The term ‘sweeteners’ encompasses both nutritive and non-nutritive sweeteners, which when added to food and beverages, can enhance the flavour and other functional properties of food and beverage products. Nutritive sweeteners (namely free sugars) provide the body with energy. Of the various terms used to define the types of sugars described in dietary recommendations (see Table 1 for a summary of these definitions), free sugars, which refers to all monosaccharides and disaccharides added to foods by the manufacturer, cook or consumer, and sugars that are naturally present in honey, syrups, fruit juices and fruit juice concentrates(1), have recently come to particular attention in relation to public health. In contrast, non-nutritive sweeteners provide a desired sweet taste without the addition of appreciable energy and can help maintain the palatability of reformulated products. Non-nutritive sweeteners have been categorised in different ways in the past but can be broadly categorised as bulk sweeteners or intense sweeteners (Table 2). For the purpose of this review, the term sugar/s will be used to describe nutritive sweeteners, as encompassed within the current WHO classification of free sugars(1) and for non-nutritive sweeteners the review will focus on intense sweeteners only, referring to these as low-energy sweeteners (LES). This review considers how dietary biomarker approaches may enhance current understanding of nutritive sweeteners (free sugars) and non-nutritive or LES intakes and how these may impact health.
Table 1. Various different definitions used for dietary sugars (adapted from SACN, 2015(4))

* The only difference between NMES and free sugars is that non-milk extrinsic sugars includes 50 % of the fruit sugars from stewed, dried or canned fruit(Reference Bates, Lennox and Prentice55), but free sugars includes none.
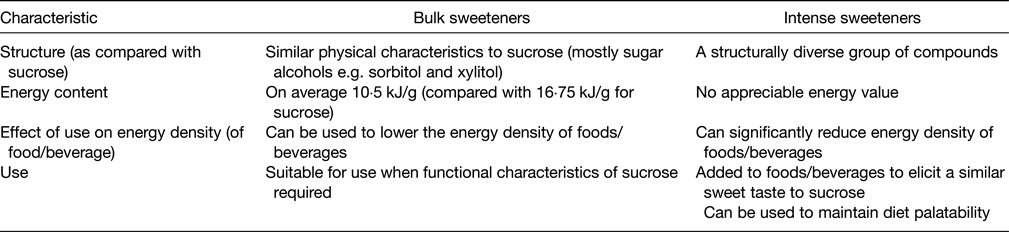
Table 2. Characteristics of bulk sweeteners v. intense sweeteners
Nutritive sweeteners
Free sugars and health
Global estimates suggest that some 650 million adults and 41 million children (<5 years) are now obese(2). The global prevalence (age-standardised) of type-2 diabetes mellitus has almost doubled over the past 20 years, up from 4·7 % in 1980 to 8·5 % in the adult population(3). The potential negative health impacts of overconsumption of free sugars, particularly from sugar-sweetened beverages (SSB), in relation to weight gain (and increased BMI), increased risk of developing type-2 diabetes and tooth decay are well documented(1,4) .
Current and recommended sugar intakes
Most recent data from the UK National Diet and Nutrition Survey indicate that in all age groups, mean intake of free sugars exceeded (more than double) the UK government recommendation of no more than 5 % of daily total energy intakes (TEI)(5). Furthermore, only 2 % of UK children aged 4–10 years, 5 % of children 11–18 years and 13 % of UK adults met these recommendations for free sugar intakes(5). In 2010, the European Food Safety Authority (EFSA) published its Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre and observed that the average intake of (added) sugars in some European Union (EU) Member States exceeded 10 % of TEI(6). The current US Department of Agriculture dietary guidelines for Americans recommended that added sugar intake should not exceed 10 % of TEI(7). Similarly, WHO strongly recommends reducing the intake of free sugars to less than 10 % of TEI in both adults and children; corresponding to about 50 g/d for females with a daily energy requirement of 8·64 MJ/d (2000 kcal/d) or about 60 g/d for males with a daily energy requirement of 10·46 MJ/d (2500 kcal/d). Furthermore, WHO(1) suggests a further reduction to below 5 % of TEI as desirable and in the UK, the Scientific Advisory Committee on Nutrition (SACN) has recommended that the average population intake of free sugars should not exceed 5 % of TEI for adults and children (aged 2 years and over)(4).
