form consisting of up to seven (possibly more) cylindrical tubes (zooids) which bud from the base and diverge at an angle of 80–135°. The zooids fit closely together at their bases, but leave narrow open interspaces higher up. Each zooid is composed of a varying number of irregular plates, which show faint straight or curved boundaries on the outer surface and zigzag sutures on the inner one. Rare spicules embedded in the calcareous endoskeleton were observed in one species (T. triplicatum).
Occurrence
Cassian Formation (lower Carnian), Dolomites (Italy).
Etymology
After Maria Luigia Toscani (Cortina d'Ampezzo, Italy), collector of fossils from the Cassian Formation, who donated the specimens for the present study. Soma (Greek) = body.
Remarks
The colonial growth form consisting of several (seven, possibly more) zooids budding from a common center shows relationships to morphologically similar living genera of Ascidiacea that, however, are soft-bodied (see above). This growth form is also typical for phaceloid rugosan and scleractinian corals, but in contrast, the new genus does not display any internal skeletal elements. Compound, solid calcareous skeletons are unknown from living genera of Ascidiacea.
Toscanisoma multipartitum new species
Figures 2, 3.1, 3.2, 5.1, 5.4, 5.5

Figure 5. Microstructures. (1, 4, 5) Toscanisoma multipartitum. (2, 3, 6) Toscanisoma triplicatum. (1, 2, 5, 6) Irregular microstructure; (3, 4) clinogonal microstructure (partly micritized). (1–4) Artificially broken cross sections; (5, 6) polished and EDTA-etched surface-parallel thin sections showing original irregular (5) and recrystallized (6) microstructure.
Holotype
Figures as above; GPIT/TU 82.
Diagnosis
A sessile colonial species consisting of seven branching tubes (zooids) composed of irregular plates that are joined by straight or curved boundaries on the outer and zigzag ones on the inner side. The only complete zooid of the colony is closed at the top by six plates that are smaller than the remaining ones. The other zooids are incomplete, but were probably slightly larger. Spicules have not been observed. For measurements see Table 1.
Occurrence
Alpe di Specie (Seelandalpe) near Cortina d'Ampezzo (Dolomites, northern Italy), Cassian Formation (lower Carnian).
Description
The only specimen available for the present study is not complete, lacking the basal portion, which probably served as a holdfast. The seven zooids, which bud from the flattened base, have different shapes ranging from almost circular to elongate or flattened in cross section. Only the smallest zooid is complete, 25 mm long and consists of ~10 plates with very faint and barely recognizable outer boundaries (exaggerated in the drawing of Fig. 2.1). The inner plate boundaries of the incomplete zooids show zigzag sutures similar to contemporaneous representatives of the order Khmeriamorpha (Wendt, Reference Wendt2018). The zooids fit closely together at the base and are separated by small open interspaces higher up.
Etymology
Multipartitum (Latin) = consisting of several parts.
Remarks
By careful manual preparing and etching with diluted (10%) acetic acid, some inner plate boundaries could be uncovered exhibiting typical zigzag boundaries (Figs. 2.1, 3.2). It must be assumed that the incomplete zooids of the colony were also closed by small plates similar to the only complete one (Fig. 2.1, 2.3). The entire colony might have reached a height of up to 5–6 cm and a width of ~7–8 cm, but their exact final size cannot be calculated. Spicules embedded in the solid skeleton have not been observed.
Toscanisoma triplicatum new species
Figures 3.3–3.5, 4, 5.2, 5.3, 5.6
Holotype
Figures as above; GPIT/TU 83.
Diagnosis
A sessile colonial species consisting of three (eventually more) zooids, which bud at a certain distance from the basal cup. The apical angle of the colony is ~80°. Rare monaxon spicules of different size and shape are embedded in the porous skeleton. The distal portion of the zooids is not preserved. The outer surface of the basal part shows irregular swellings and faintly recognizable straight boundaries of individual plates with polygonal outlines. For measurements see Table 1.
Differential diagnosis
The species is distinguished from T. multipartitum by the presence of spicules, the different kind of budding, in which the separation into three individual zooids starts at a distance of ~2 cm above the base. Thus, in their early growth stage, the individual zooids share the outer walls of the adjacent ones, but higher up than in T. multipartitum.
Occurrence
Alpe di Specie (Seelandalpe) near Cortina d'Ampezzo (Dolomites, northern Italy), Cassian Formation (lower Carnian).
Etymology
Triplicatum = triplicate (consisting of three zooids).
Remarks
Because of the fragileness of the specimen, the inner side of the basal cup and the individual zooids could not be sufficiently prepared, but it is assumed that the inner plate boundaries show zigzag sutures similar to T. multipartitum. Due to the incomplete state of preservation and the lack of comparable material, speculations about the final growth form and the number of zooids are premature.
