- AL
ad lib
- CER
continuous energy restriction
- ER
energy restriction
- FFM
fat-free mass
- IER
intermittent energy restriction
- IF
intermittent fasting
- IGF-1
insulin-like growth factor 1
- IGFBP
IGF binding protein
- RR
relative risk
Breast cancer is the most common malignancy among women and its incidence is increasing in the UK and worldwide. These increases are thought to be driven partly by reproductive factors, such as late age of first pregnancy, and also aspects of the western lifestyle including excessive energy intake and sedentary behaviour. The proportion of breast cancer cases attributable to overweight and obesity is estimated to be approximately 16%(1, Reference Renehan, Soerjomataram and Leitzmann2).
The anticancer effects of energy restriction (ER) were first identified in animal studies by Moreschi in 1909(Reference Kritchevsky3). More recent evidence that weight reduction and ER reduces risk of breast cancer in women has come from a number of observational studies, a large randomised dietary trial and studies of the results of bariatric surgery(Reference Harvie, Howell and Vierkant4–Reference Ostlund, Lu and Lagergren9).
These studies indicate that ER has the potential for breast cancer prevention, but it is well known to be difficult to achieve and to maintain(Reference Dansinger, Gleason and Griffith10). Most studies of ER have involved habitual, daily continuous energy restriction (CER). The question arises whether compliance and efficacy can be improved by other approaches such as intermittent energy restriction (IER) or intermittent fasting (IF) where spells of restriction are interspersed with normal food intake. These approaches even have the potential to be superior to CER since they are associated with more profound ER albeit for short periods.
Here, we summarise the effects of CER, IER or IF in women at risk of the disease and in animal models of breast cancer and speculate whether their use may be worthwhile and safe for the prevention of breast cancer and other weight-related diseases.
Comparison between intermittent energy restriction, intermittent fasting and continuous energy restriction
Interest in studying IER or IF comes from two quarters. Firstly to test their possible beneficial effects since they may be better suited to our physiology, possibly mimicking the periods of food abundance and scarcity seen in the Palaeolithic period before the onset of agriculture and consistent food availability. Secondly, because of potential adverse effects of IER or IF since they may be construed as weight cycling (yo-yo dieting) and erratic meal patterns, and thus perceived to be harmful.
A series of experimental protocols have been studied which include periods of either IER (most commonly 50–70% restriction) or IF (most commonly alternate day fasting). Since the difference in severity of restriction between IER and IF may elicit different biological responses, we will summarise the data from these approaches separately. The distinct spells of restriction and refeeding within IER or IF are likely to elicit numerous and distinct physiological and metabolic effects as compared with CER. A true evaluation of IER and IF thus requires assessment of its overall effects in addition to assessments during both the restricted and refeeding phases. Effective regimens are those in which the beneficial effects of restricted periods outweigh potential harmful effects of the refeeding periods. It is also important to determine whether IER or IF is associated with a reduction in effectiveness with time (tachyphylaxis).
Weight change, energy intake and breast cancer risk in women
Several cohort studies indicate that mid-life weight gain and adiposity increase the risk of post-menopausal, but not pre-menopausal breast cancer. A gain of 20 kg during adult life doubles the risk of post-menopausal breast cancer compared with women who maintain weight. However, this risk increase is not detectable in women taking hormone replacement therapy, indirectly suggesting that the risk may be related to oestrogen production from excess adipose tissue(Reference Vrieling, Buck and Kaaks11).
Some of the increased breast cancer risk in heavier women is also thought to be mediated directly by higher energy intakes and positive energy balance. High-energy intake has been linked to breast cancer risk in several, but not all cohort studies (Reference Sue, Schairer and Ma12–Reference Prentice, Shaw and Bingham14). Most studies are based on self-report data that are confounded by the well-documented bias of energy under-reporting among subjects with the highest dietary intake(Reference Mattisson, Wirfalt and Aronsson15). Notably self-reported energy intake has only been linked to risk in studies which have calibrated and validated reported energy intakes against doubly-labelled water measurements (hazard ratio 1·24 for 20% greater consumption)(Reference Prentice, Shaw and Bingham14).