Strategies for reducing intakes of free sugars
Considering current consumption of free sugars within the population, achieving the recommended reductions in free sugar intakes are likely to prove challenging. Strategies that have been endorsed include promoting healthier options (e.g. ‘sugar swaps’), reducing the sugar content of commonly consumed products, reducing portion size, and/or shifting purchasing towards lower sugar alternatives(8). In the UK, efforts to achieve these public health targets for sugar intakes have also included the introduction of a SSB levy together with guidelines for all sectors of the food industry in relation to sugar (and energy) reduction of selected food products. These guidelines aim to encourage industry to achieve a 20 % sugar reduction by 2020 across the top nine categories of food products that contribute most to intakes of children up to age 18 years (based on the 2015 sugar content of these food products)(8). It is interesting to note that, ahead of the UK introduction of the SSB drink tax (introduced on 6 April 2018), an estimated 50 % of all beverages already had reduced sugar content. Furthermore, early assessment of progress by the food industry in relation to sugar reduction targets show good progress towards the first 5 % reduction in sugar content across a number of food categories(9). Using strategies such as promoting healthier choices, reducing portion size and reformulating products are also likely to go some way for achieving these sugar reduction targets. However, given the very significant health and economic implications of chronic conditions such as obesity and diabetes, it is essential that the effectiveness of such approaches aimed at reducing sugar consumption is robustly evaluated.
Limitations with current research approaches to investigating the health impacts of sugars
Current recommendations for sugar intakes are largely based on evidence prospective cohort studies and randomised clinical trials (RCT)(1,4,7) . A recognised weakness of using epidemiological data is that prospective cohort studies rely on unreliable quantitative assessment of food intake, and hence, may yield erroneous estimates of dose–response relationships for sugar intake(Reference Tappy, Morio and Azzout-Marniche10). An alternative approach employed by Tappy et al.(Reference Tappy, Morio and Azzout-Marniche10) focused on data from mechanistic studies and RCT to determine intake thresholds above which sugar intake may negatively impact on health and hypothesised that the specific effects of sugars were attributed to their fructose component and thereby focused on studies which had assessed the effects of pure fructose. Clearly, a limitation of this approach was that the authors assumed that the effect of 50 g fructose can be extrapolated to the effect of 100 g sucrose(Reference Tappy, Morio and Azzout-Marniche10), which may not be the case. Another limitation when considering such studies relates directly to how sugars are defined, for example as ‘added sugars’ (USA(7)) or ‘free’ sugars (WHO(1), SACN(4)). There has been considerable debate on these definitions which can result in significant variation in findings(Reference Erickson and Slavin11). Based on available data, it is not possible to distinguish the health effects of sugars naturally present in food (e.g. fruit, vegetables, milk/dairy) from those of added sugars(Reference Tappy, Morio and Azzout-Marniche10). In the case of foods which naturally contain sugars, strong evidence exists for the protective effect of high fruit and vegetable consumption in relation to non-communicable diseases, which likely reflects other beneficial constituents within these foods (such as NSP, vitamins, minerals and in the case of fruit/vegetables phytochemicals). Recently published guidance on sugar consumption in France sets an upper intake limit of 100 g total sugar/d while continuing to recommend promotion of fruit and vegetable intakes(12). Food consumption data estimated that within the French diet, fruit intakes provide about 40 g sugars, and intakes of vegetables provide 6 g sugars; this is consistent with a daily amount of free or added sugars in the 5–10 % TEI range, and thus these recommendations align with the WHO(1) and SACN(4) recommendations. When EFSA published its Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre in 2010(6), the available evidence at that time was deemed to be insufficient to set an upper limit for the daily intake of total or added sugars. It is expected that EFSA will provide scientific advice on a tolerable upper intake level for (total/added/free) sugars by early 2020 with the aim of establishing science-based cut-off values for daily exposure to added sugars from all sources which are not associated with adverse health effects (to include body weight, glucose intolerance and insulin sensitivity, type-2 diabetes, cardiovascular risk factors and dental caries)(13). It is also anticipated that in this context EFSA will consider the general healthy population, including children, adolescents, adults and the elderly.
