Nutritional disorders, broadly defined as malnutrition, represent a syndromic continuum ranging from severe undernutrition to morbid overnutrition (Fig. 1). The development of over- and undernutrition is robustly associated with increased morbidity, mortality and healthcare costs( Reference Alpert, Lavie and Agrawal 1 , Reference Gastalver-Martín, Alarcón-Payer and León-Sanz 2 ). Therefore, over- and undernutrition should represent a clinical priority. Unfortunately, only the obesity pandemia has so far received attention by clinicians, researchers and politicians. In contrast, undernutrition remains a neglected issue in daily practice and in political agenda.

Fig. 1. Malnutrition is a syndromic continuum, ranging from severe undernutrition, when intake is insufficient compared with expenditures, to morbid overnutrition, when intake greatly exceeds expenditure.
The main phenotypic feature of undernutrition is weight loss. However, the clinical impact of weight loss is different according to the different underlying pathogenic mechanisms (Fig. 2). In fact, weight loss can be secondary to insufficient food ingestion or malabsorption or loss of nutrients, resulting in starvation. In contrast, disease-associated weight loss, i.e. cachexia, results from the metabolic and behavioural effects of increased inflammatory response triggered by the underlying illness. Although both starvation and cachexia promote weight loss, their impact on body composition, i.e. on fat mass and muscle mass, is different. During evolution, human metabolism has been primed by periods of famine, yielding to the emergence of compensative and adaptive biochemical pathways( Reference Higginson, McNamara and Houston 3 ). Therefore, during starvation, human metabolism minimises the impact of restricted feeding on body composition and particularly protects muscle mass. In contrast, the inflammatory response characterising cachexia prevents the activation of protective mechanisms, leading to accelerated muscle and adipose tissue wasting( Reference Argilés, Busquets and Stemmler 4 ). Consequently, cachexia has a more profound and rapid impact on patients’ outcome than simple starvation.
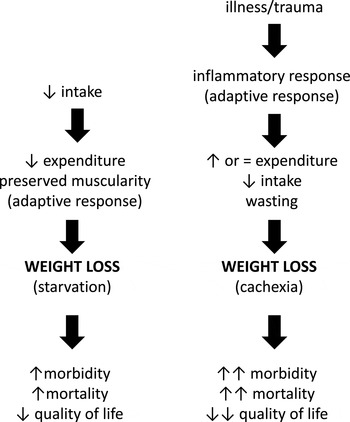
Fig. 2. Although weight loss is the main clinical sign of cachexia and starvation, their impact on body composition and therefore on outcome, is different.
From an evolutionary standpoint, it may appear inconsistent that the molecular mechanisms leading to waste during illness or trauma, and contributing to reduced long-term survival, have not been suppressed during thousands of years of evolution. However, it should be noted that disease-induced inflammatory response is a protective mechanism, which confers a survival advantage in the first hours after insult( Reference Laviano, Inui and Marks 5 , Reference Namas, Ghuma and Torres 6 ). Only recently, survival has been improved in patients with acute or chronic diseases, who would have died without the currently available clinical and technological advancements of medicine. Therefore, disease-induced inflammation, which for millennia helped a few to recover, is now cannibalising the most who are surviving despite their illness.
Counteracting starvation and cachexia involves different approaches. During starvation, the main pathogenic factor is insufficient intake, since the inflammatory response is minimal, if any. Consequently, provision of energy and proteins to meet requirements yields to restoration of body weight and composition. In contrast, the inflammatory response underlying cachexia impairs the correct utilisation of nutrients( Reference Haran, Rivas and Fielding 7 ). Therefore, meeting energy and protein requirements in cachectic patients without resolving inflammation yields to body weight gain, but not necessarily restoration of body composition, since most of the proteins and energy delivered are diverted to the synthesis of acute-phase proteins and adipose tissue( Reference Argilés, Busquets and Stemmler 4 ).
It is becoming increasingly acknowledged that cachexia is clinically relevant. We therefore aimed at reviewing the most recent updates on the clinical features and implication of cachexia, in order to highlight the importance of its recognition as a determinant of patients’ outcome.