Continuous and intermittent energy restriction, intermittent fasting and breast cancer prevention
Indication of the effectiveness of CER on breast cancer risk is derived from large observational studies where weight change is related to risk. Three such studies indicate that intentional and maintained weight loss is related to reduced risk. In the Iowa Women's Health Study based on 34 000 women, we demonstrated that maintaining ≥5% weight loss reduced post-menopausal breast cancer risk by approximately 25%(Reference Harvie, Howell and Vierkant4), a figure that was confirmed in two other large US population studies(Reference Eliassen, Colditz and Rosner5, Reference Teras, Goodman and Patel6). These studies are consistent with findings in the Women's Health Initiative randomised trial of a low-fat diet compared with control(Reference Prentice, Caan and Chlebowski7). Although fat reduction was the end point of the study women lost 2 kg in weight which was associated with a non-significant reduction of breast cancer risk by 9% (hazard ratio, 0·91; 95% CI 0·83, 1·01).
Bariatric surgery cohorts have been studied as a model of whether large weight loss and severe CER can reduce cancer risk. Typically these operations are associated with a 30% weight reduction alongside a CER of 65%(Reference Camastra, Gastaldelli and Mari16). Three large bariatric surgery studies reported reduced incidence of female (but not male) cancers compared with non-randomised comparison groups. Reduced breast cancer rates based on recorded breast oncology visits were reported in a Canadian cohort (relative risk (RR) 0·17 (95% CI 0·098, 0·311), P=0·001)(Reference Christou, Lieberman and Sampalis8), but specific risk reduction of breast cancer incidence was not found in the Utah (RR 0·96 (95% CI 0·57, 1·63), P=0·89)(Reference Adams and Hunt17) or the Swedish Obesity Study cohorts (RR 0·71 (95% CI 0·4, 1·25), P=0·24)(Reference Sjostrom, Narbro and Sjostrom18). A different Swedish study reported decreased pre- and post-menopausal breast cancer incidence compared with the background population of Sweden (RR 0·54 (95% CI 0·43, 0·68))(Reference Ostlund, Lu and Lagergren9). Further follow up may help to clarify these divergent results.
There are no data of the effects of IER in relation to breast cancer risk in human subjects. Population studies among cultural and religious groups with IER practices have not been undertaken, but are unlikely to disentangle the specific benefits of IER compared with other holistic healthy lifestyle practices and reduced body weight within these groups. Weight cycling within epidemiological cohorts has been cited as a model of the effects of IER on risk(Reference Thompson and McTiernan19). These data are likely to be a poor indicator of the effects of IER since they describe periods of restriction interspersed with longer spells of normal or increased intake, and are also likely to be confounded by weight cycling being more common among heavier women. For completeness, we report these weight cycling studies. The largest prospective cohort included 33 529 post-menopausal women and found no significant associations between either large weight cycles (gains of >10% body weight during one interval and loss of >10% during another interval) or small weight cycles (gained 5–10% during one interval, lost 5–10% during another interval) and breast cancer risk (RR 95% CI respectively 0·80 (0·59, 1·10) and 0·84 (0·57, 1·23))(Reference French, Folsom and Jeffery20). One case–control study of 5031 post-menopausal women failed to link weight cycling (losing 9 kg or more and gaining at least half back within a year) to risk (OR 1·0 (95% CI 0·9, 1·1))(Reference Trentham-Dietz, Newcomb and Egan21). In another study, a non-significant increased risk was reported in post-menopausal women (n 1996) whose weight had fluctuated from greater than to less than the median weight of the control group throughout adult life (OR 2·11 (95% CI 1·00, 4·44))(Reference Eng, Gammon and Terry22).
Thus, observational studies and the effects of bariatric surgery on human subjects suggest that CER may be effective in reducing the incidence of breast cancer although more data are required. The long-term effects of IER and IF in women are not known, but it is helpful to know that weight cycling does not appear to be associated with increased risk of breast cancer.
Continuous energy restriction, intermittent energy restriction and intermittent fasting for the prevention of mammary tumours in rodent models
CER of 30% or greater consistently reduces mammary tumour development in spontaneous(Reference Dirx, Zeegers and Dagnelie23), chemically induced(Reference Klurfeld, Welch and Davis24) and radiation-induced tumour models(Reference Gross and Dreyfuss25). The question of whether IER or IF has comparable, superior or reduced cancer protective effects to CER has been investigated in spontaneous and chemically induced mammary tumour models over the past 65 years.