Non-nutritive sweeteners
Safety and regulation
The safety of non-nutritive sweeteners is rigorously evaluated prior to approval for use; the responsibility for these evaluations lies with regulatory bodies such as EFSA, the Joint Expert Committee on Food Additives and the Food and Drug Administration and safety evaluations usually result in the assignment of an acceptable daily intake (ADI). The ADI, which is calculated following the application of large safety factors to the no observed adverse effect level (Fig. 1), is regarded as the quantity of a food additive that can be ingested on a daily basis over a lifetime without appreciable health risk. The ADI is an often mis-interpreted value; it does not represent a threshold between safe and unsafe but rather, it is a calculated value, derived by dividing the NOAEL (no observed adverse effect level) observed in toxicology studies by a safety factor(Reference Magnuson, Carakostas and Moore14). The NOAEL is the daily amount consumed in long-term, repeated-dose studies that was shown to have no adverse effects in the most sensitive animal model; in other words, it is a level of daily intake that is too low to cause any biological effects. The safety factor (often a factor of 100 times lower than the NOAEL) is then applied to ensure protection of the most susceptible and sensitive individuals in an entire population, including children and pregnant women (Fig. 1). This calculation for ADI represents a much greater safety factor than exists for most nutrients and other naturally occurring food components. Therefore, the ADI is a level of daily intake considered safe for everyone, including those with the highest potential exposure to an ingredient (or LES).(Reference Benford15)

Fig. 1. (Colour online) Safety factors applied to establish the acceptable daily intake (ADI). NOAEL, no observed adverse effect level. Source: Logue et al.(Reference Logue, Dowey and Strain21)
Within the EU there are currently eleven LES approved for use (Table 3) and, in accordance with the EU Regulation 1333/2008 on food additives(16), the use of LES in a food or beverage, in most cases, must also result in an energy reduction of at least 30 % in the food/beverage product. For consumers this means that where LES are present a significant reduction in energy intake is possible with like-for-like product swaps. However, some regulatory constraints regarding the use of LES may limit the opportunities of food reformulation(Reference Gibson, Ashwell and Arthur17). For example, under EU Regulation 1333/2008, permitted use depends on the food category or categories into which the product falls and at present LES cannot be incorporated into fine baked products (e.g. cakes or biscuits).
Table 3. Intense sweeteners approved for use in Europe

ADI, acceptable daily intake; BW, body weight.
* Sweetness relative to sucrose.
Source: Adapted from Logue et al.(Reference Logue, Dowey and Strain21)
Current intakes of low-energy sweeteners
The most recent review of available data on global intakes of LES(Reference Martyn, Darch and Roberts18) raised no concerns with respect to exceedance of the ADI for individual LES across healthy population groups. However, the authors recommended that quality dietary data were needed and a more standardised approach be adopted to allow monitoring of potential changes in exposure over time (e.g. in response to sugar reduction strategies or change in product formulation). In particular, the authors recommended that consideration should be given to intakes in individuals likely to be higher consumers of LES, such as those with type-2 diabetes or those on weight-loss diets(Reference Martyn, Darch and Roberts18). Recent trend data from the US National Health and Nutrition Examination Survey cohort, collected in five 2-year cycles from 1999–2000 to 2007–2008 using a single 24-h recall, was used to determine trends in LES intakes over time(Reference Sylvetsky, Welsh and Brown19). Whilst no observed changes in energy intakes over time were observed, the percentage of children consuming foods and beverages containing LES nearly doubled (8·7 % in 1999–2000 to 14·9 % in 2007–2008) with the percentage of the adults consuming LES-containing foods/beverages increasing from 26·9 to 32·0 %(Reference Sylvetsky, Welsh and Brown19). The data also suggested that the consumption of LES-containing beverages also doubled among US children over the past decade with an estimated 12·5 % of children consuming beverages with LES in 2007–2008(Reference Sylvetsky, Welsh and Brown19). Increased consumption has been sustained with cross-sectional National Health and Nutrition Examination Survey data from 2009 to 2012 suggesting that 25·1 % of children and 41·4 % adults reported consuming foods/beverages containing LES (obtained from two 24-h recalls)(Reference Sylvetsky, Jin and Clark20). However, it was acknowledged that these self-reported intake data (obtained from one–two 24-h recalls), and the lack of information on the specific type or quantity of LES in foods or beverages in the US Department of Agriculture database, mean that it is not possible to determine actual intakes of LES (either as total LES intake or intakes of individual LES) within this cohort. European intake assessments generally do not indicate a concern among average or high-level healthy consumers of all ages in Europe under the typical conditions of use for all LES considered using tiered assessment approached (i.e. Tier 3 using estimates of actual food consumption and usage levels). More recent studies have generally indicated a reduction in LES intakes in various EU countries, although differences in study design limit comparisons between these studies and thus should be interpreted with caution(Reference Martyn, Darch and Roberts18). It has been highlighted that certain subgroups of the population such as young, individuals with diabetes, phenylketonuria or with severe cow's milk protein allergy and who are consumers of foods for special medical purposes that are sweetened to increase palatability may have higher intakes of specific LES(Reference Martyn, Darch and Roberts18) and may thus warrant closer monitoring.