Cachexia definition and diagnosis
The term cachexia derives from the Greek words ‘kakos’ and ‘hexis’, which mean ‘bad conditions’. However, a more clinically relevant definition was needed in order to design useful clinical trials and improve our understanding of the pathogenic mechanisms. In 2008, an international consensus defined cachexia as ‘a complex metabolic syndrome associated with underlying illness and characterised by loss of muscle with or without loss of fat mass. The prominent clinical feature of cachexia is weight loss in adults (corrected for fluid retention) or growth failure in children (excluding endocrine disorders)’( Reference Evans, Morley and Argilés 8 ). Although Evans et al. focused their definition mainly on chronic diseases( Reference Evans, Morley and Argilés 8 ), it is important to note that cachexia, i.e. disease-associated malnutrition characterised by muscle loss, is prevalent also in patients with acute and critical illness, although the identification of the specific contributions of inflammation and disuse to muscle loss is almost impossible( Reference Schefold, Bierbrauer and Weber-Carstens 9 ). According to the Evans et al. consensus, cachexia is diagnosed in the presence of significant weight loss (i.e. >5 % in the previous 12 months or less) associated with at least three of the following markers: decreased muscle strength, fatigue, anorexia, low fat-free mass index and abnormal biochemistry( Reference Evans, Morley and Argilés 8 ).
Although supported by clinical reasoning and molecular evidence, the Evans et al. definition and its attending diagnostic criteria may not precisely assess the clinical conditions of cachectic patients nor predict their outcome, since they were not validated by a large prospective clinical trial. However, many studies have consistently confirmed that the main clinical feature of cachexia is muscle loss, independently of the underlying disease( Reference Palus, von Haehling and Springer 10 ). Therefore, it should not be surprising that in daily practice the recent and specific definition of cancer cachexia is frequently used also to define cachexia associated with other acute or chronic diseases. In fact, cancer cachexia has been defined as ‘a multifactorial syndrome characterised by an ongoing loss of skeletal muscle mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutritional support and leads to functional impairment. The pathophysiology is characterised by a negative protein and energy balance driven by a variable combination of reduced food intake and abnormal metabolism’( Reference Fearon, Strasser and Anker 11 ). This definition elaborated for cancer wasting highlights the key features of cachexia, and therefore is applied to other acute and chronic diseases in which muscle loss develops. Supporting the use of this definition under different clinical conditions, experimental evidence shows that most of the molecular pathways responsible for cancer cachexia are also activated during other diseases( Reference Cohen, Nathan and Goldberg 12 ).
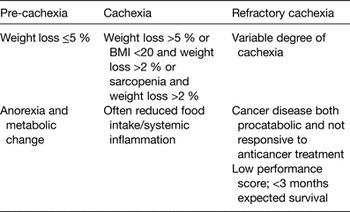
Cancer cachexia is a progressive disease. Therefore, the clinical phenotypes of cachectic patients may vary amply, ranging from reduced food intake and minimal weight loss to severe anorexia, wasting and fatigue. To correctly identify cachectic patients, it has been proposed that cachexia is divided into three stages: pre-cachexia, cachexia and refractory cachexia( Reference Fearon, Strasser and Anker 11 ) (Table 1). This classification highlights the importance of the early recognition and treatment of cachexia, before reaching the stage of refractory cachexia when treatments appear futile.
Table 1. Clinical features and diagnostic criteria of cachexia stages( Reference Fearon, Strasser and Anker 11 )
This conceptual framework has been recently validated in a large cohort of cancer patients( Reference Blum, Stene and Solheim 13 ). Results prospectively obtained show that by applying the definition of cachexia to cancer patients, cachectic patients have shorter survival( Reference Blum, Stene and Solheim 13 ). More importantly, by stratifying cancer patients according to the stages of cachexia, survival of refractory cachectic patients is shorter than cachectic and pre-cachectic patients( Reference Blum, Stene and Solheim 13 ). Interestingly, no significant difference in survival was observed between cancer patients with no cachexia and cancer patients with pre-cachexia, which underscores the clinical benefits associated with the early recognition and treatment of nutritional deterioration during disease.