Spontaneous models are presumed to be more akin to the human situation than chemically induced models where large doses of carcinogen are used to induce tumours with a short follow up. The earliest studies of spontaneous mammary tumour models tested IF with fasting on alternate days or for 2 or 3 d periods each week (Table 1). Both IF approaches reduced tumour rates by 40–80% compared with ad lib (AL) feeding in a number of models(Reference Carlson and Hoelzel26–Reference Chen, Good and Engelman28). The effects of IF were most evident using alternate day fasting and when there was an overall ER and weight reduction. Reduced tumour rates were not reported with an IF regime which allowed overfeeding on feeding days and had a minimal overall ER (4%) and weight reduction(Reference Tannenbaum and Silverstone29). Good and colleagues at University South Florida used an intermittent feeding pattern (feeding Monday and Thursday only, interspersed with a 2 and a 3 d fasting spell per week) in both their AL and ER CB3H mice, and found tumour rates were only reduced when intake on feeding days was also restricted (tumour rates: AL 90% ER 20%)(Reference Shao, Dao and Day30, Reference Engelman, Day and Chen31). No studies have directly compared the effects of IF and CER on rodents.
Table 1. Intermittent fasting (IF) and spontaneous mammary tumour development in laboratory rodents
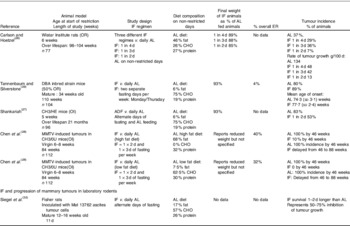
AL, ad lib; ER, energy restriction; MMTV, mouse mammary tumour virus; OR, ovarian responsive animal model; DBA, Dilute brown non-agouti; OI, ovarian independent animal model; ADF, alternate day fasting; CHO, carbohydrate.
IER has mainly been studied in spontaneous mammary tumour models by Cleary and Grossmann, using 3-week cycles of 50% restriction and 3 weeks of AL feeding (Table 2). Four studies in the oestrogen responsive mouse mammary tumour virus transforming growth factor-α model demonstrated that IER was superior to isoenergetic CER (AL 71–84% v. IER 3–15% and CER 20–44%). Two other studies in the oestrogen unresponsive MMTV–neu model reported IER to be comparable to CER in (AL 37·5% v. IER 22·5% and CER 33%)(Reference Pape-Ansorge, Grande and Christensen33) and (AL 86·7% v. IER 59·6% and CER 47·2%)(Reference Cleary and Grossmann34). The greater reductions in tumour rates in IER compared with CER occurred despite these groups having a comparable overall ER and adiposity(Reference Cleary, Hu and Grossmann35). These studies suggest that the spells of IER offer additional cancer protective effects that are independent of weight and overall energy intake in the oestrogen receptor positive model. These effects may be particularly important in preventing the initiation rather than the progression of tumours since tumour size of the ones that did develop were not different.
Table 2. Intermittent energy restriction (IER) and spontaneous mammary tumour development in laboratory rodents

MMTV, mouse mammary tumour virus; TGF, transforming growth factor; ER, energy restriction; CER, continuous energy restriction; AL, ad lib; OR, ovarian responsive animal model; OI, ovarian independent animal model; CHO, carbohydrate.
In contrast, IER seems somewhat less effective than CER in carcinogen-induced tumour models. This appears to be because IER initiated soon after carcinogen exposure may promote the carcinogenic process (Table 3)(Reference Klurfeld, Welch and Davis24). Mehta et al. reported repeated cycles of 2 d of 40% ER and 2 d AL feeding (equivalent to an overall 20% ER), was associated with comparable rates of tumour induction to AL feeding in the 7,12-dimethyl-benz(a)anthracene-induced model, whereas 40% CER reduced rates by 57%(Reference Mehta, Harris and Gunnett39). Tagliaferro, in a 1-methyl-1-nitrosourea-induced tumour model, reported a 12% greater incidence of mammary tumour with an IER of 1 week 33% restriction and 3 weeks AL feeding (equivalent to an overall 14% ER) compared to AL-fed animals after adjusting for the effect of final body weight(Reference Tagliaferro, Ronan and Meeker40). In contrast Buison et al.(Reference Buison, Pellizzon and Brogan41), in a 7,12-dimethyl-benz[a]anthracene model demonstrated that an IER of 50% intake for 3 weeks interspersed with 2 weeks of AL to regain weight led to a 50% reduction in mammary tumours compared to AL-fed animals. The adverse effects of IER in the earlier carcinogen studies may reflect the timing of either restricted periods or refeeding phases at the critical promotion phase after carcinogen exposure(Reference Kritchevsky, Welch and Klurfeld42, Reference Sylvester, Aylsworth and Van Vugt43). The first two studies initiated IER in the 7–10 d directly after carcinogen exposure, whereas Busion et al. delayed IER until 13 weeks after exposure (see possible problems of IER and IF).