It is worth noting that LES are now more commonly used as part of blends in food/beverage products to achieve desired taste profiles of products. This should be of interest when investigating the potential health impacts of LES-use as LES have different biological fates following ingestion(Reference Magnuson, Carakostas and Moore14). As such, research approaches should aim to discern intakes of individual and blends of LES so that potential relationships with health can be properly explored. However, achieving this in practice using traditional methods of intake assessment may prove challenging as the use of LES is continuously evolving and manufacturers in most countries are not required to reveal the quantities of LES used in products.
Several methodologic issues have been highlighted in relation to assessing LES intakes(Reference Logue, Dowey and Strain21). In addition to foods/beverages, LES may also be present in a variety of personal care products such as toothpaste, mouthwash, dietary supplements and over-the-counter medications(Reference Logue, Dowey and Strain21). This can pose difficulties for researchers who may wish to include a non-consumer group in that instructions to avoid LES need to consider both dietary and non-dietary sources (e.g. personal care products)(Reference Sylvetsky, Blau and Rother22). For example, a recent study which aims to recruit healthy volunteers who self-reported to be non-consumers of LES, found that over 30 % were exposed to sucralose at baseline and/or before randomisation, and nearly half were exposed after assignment to the control(Reference Sylvetsky, Walter and Garraffo23). This shows that instructions to avoid LES are not effective and that non-dietary sources (e.g. personal care products) may be important contributors to overall exposure. In a small study of twenty lactating mothers, LES (namely sucralose, acesulfame-K and saccharin) have also been shown to pass into breast milk(Reference Sylvetsky, Gardner and Bauman24). In addition, these LES were found in the breast milk of 65 % of these lactating women who had been enrolled in the study, irrespective of their history of LES usage; again highlighting the ubiquitous nature of LES. Furthermore, several LES have gained attention as potential environmental contaminants owing to their persistence in the environment, which in turn may, over time, lead to further inadvertent exposure to certain LES. Concentrations of some LES (e.g. sucralose, acesulfame-K) of up to µg/l have been observed in soil, waste water and surface water with the measured concentrations(Reference Lange, Scheurer and Brauch25). For LES with the highest environmental concentrations (namely acesulfame-K and sucralose), levels in drinking-water (and other tested matrices) are three to four orders of magnitude below the ADI values for LES(Reference Lange, Scheurer and Brauch25). However, with the persistence of these LES within the environment, along with increasing use, this source of inadvertent exposure may become more significant in the coming years and therefore be of interest as part of investigations of the health impacts of LES.
Health impacts of low-energy sweeteners
The potential health impacts of using LES is a topic of ongoing debate despite the stringent safety evaluations prior to their approval for use. A significant number of reviews have been published dealing with a range of health outcomes such as weight status(Reference Rogers, Hogenkamp and de Graaf26,Reference Azad, Abou-Setta and Chauhan27) , type-2 diabetes(Reference Imamura, O'Connor and Ye28), cancer(Reference Weihrauch and Diehl29) and preterm delivery(Reference La Vecchia30). However, to date, adverse health impacts of LES use have not been conclusively established, although significant gaps in the literature exist that should be addressed going forward. One important and widely accepted benefit that has been endorsed by EFSA(31) is the reduction in tooth demineralisation when LES are used in place of nutritive sweeteners.
A brief summary of the evidence in relation to several selected health outcomes is provided below.