Sarcopenia and cachexia
Muscle loss is the key feature of cachexia. However, muscle loss is not exclusively found in cachectic patients. Ageing is characterised by the profound rearrangement of human metabolism and thus body composition( Reference Laviano, Gori and Rianda 14 ). Age-dependent muscle loss is defined as sarcopenia. To differentiate age-dependent from disease-associated muscle loss, it has been proposed that muscle paucity of cachexia is defined as myopenia( Reference Fearon, Evans and Anker 15 ). Although it is acknowledged that sarcopenia and myopenia recognise different pathogenic mechanisms, in clinical practice it could be extremely difficult to ascertain the specific contribution of sarcopenia and cachexia to muscle loss in elderly patients suffering from chronic diseases. Therefore, in clinical practice, the term myopenia is scarcely used and sarcopenia is often used also to define disease-associated muscle loss.
BMI as a contributory factor in determining outcome
Muscle loss is the key feature of cachexia. When body composition analysis is not available, involuntary weight loss allows diagnosis of cachexia( Reference Fearon, Strasser and Anker 11 ). Robust data show that the severity of weight loss is a reliable, yet negative, prognostic factor in cancer patients and in other clinical conditions as well( Reference Martin, Senesse and Gioulbasanis 16 ). However, BMI also influences survival in cancer patients, higher BMI being associated with longer survival( Reference Martin, Senesse and Gioulbasanis 16 ). In order to obtain a more accurate classification of weight loss, and therefore of cachexia, which encompasses muscle and fat mass changes, Martin et al. classified a large cohort of cancer patients (n 8160) not only according to the severity of weight loss but BMI as well( Reference Martin, Senesse and Gioulbasanis 16 ). In particular, anthropometric characteristics of patients were used to fill a 5 × 5 matrix as outlined in Table 2.
Table 2. Conceptual framework for classification of cancer patients based on their weight loss and BMI and relative class of risk for shorter survival (adapted from( Reference Martin, Senesse and Gioulbasanis 16 ))

By assessing the mean survival time for each class of patients, Martin et al. found five specific patterns, with significant different survival, ranging from zero, with the longest survival, to four, with the shortest survival (Table 2)( Reference Martin, Senesse and Gioulbasanis 16 ). Based on these robust data, it could be proposed that cancer cachexia could be classified in five-stages, stage-0 being pre-cachexia and stage-4 being refractory cachexia. Whether this classification can be applied to other clinical conditions in which cachexia develops is presently being tested.
Pathogenesis and clinical features
The pathogenesis of cachexia is complex and involves a number of mechanisms. The main driving mechanism is the increased inflammatory response which triggers a cascade of molecular events, ranging from increased muscle proteolysis without compensatory anabolism and increased lipolysis, to functional impairment of the hypothalamic areas controlling food intake( Reference Argilés, Busquets and Stemmler 4 , Reference Cohen, Nathan and Goldberg 12 ). Of specific interest is the investigation of the early events leading to progressive muscle loss. In this regard, recent data in experimental models of cancer seem to suggest that hyperactivation of lipase activity yields to increased circulating levels of NEFA, which infiltrate muscles, causing or exacerbating proteolysis( Reference Arner 17 ). Whether this early crosstalk between adipose tissue and muscle mass is operating also in cachexia of diseases other than cancer, remains to be tested( Reference Fearon 18 ).
Muscle loss is the key feature of cachexia. However, cachexia cannot be defined as a syndrome involving only muscularity. In fact, other tissues and organs are affected by the presence of cachexia. Gut barrier dysfunction, myocardial decreased innervations, reduced hepatic synthesis of albumin and increased thermogenesis are just a few alterations observed and described in cachectic patients( Reference Argilés, Busquets and Stemmler 4 ). Also, it appears that sexual dimorphisms exist when the impact of cachexia on muscle function is considered. Consistent evidence shows that in the presence of cancer-associated moderate weight loss, muscle function loss is similar between males and females( Reference Stephens, Gray and MacDonald 19 , Reference Norman, Stobäus and Reiß 20 ). In contrast, when weight loss is severe, muscle function is more preserved in females than in males( Reference Stephens, Gray and MacDonald 19 , Reference Norman, Stobäus and Reiß 20 ). This evidence highlights the relevance of sexual hormones in the pathogenesis and clinical feature of cachexia.