Table 3. Intermittent energy restriction (IER) and carcinogen-induced mammary tumour development

ER, energy restriction; CER, continuous energy restriction; AL, ad lib; OR, ovarian responsive animal model; DMBA, 7,12-dimethyl-benz(a)anthracene; MNU, 1-methyl-1-nitrosourea; CHO, carbohydrate.
In summary, studies in rodent mammary tumour models indicate IER to be superior or equivalent to CER except in carcinogen-induced tumour models when ER is initiated shortly after carcinogen exposure. Alternate day fasting also reduces tumour formation as long as there is no compensatory overfeeding on fed days. Future randomised comparisons of other IER or IF regimes v. CER are required in other spontaneous animal models to evaluate the full effectiveness of the intermittent regimens.
Possible mechanistic differences between continuous energy restriction, intermittent energy restriction and intermittent fasting
The potential breast cancer protective effects of CER, IER and IF regimens may be mediated by reductions in adiposity or other potential mechanisms directly brought about by ER, or a combination of the two. Reductions in subcutaneous, visceral, hepatic, muscle and breast fat stores will lead to lower levels of mitogens including insulin, oestradiol (post-menopausal women), androgens and inflammatory cytokines, e.g. TNFα and IL-6(Reference Morris, Hudis and Giri44). Reduced adiposity will also result in decreased leptin and increased adiponectin and a reduced adiponectin:leptin ratio, which is considered more important in the development of breast cancer than their absolute amounts(Reference Grossmann, Ray and Dogan45). As well as reducing adiposity, ER also directly induces a number of cancer protective effects, and brings about reductions in insulin, adipokines, steroid hormones, insulin-like growth factor 1 (IGF-1), oxidative stress and inflammation and down-regulation of the Protein kinase B–AMP-activated protein kinase pathway, mammalian target of rapamycin signalling and cell proliferation. Differences in some of these possible cancer protective mechanisms have been identified between CER and IER and IF-fed animals. The relevance of these animal data to the human situation is considered by summarising the likelihood of differential effects of CER, IER and IF in human subjects, with available corroborative human weight and biomarker data.
Differential effects of continuous energy restriction, intermittent energy restriction and intermittent fasting on adiposity and body fat stores
CER, IER and IF have been investigated in overweight or obese adults (Table 4)(Reference Hill, Schlundt and Sbrocco46–Reference Varady, Bhutani and Church49). The question of whether IER is easier to follow and more effective than CER has been addressed in three randomised trials. These demonstrated comparable reductions in body weight and body fat. The largest study, in overweight pre-menopausal women, demonstrated that 2 d of a 70% ER (2·71 MJ/d) on restricted days and 5 d AL Mediterranean-type diet per week for 6 months reduced body fat by 6·4 (sd 1·5) kg compared to 5·6 (sd 1·3) kg using an isoenergetic 25% daily energy restricted Mediterranean-type diet (6·8 MJ/d 7 d per week) (P=0·34)(Reference Harvie, Pegington and Mattson50). Ash et al. compared an IER (4·18 MJ for 4 d and 3 d AL/week) v. CER (6·0–7·0 MJ/d) among fifty-one men with type 2 diabetes and showed no difference in terms of weight or fasting insulin(Reference Ash, Reeves and Yeo47). Hill et al.(Reference Hill, Schlundt and Sbrocco46) compared alternating weeks of 2·5, 3·8, 5·0 or 7·25 MJ/d as compared to a constant restriction of 5·0 MJ/d in thirty-two moderately obese women and reported comparable weight loss, but greater reductions in cholesterol in the IER group compared with the CER group (−14 v. −6%). There have been no direct comparisons of weight loss between IF and CER.