Energy intake, body weight and cardiometabolic health
Evidence from intervention studies has tended to indicate beneficial effects of LES with regard to weight status when used in place of sugar; however, debate on this topic persists, partly because of limitations with current research, but also as a result of more mixed findings from observational research. Short-term RCT have tested the impact of the consumption of LES-preloads on the subsequent energy intake in an ad libitum meal as compared with the sugar or v. unsweetened products, water, placebo or nothing (controls). While studies have shown that there is some compensation for the ‘missing’ energy when LES are used to replace sugar, this compensation is only small, partial and incomplete resulting in a significant energy deficit with LES use, and thus a decrease in overall energy consumed in the subsequent meal(s) and overall within the day(Reference Anton, Martin and Han32–Reference Rogers34). A recent comprehensive systematic review with meta-analyses by Rogers et al.(Reference Rogers, Hogenkamp and de Graaf26), which included evidence from animal and human studies over a wide range of study durations, provides considerable weight of evidence favouring consumption of LES in place of sugar as helpful in reducing relative energy intake and body weight. Notably, the effects of LES-beverages on body weight also appear neutral relative to water, or even beneficial; however, further work is required to conclusively establish this potentially significant benefit. Contrary to the conclusions of Rogers et al.(Reference Rogers, Hogenkamp and de Graaf26), Azad et al.(Reference Azad, Abou-Setta and Chauhan27) conducted a systematic review with meta-analyses focusing on longer-term consumption of LES and whether this was associated with cardiometabolic effects on adults and adolescents. They reported no beneficial effects of LES in relation to a range of outcomes including BMI, body weight and other relevant cardiometabolic risk factors. They included evidence only from longer-term (at least 6 month duration) RCT and prospective cohort studies, which, it is argued, may be more relevant to conditions that might develop over longer periods. It should be noted that the authors acknowledged several limitations with the current evidence and which should be addressed in future work in this area. This ongoing debate has led some to explore mechanisms through which LES may elicit clinically meaningful effects within the body, some of which are plausible and indeed supported by in vitro and animal studies(Reference Burke and Small35). Examples of potential mechanisms include interactions with the gut microbiota, stimulation of the sweet receptors (oral and extra-oral) and changes in the physiological response to sweet compounds in the diet. Contrary to the concern that LES might increase subsequent energy intake by stimulation of oral or gut sweet taste receptors or via other mechanisms, energy intake does not appear to differ for LES v. water, v. unsweetened product or v. placebo or nothing(Reference Mattes and Popkin36), LES do not appear to affect energy intake differently when compared with a control different from sugar such as water, or placebo, or unsweetened products, or nothing(Reference Rogers, Hogenkamp and de Graaf26).
LES and glycaemia
The impact of LES on glycaemia is particularly topical given the increased prevalence of type-2 diabetes along with recent public health efforts to reduce free sugar consumption within the population. In a well-publicised study by Suez et al.(Reference Suez, Korem and Zeevi37), LES were implicated in impaired glucose tolerance with the authors concluding that this effect was mediated by changes to the gut microbiota(Reference Suez, Korem and Zeevi37); however, several important limitations should be highlighted to facilitate appropriate interpretation of the results. Although the dose of saccharin was theoretically within physiologically relevant limits (5 mg/kg body weight), it would represent a very high dose (equivalent to 100 % of the ADI), which is unlikely to reflect actual exposure within the population. Furthermore, the human intervention that followed the animal studies involved a small number of individuals (n 7) and lacked a control group; therefore, future work should aim to address these limitations and over a longer-term. Other potential mechanisms by which LES may impact glycaemia have been discussed including enhanced gut hormone release and altered glucose absorption(Reference Rogers34); however, these mechanisms have not been conclusively established in human subjects. Epidemiologic studies investigating LES consumption and incidence of type-2 diabetes have reported mixed findings(Reference Imamura, O'Connor and Ye28,Reference O'Connor, Imamura and Lentjes38) whilst a recent systematic review of RCT report no effect on glycaemia; therefore, as with body weight and weight status, further well designed RCT are warranted to conclusively establish the impact of LES on glycaemia(Reference Nichol, Holle and An39). It is also important to state that there is clearly a lack of evidence on the benefits or otherwise of LES use on glycaemic control in clinically relevant populations as much of the research published to date is in healthy populations and therefore, future work should be conducted in clinically relevant populations such as in those living with diabetes.
