Epidemiological studies have shown that diets rich in fruits and vegetables are associated with a reduced risk of CVD and certain cancers(Reference Kelley, Rasooly, Jacob, Kader and Mackey1). Studies suggest that the beneficial health effects of plant foods may be in part associated with phytochemicals, which are bioactive non-nutritive compounds, not classified as vitamins, which may be effective as antioxidants(Reference Scalbert, Johnson and Saltmarsh2) as well as having other effects. Oxidative modification of LDL has been recognised as an early stage in the development of atherosclerosis that leads to CVD(Reference Jeong, Choi, Kwon, Kang, Park, Lee and Kang3). An increasing number of studies have reported the antioxidant effect of phytochemicals in fruits and vegetables, which includes the retardation of the susceptibility of LDL to oxidation both in vitro and ex vivo (Reference Laponite, Couillard and Lemieux4).
The present study has investigated the effects of incubating pure anthocyanins and fruit and vegetable extracts with human plasma or isolated human LDL on the reduction of LDL oxidation. An in vitro study involving the addition of pure flavonoids directly to plasma or isolated LDL was performed. The isolated LDL was mixed with anthocyanins to achieve a final concentration of 0.025, 0.1 and 0.5 mol/50 μm-LDL-protein. The mixtures were incubated at 37°C for 30 min before starting oxidation. Cyanidin-3-glucoside and delphinidin-3-glucoside (0.5 μm) reduced the susceptibility of LDL to oxidation by 58% and 30% respectively when incubated directly with isolated LDL without the removal of the free anthocyanins. When these flavonoids were added to plasma or LDL and free anthocyanins were removed the effects on the LDL-oxidation lag phase were smaller, although both cyanidin-3-glucoside and delphinidin-3-glucoside caused significant increases in the LDL-oxidation lag phase at higher concentrations (P<0.01).
In a second study the effect of flavonoid-rich fruit and vegetable extracts on LDL oxidation in vitro was investigated. The phytochemical content and antioxidant activity of extracts from red leaf lettuce (‘Lollo Rosso’), blueberries (‘Bluecrop’), raspberries (‘Joan Squire’) and strawberries (‘Elsanta’) grown under plastic films in polytunnels were determined. Flavonoid composition was determined by HPLC, total phenolics by the Folin-Ciocalteu assay, anthocyanins by the pH differential method and antioxidant activity by both the oxygen radical absorbance capacity (ORAC) assay and the Cu-catalysed oxidation of LDL in vitro (Reference Leigh-Firbank, Minihane, Leake, Wright, Murphy and Williams5). The results are summarised in the Table.
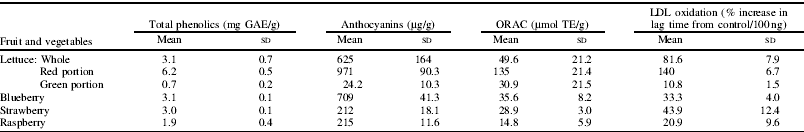
GAE, gallic acid equivalent; TE, Trolox equivalent.
In conclusion, these in vitro studies demonstrate that pure flavonoids, and extracts from fruits and vegetables rich in these components can significantly reduce the susceptibility of LDL to oxidation. This finding may have implications for the potential benefit of flavonoid-rich foods on oxidative status in vivo. Further human studies are required to confirm these in vitro data.
Funding provided by Research Council UK and The Royal Thai Government is gratefully acknowledged.