Zardini (Reference Zardini1981, pl. 23, fig. 10) figured a specimen determined as ”?Spondylus zardinii (Leonardi)” from the Cassian Formation near Cortina d'Ampezzo, which bears a certain resemblance to the new species. It consists of four (or six?) closely fitting tubes of similar size as those in T. triplicatum, which are open at their distal parts and thus are probably incomplete. Unfortunately, the original specimen could not be traced in the non-cataloged and rather disordered collections of the author deposited in the Museo Zardini at Cortina d'Ampezzo. I suppose that additional specimens that might show a much more reliable relationship to the here established new species (and to T. multipartitum) could be rediscovered in the immense collections of the late Rinaldo Zardini, which contains far over a million specimens and is worldwide unique.
Discussion
The above sketched morphological and mineralogical features raise the pivotal question of the systematic attribution and the functional morphology of these enigmatic remains. Solid skeletons (whether exo- or endo-) composed of irregular plates that consist of acicular aragonite crystals are unknown in the animal kingdom—with one exception: Permian/Triassic ascidian tunicates with a compound calcareous skeleton, which appeared in the early(?) Permian and became extinct during the Late Triassic (Wendt, Reference Wendt2018). The reason for this surprising taxonomic attribution has been discussed at length in a previous article (Wendt, Reference Wendt2018). These remains reveal a certain relationship to some living ascidians (e.g., Chelyosoma and Forbesella, later attributed to Pyura; Bronn, Reference Bronn1893–1911; Bancroft, Reference Bancroft1898), although these lack any hard parts. But they are partially composed of irregular soft plates that can be moved by muscles (Van Name, Reference Van Name1945). The hinge-like sutures on the inner plate surfaces of the newly described fossil endoskeletons also suggest a certain flexibility of the compound skeleton, which is indispensable for their here proposed assignment to ascidian tunicates. The latter are filter-feeders in which a steady flow of seawater moves through an atrial and a branchial siphon. If we assign a similar function to the fossil counterparts described here, it must be required that one or two of the top plates could be opened by muscles during the life-time of the organism because this is the case in the two above-mentioned recent genera. Unfortunately, the upper part of the examined specimens generally is not preserved. But one zooid of Toscanisoma multipartitum is complete and exhibits a mosaic of tiny plates (Fig. 2.1, 2.3), which probably could be opened by muscles to allow access for the protruding siphons.
At a first glance, the presence of a solid calcareous endoskeleton in Cassianomorpha might be surprising. However, one should bear in mind that, apart from Arthropoda (which have a totally different kind of growth), all invertebrate classes with a calcareous exoskeleton show well-developed growth lines reflecting an intermittent growth at the edge of the skeleton-secreting tissue. This is not the case in the Cassianomorpha in which the skeleton is formed within the mantle (tunica) starting more or less simultaneously at several nucleation points. Faced with these observations, it is less surprising that the extinct Cassianomorpha developed an endoskeleton, as did the other main deuterostome phyla or classes (Echinodermata, Vertebrata, Jurassic to recent Ascidiacea), than the fact that the other contemporaneous fossil ascidian order Khmeriamorpha strangely developed an exoskeleton (Wendt, Reference Wendt2018).
Conclusions
By mid-Cambrian times, representatives of almost all metazoan phyla and subphyla had reached a high degree of biomineralization in such a way to enable a reliable reconstruction of their evolution. Why is this not the case with tunicates, which must have existed contemporaneously? One could speculate about a change in seawater chemistry or a possible global perturbation of the carbon cycle near the Carboniferous/Permian boundary (Sandberg, Reference Sandberg1983; Stanley, Reference Stanley2006), but such speculations about the appearance of mineralized (aragonitic) tunicate skeletons at this interval appear rather theoretical, because the time-span of an “aragonitic ocean” (Mississippian to Middle Jurassic; Stanley, Reference Stanley2006) is not consistent with the existence of aragonitic ascician skeletons.
A possible answer to this fundamental question is that compound pre-Permian tunicate skeletons might have existed in earlier Paleozoic times, but they have not yet been discovered or recognized as such. It can also not be totally dismissed that they are hidden among the great number of previously described fossil Problematica or those of incertae sedis. The discovery of rare spicules embedded in the solid skeleton clearly points to a new and successful attempt of tunicate biomineralization in the Late Triassic, which persisted until recent. However, post-Triassic survivors of tunicates with compound calcareous, either endo- or exoskeletons, are unknown from the fossil record. One may speculate that post-Triassic ascidians developed other protective strategies that served as a defensive function (e.g., secretion of indigestible chemicals), which, of course, cannot be detected in fossil remains. Not taking into account these theoretical considerations, the total loss of a compound calcareous skeleton in ascidians during the Late Triassic in favor of a much less stable one consisting of isolated spicules only remains an unresolved question.
Acknowledgments
I am indebted to the following persons for their support of the present study: the late R. Zardini and M.L. Toscani (both Cortina d'Ampezzo, Italy) collected and donated the majority of the studied material. W. Gerber (Tübingen) produced the photographs, N.M. Lesic (Tübingen) made the drawing of Figure 2.1., H. Schulz (Tübingen) photographed several samples under the SEM, Ch. Berthold (Tübingen) performed the X-ray diffraction analysis, P. Jeisecke (Tübingen) made the thin sections. The manuscript benefitted from the corrections and critical comments of R.D.K. Thomas (Lancaster, PA), A. Pisera (Warszawa, Poland), and an anonymous reviewer.