Other health outcomes
LES use has also been investigated in relation to several other health outcomes including cancer and preterm deliveries; however, no convincing evidence has been presented to date to support these theories(Reference Logue, Dowey and Strain21). Indeed, the French Agency for Food, Environmental and Occupational Health and Safety (ANSES), in its review of the evidence, concluded that, although further research is required to establish the long-term beneficial effects of LES consumption on health, the available data would endorse the safety of LES(40). Notably, ANSES also recommended that future cohort studies should aim to distinguish the intakes of individual LES, so that the effects of single and multiple LES use can be investigated more effectively(40).
Whilst associations between LES consumption, weight gain and metabolic abnormalities have been reported in epidemiologic studies and have been observed in rodent models (as highlighted earlier and discussed in detail elsewhere), Sylvetsky et al.(Reference Sylvetsky, Blau and Rother22) concluded that in their review of the evidence that robust intervention studies in human subjects to evaluate the health effects of LES, in particular the potential impact of LES on metabolism and weight are needed. Indeed, a recent systematic review with meta-analyses investigated the association of LES use with various health outcomes including changes in body weight and glycaemic control with no consistent findings(Reference Toews, Lohner and Küllenberg de Gaudry41). Several limitations of epidemiologic studies have previously been highlighted and should be addressed: given the differential metabolic fates and potential mechanistic effects of LES, intakes of specific LES should be investigated rather than generalising findings of studies to all LES; it is also important that habitual exposure is reliably assessed both in the context of cohort studies as well as in RCT; and finally, the longer-term impact of LES should be assessed in appropriately designed long-term RCT that reflect typical use within a free-living population.
Limitations of current approaches to investigating the health impacts of low-energy sweeteners
As highlighted by ANSES(40) future research should aim to more effectively evaluate the intakes of individual LES with consideration given to the potential effects of individual LES as well as multiple LES use. Since current assessment investigations of LES intakes in relation to health often only consider certain sources of LES (such as LES-beverages) and/or consider LES as a homogeneous group despite their known differing biological fates(Reference Magnuson, Carakostas and Moore14), it is unlikely that these approaches adequately capture intakes of individual LES, or allow reliable estimation of overall intakes, thus limiting the possibility of robust evaluation of their potential impact of health. Biomarker approaches for sugar consumption and LES consumption and represent interesting developments in an area of significant public health interest(Reference Logue, Dowey and Strain42).
Biomarker approaches to dietary intake assessments
Biomarker approaches have been developed for several dietary components including salt, protein or specific foods such as coffee and garlic. The main advantage of implementing such an approach to assessing dietary intake is that it can eliminate many of the sources of bias associated with self-reported intake data. A biomarker approach must be properly validated by demonstrating a dose–response relationship between the putative biomarker and intake of the dietary component of interest. Other considerations include the specificity of the biomarker, the suitability of the sampling protocol in terms of burden on the individual and protecting the integrity of the sample, and the subsequent storage and analysis of the sample should be appropriate.
Biomarker approaches to the assessment of sugar intakes
Exposure biomarkers for the intake of sugars have been described using two different approaches, either through the measurement of the carbon isotope ratio 13C:12C (expressed as δ 13C value) or through the determination of sugars in urine(Reference Rothwell, Madrid-Gambin and Garcia-Aloy43).
Estimation of δ 13C value
The first approach is based on different discrimination against carbon dioxide formed from the 13C and 12C isotopes in plants(Reference Jahren, Saudek and Yeung44). In brief, crop species have been classified as C3 and C4 plants depending on their photosynthetic pathway. The photosynthetic pathway of C3 plants, such as sugarbeet, discriminates against 13CO2 compared with 12CO2, and the resulting plant mass carbon has a lower δ 13C than atmospheric CO2. In contrast, the photosynthetic pathway of C4 plants, such as sugarcane and maize, is almost all is almost non-discriminating against 13C, resulting in a plant mass higher in 13C compared with C3 plants. In the USA added sugar is mostly derived (78 %) from maize or maize derivatives (such as high-fructose maize sugar) or sugarcane (i.e. C4 plants) whereas in Europe it is the opposite with the majority of added sugar (80 %). Thus as a biomarker of added sugar intake, blood plasma or finger prick δ 13C has been largely limited to use in US populations(Reference Hedrick, Davy and Wilburn45) since in the US added sugar is mostly derived (78 %) from sugarcane or maize (i.e. coming from C4 crops), whereas in Europe it is the opposite with the majority of added sugar (80 %) derived from sugarbeet which are C3 crops.