Obesity paradox in cachexia
As previously mentioned, high BMI is associated with better outcome in cancer patients( Reference Martin, Senesse and Gioulbasanis 16 ). This evidence appears to support the concept that obesity may exert a protective role in chronic diseases( Reference Lainscak, von Haehling and Doehner 21 ). However, it has also been proposed that the obesity paradox may not be a true phenomenon, since large adipose tissue is frequently associated with large muscularity, which may be the real reason for the better outcome. To address this uncertainty, Gonzalez et al. analysed the survival of cancer patients( Reference Gonzalez, Pastore and Orlandi 22 ). When stratified according to BMI, the longest survival was observed for those cancer patients with BMI > 25. However, when muscle mass and fat mass were measured, the shortest survival was observed for those patients with obesity and muscle loss, i.e. with sarcopenic obesity( Reference Gonzalez, Pastore and Orlandi 22 ). These results demonstrate that adipose tissue plays a protective role only in the presence of normal or increased muscle mass. In fact, the combination of obesity and sarcopenia or myopenia is a severe negative prognostic factor in patients with chronic diseases( Reference Koo, Park and Park 23 , Reference Prado, Lieffers and McCargar 24 ).
Clinical relevance of cachexia
Cachexia is clinically relevant since it impacts on patients’ quality of life, morbidity and mortality( Reference LeBlanc, Nipp and Rushing 25 – Reference Habedank, Meyer and Hetzer 27 ). Nevertheless, the assessment of patients’ nutritional status does not represent a priority in many clinical settings( Reference Schindler, Pernicka and Laviano 28 ). It could be speculated that the lack of interest in the evaluation of the presence of cachexia could be related to the fact that other prognostic factors are usually considered by healthcare professionals in their clinical practice, making the diagnosis of cachexia apparently futile. However, it should be noted that cachexia is a more robust prognostic factor than the traditional ones, at least in cancer. In a large cohort of gastrointestinal and lung cancer patients (n 1473), a survival model containing conventional variables (i.e. cancer diagnosis, stage, age and performance status) revealed a c statistic of 0·73( Reference Martin, Birdsell and Macdonald 29 ). However, a survival model tested in the same cohort and including only BMI, weight loss, muscle index and muscle attenuation revealed a c statistic of 0·92( Reference Martin, Birdsell and Macdonald 29 ). These results suggest that cachexia is a powerful predictor of outcome in cancer, possibly superior to conventional variables. Confirming this concept, Stene et al. have recently demonstrated in advanced lung cancer patients that increase in muscle mass during chemotherapy, but not sarcopenia at baseline, is a significant prognostic factor predicting better survival( Reference Stene, Helbostad and Amundsen 30 ). These results point the importance of monitoring cachexia during the clinical journey of cancer patients, and likely of patients with other acute and chronic diseases.
Consistent evidence shows that weight loss during therapy is associated with worse outcome independently of the presence of sarcopenia at baseline, at least in cancer patients. Lu et al. have showed in 384 patients with gastric cancer that weight loss as minimal as 3 % during chemotherapy was associated with reduced survival independently from the presence of weight loss before starting anti-cancer therapies( Reference Lu, Yang and Yu 31 ). Kimura et al. regularly assessed 134 newly diagnosed non-small-cell lung cancer patients during their journey and demonstrated that at all time points, patients with cancer cachexia had shorter survival times than those without cachexia( Reference Kimura, Naito and Kenmotsu 32 ). Therefore, the nutritional status of patients suffering from chronic diseases should be regularly monitored in order to early detect any change which may negatively influence patients’ outcome.
The mechanisms by which cachexia negatively influences clinical outcome remain to be fully elucidated. In cancer patients, it has been shown that cachexia is associated with increased incidence of dose-limiting toxicity which yields to incomplete delivery of the planned treatment schedules( Reference Mir, Coriat and Blanchet 33 – Reference Lieffers, Bathe and Fassbender 35 ). This effect could be related to the different distribution volume of drugs caused by sarcopenia when dosing is calculated based on the body surface area( Reference Laviano, Rianda and Rossi Fanelli 36 ). The impairment of drug distribution volume may also represent a contributing factor for increased morbidity in other clinical conditions as well.
Anabolic potential in cachexia
Cachexia is associated with worse outcome( Reference LeBlanc, Nipp and Rushing 25 – Reference Habedank, Meyer and Hetzer 27 ). In order to develop effective therapies, it should be first assessed whether anabolic potential is still exploitable in patients with acute and chronic diseases. To address this key issue, Prado et al. measured muscle mass of 368 cancer patients at different time points during their clinical journey( Reference Prado, Sawyer and Ghosh 37 ). Results obtained showed that the overall frequency of muscle gain was 15·4 %, and muscle was stable in 45·6 % of intervals between any two scans, which made the maintenance or gain of muscle the predominant behaviour( Reference Prado, Sawyer and Ghosh 37 ). Also, multinomial logistic regression revealed that being within 90 d (compared with >90 d) from death was the principal risk factor for muscle loss( Reference Prado, Sawyer and Ghosh 37 ). The authors then concluded that ‘a window of anabolic potential exists at defined early phases of the disease trajectory (>90 d survival), creating an opportunity for nutritional intervention to stop or reverse cachexia. Cancer patients within 90 d of death have a low likelihood of anabolic potential’( Reference Prado, Sawyer and Ghosh 37 ). Based on these results, it could be speculated that refractory cachexia coincides with the last 90 d of survival, at least in cancer patients. Also, these results highlight the importance of starting any anti-cachexia therapy early in the clinical course of the underlying disease.
Confirming the possibility that cachexia can be prevented and treated, Stene et al. have demonstrated that muscle mass does increase during chemotherapy, which is associated with longer survival( Reference Stene, Helbostad and Amundsen 30 ). Also, Lu et al. reported that cancer patients increasing their body weight during chemotherapy significantly improve their survival, even if they were losing weight at baseline( Reference Lu, Yang and Yu 31 ). Similarly, Kimura et al. reported an intermediate survival time for those patients who became cachectic or reverted from cachexia during chemotherapy, when compared with persistently cachectic and persistently well-nourished cancer patients( Reference Kimura, Naito and Kenmotsu 32 ). It is acknowledged that the study designs of these trials does not allow us to ascertain whether body weight gain is the consequence of effective nutrition therapy or effective anti-cancer therapies leading to reduced tumour mass and decreased inflammatory response. Nevertheless, it remains imperative that supportive care is started early to maintain body weight or facilitate recovery from weight loss.
Further supporting the relevance and potential of anti-cachexia therapies, the concept of anabolic resistance associated with increased inflammatory response has been recently challenged. In their elegant study, Winter et al. tested whether insulin-mediated resistance of protein anabolism could underlie the muscle degradation associated with cancer cachexia and whether a sustained, physiological elevation of amino acids with hyperinsulinaemia would compensate for it( Reference Winter, MacAdams and Chevalier 38 ). Results obtained in lung cancer patients showed that cachexia was associated with insulin resistance and impaired whole-body protein anabolism. However, they also revealed that patients’ anabolic protein response was stimulated normally by hyperaminoacidaemia( Reference Winter, MacAdams and Chevalier 38 ). Therefore, ample provision of amino acids appears a promising and effective strategy to overcome the protein anabolic resistance of cachexia. Similarly, Deutz et al. showed that the use of selected nutrients, including leucine and n 3 fatty acids, is able to stimulate protein synthesis in cancer patients, reverting catabolism( Reference Deutz, Safar and Schutzler 39 ).
Thus, cachexia is a syndrome which increases morbidity and mortality, but it can be effectively prevented and treated.
Conclusions
Cachexia is a clinically relevant syndrome, whose key feature is muscle loss. Prevention and effective treatment have been shown to improve clinical outcome. However, early recognition is key to obtain clinically meaningful results. More studies are needed to further explore the potential of anti-cachexia therapies in different clinical conditions, but the way has been set. It is important to remember that patients’ outcome can be improved by not only addressing their nutritional status but any other patient-centred need. Considering the financial impact of developing, testing and delivering new drugs to patients, it appears unwise not routinely assessing patients’ needs and implementing supportive care, of which nutritional support is a pillar.
Financial support
None.
Conflicts of interest
None.
Authorship
A. L. wrote the manuscript. A. K. and A. M. reviewed the literature and prepared the figures/tables. All authors reviewed the manuscript and agreed on its content.