Estimation of urinary sugars
The second approach for estimating sugar intake uses urinary sucrose and fructose as exposure markers(Reference Luceri, Caderni and Lodovici46). Urinary sugars have been validated(Reference Tasevska, Runswick and McTaggart47) as dietary biomarkers of total sugars (i.e. the sum of intrinsic, milk and free sugars) and sucrose(Reference Tasevska48), and can help to resolve the discrepancy between self-reported and actual intake. This biomarker relies on the total excretion of sucrose and fructose within 24-h and therefore requires complete 24-h urine samples. More recently, Campbell et al.(Reference Campbell, Tasevska and Jackson49) used this urinary biomarker in a nationally-representative sample of the UK adults and demonstrated positive associations between total sugar intake, measures of obesity and likelihood of being obese within the cohort. The authors noted that this was the first time such an association has been demonstrated using a validated biomarker approach(Reference Campbell, Tasevska and Jackson49). However, it is worth noting that no comprehensive dietary intake data was collected in this study and it did not set out as a validation study and as acknowledged by the authors this biomarker of total sugars is potentially useful but remains to be fully validated and characterised in different population groups and to be characterised further in terms of its biases, performance, reliability, sensitivity etc. A metabolomics-based strategy was applied to objectively assess intakes of SSB and identified a panel of four urinary biomarkers (formate, citrulline, taurine and isocitrate) indicative of SSB consumption from a national food consumption survey and have subsequently validated this panel of urinary biomarkers in an acute intervention study as markers of SSB intake showing that following acute consumption of an SSB drink, all four metabolites increased in the urine(Reference Gibbons, McNulty and Nugent50). However, future work is needed to ascertain how to translate this panel of markers for use in nutrition epidemiology. More recently, metabolomics to investigate the metabolic impact of acute sucrose exposure and highlighted sixteen major metabolite signals in urine and twenty-five metabolite signals in plasma that were discriminatory and correlated with acute exposure to sucrose intake(Reference Beckmann, Joosen and Clarke51). However, as only one component of total/added/free sugars, the application of this method in relation to sugar intakes is perhaps limited.
Biomarker approaches to the assessment of low-energy sweetener intakes
A challenge of assessing the potential health impacts of LES, which are now ubiquitous in today's society, in free-living populations is the reliable estimation of intakes. The use of LES extends to a wide range of foods and beverages, as well as non-dietary products such as oral hygiene products and e-cigarette fluids(Reference Logue, Dowey and Strain21). As noted previously, many current approaches consider only certain sources of LES, such LES-beverages, and often consider LES as a homogeneous group despite their differing biological fates. Such approaches, which do not adequately capture intakes of individual LES or indeed allow for reliable estimation of overall intakes, will not facilitate robust investigations of the health impacts and therefore, alternative approaches should be explored.
Urinary biomarker approaches have been developed for investigation of LES consumption and may provide more objective LES intake data(Reference Logue, Dowey and Strain42). A novel analytical method has been developed and validated to simultaneously assess the urinary excretion of five commonly consumed LES, namely, acesulfame-K, saccharin, sucralose, cyclamate and steviol glycosides (which are all excreted via the urine)(Reference Logue, Dowey and Strain42). A double-blind, randomised crossover dose–response study was subsequently conducted to assess the usefulness of urinary LES excretions (from both fasting spot and a full 24-h urine collection) for investigating recent intakes. Both modes of sampling were useful for distinguishing between the three short-term intakes of acesulfame-K, saccharin, cyclamates and steviol glycosides, whereas for sucralose, urinary concentrations were useful for distinguishing between low (0·1 % ADI) and high doses (10 % ADI) only. Given the high level of specificity of these putative biomarkers, along with promising initial validation data, a urinary biomarker approach may be useful for assessing intakes of these five commonly consumed LES. However, to conclusively demonstrate the usefulness of these biomarkers as indicators of intake, further validation work should be conducted to characterise the relationship between urinary excretion of the LES with intake e.g. in population groups of interest and over longer periods of time. Therefore, a biomarker approach could potentially be used in both experimental and observational research to objectively assess recent intakes of the respective LES thereby facilitating more effective investigations of the health impacts of LES-use. Outside of this panel of five commonly consumed LES, biomarker approaches to assessing intakes of other LES may be more problematic because of their metabolic fate following ingestion. For example, aspartame undergoes digestion in the gastrointestinal tract and is converted to methanol (10 % weight of aspartame), and the two amino acids aspartic acid (40 % by weight of aspartame) and phenylalanine (50 % by weight of aspartic acid), which are then absorbed and reaches the circulation(Reference Magnuson, Carakostas and Moore14). Since aspartic acid and phenylalanine are also absorbed in the same form following digestion of natural food sources (such as protein-rich foods such as meat, fish, eggs, dairy or legumes), against the very much lower concentrations of aspartame in the diet a urinary biomarker approach or metabolomics for assessing aspartame intakes is not likely to be feasible; however, one (speculative) approach might be to utilise a metabolomics approach to identify potential effect biomarkers. It should be noted that using such biomarkers for assessing intakes may be limited owing to likely inter-individual variation in potential effects of LES consumption.
Opportunities and challenges
While biomarker approaches to assessing dietary intakes can help generate objective intake data and therefore facilitate more robust investigations of relationships with health, they cannot allow for the identification of the sources of intake. Such information may be useful for assessing dietary patterns associated with the use of sugars and LES within the diet. As such, a worthwhile approach might be to combine biomarker approaches with more traditional methods of assessing dietary intakes in order to facilitate more robust investigations of the relationships between diet and health. Currently, the optimal sampling protocol for assessing LES-use is likely to be the collection of 24-h urine samples, which can be burdensome on participants resulting in incomplete collections. The collection of 24-h urine samples has been carried out in numerous epidemiological studies; therefore, the perceived challenges can be overcome. However, it should be noted that specific population groups of interest include children so more appropriate sampling protocols should be investigated for this cohort.
As noted previously, with the increasing use of blends of LES (rather than single LES) within food/beverage products, reliance on exposure assessment which focus on single LES have limited use. The range and type of LES available are likely to change over time in response to consumer demand. For example, since EU regulatory approval for use in 2011, steviol has been marketed as a natural sweetener (v. an artificial sweetener) and its use has increased year-on-year since its introduction in Europe. Other natural sweeteners are likely to follow as illustrated by the recent application for EU novel food application for Monk fruit extract/Luo han guo extract (already approved for use in the USA). As new LES are approved and come to market, approaches to assessing and monitoring their intake within the population will be needed. Regardless of which biomarker approaches are developed to address these needs, attention is needed in relation to the sensitivity, specificity and repeatability of these assessment approaches to ensure the usefulness of putative biomarkers for assessing intakes.
Future research needs
Implementing a biomarker approach to assessing intakes of sweeteners offers an exciting and timely alternative to current intake assessment methodologies in that it can overcome many of the challenges associated with assessing dietary intakes in free-living populations. Further biomarker validation research is warranted for LES and this work should aim to investigate excretion patterns following chronic intakes, excretion in high consumers as well as excretion in those populations in which excretion may be altered such as those with renal impairment.
In conclusion, given the current public health interest in the role of free sugars in the development of non-communicable diseases and the possible role that LES play in helping to achieve important public health objectives in relation to free sugar consumption, future research should aim to implement biomarker approaches to investigating sugars and LES intakes.
Acknowledgements
C. L. was previously supported by a PhD studentship by the National Institute for Public Health and the Environment, The Netherlands for the urinary biomarker approach for LES assessment.
Financial Support
None.
Conflict of Interest
A. M. G. is member of a Scientific Advisory Panel to the International Sweeteners Association (Brussels, Belgium) and has received an honorarium for her participation. C. L. has previously received speaker fees from the International Sweeteners Association (Brussels, Belgium). Preparation of the present review received no input from any funding agency, commercial or not-for-profit sectors.
Authorship
A. M. G. and C. L. contributed equally to the content with A. M. G. primarily responsible for the writing and editing of the text.