Table 4. The effects of intermittent energy restriction (IER) on weight loss in human subjects

AL, ad lib; ER, energy restriction; CER, continuous energy restriction; VLCD, very low energy diet; CHO, carbohydrate.
Loss of body fat during ER ideally occurs with the preservation of fat-free mass (FFM) and metabolic rate. A potential concern is that IER or IF regimens which include periods of severe restriction, i.e. fasting or intakes of less than 2·0 MJ/d could lead to a greater proportion of weight lost as FFM. This is known to be the case with severe CER, where regimens of <2·0, 2·0–4·0 and >4·0 MJ/d respectively result in 60, 35 and 20% of weight to be lost as FFM(Reference Hall51). Short intermittent periods of severe restriction with IER or IF do not, however, appear to be a problem. Our 2 d/week 70% ER found the proportion of weight lost as FFM to be comparable to a 25% restricted CER group; 21 (sd 6) v. 21 (sd 7) %. Likewise, Varady et al. found alternate days of a severe IER (80% restriction, 1·89 MJ on fast days) reported only 10% weight loss to be lost as FFM(Reference Varady52).
Animal studies suggest that the acute spells of negative energy balance with IER and IF may preferentially mobilise hepatic and visceral fat stores, due to the acute sensitivity of these stores to ER(Reference Kirk, Reeds and Finck53). Varady et al. reported that alternate days of either fasting or of a 75 or 85% ER did not change total amounts of body fat, but led to redistribution from visceral to subcutaneous depots. The alternate day regimens brought about a 25% reduction in visceral fat which was comparable to the 25% weight reduced CER animals which had reduced adiposity from a number of sites(Reference Varady, Allister and Roohk54).
In another animal experiment, alternate days of a 50% ER (but not 25% ER) and AL feeding did not change overall fat mass but reduced fat cell size in inguinal (subcutaneous) and epididymal (visceral) stores by 35–45% which, in turn, is believed to reduce risk of inflammation and metabolic diseases(Reference Varady, Roohk and Loe55).
IER regimens in women show comparable reductions in weight and total body fat to CER (Table 4), and a possible fat redistribution. In our pre-menopausal study, women following IER (2 d/week 70% ER) had a non-significant greater decline in waist measurement compared to CER; mean difference between IER and CER at 3 months; −1·1 (95% CI −2·3, 0·1) cm (P=0·07)(Reference Harvie, Pegington and Mattson50).
Larger randomised trials with accurate uptake figures and longer term follow up are needed to test the wider and longer term acceptability of IER and IF. The effect of IER or IF on visceral fat stores and fat cell size in human subjects needs to be explored.
Effects of continuous energy restriction, intermittent energy restriction and intermittent fasting on insulin sensitivity and the insulin-like growth factor-1 axis
In our randomised trial of CER v. IER, with overweight and obese healthy women, 6 months of IER (2 d 70% ER per week) led to greater improvements in insulin sensitivity even on non-restricted days compared to an isoenergetic CER; mean (95% CI difference) −23 (−38·1, −8·6)% (P=0·001), with a further 25% reduction on the restricted days(Reference Harvie, Pegington and Mattson50). The mechanism for this greater improvement of sensitivity is not known but may involve altered insulin receptor affinity(Reference Bar, Gorden and Roth56), and possible reductions in hepatic(Reference Kirk, Reeds and Finck53) and visceral fat stores(Reference Varady, Roohk and Loe55) and fat cell size(Reference Varady, Roohk and Loe55) with the greater nadir of ER seen with IER.
Reductions in IGF-1 are believed to mediate a significant part of the effects of CER, IER and IF in animal studies. IGF-1 concentrations are on average lower in IER-fed animals than their CER-fed counterparts, with lower levels than CER on restricted IER days(Reference Cleary, Jacobson and Phillips36, Reference Rogozina, Bonorden and Grande37, Reference Dogan, Johannsen and Grande57, Reference Pape-Ansorge, Grande and Christensen58) and comparable levels to CER on refeeding days. Dogan et al. reported decreased IGF-1 receptor expression in the mammary fat pad and down-regulation of mammalian target of rapamycin during IER restricted days and during CER, but not during IER refeeding days(Reference Dogan, Johannsen and Grande57). In this study, elevated serum IGF-1 levels preceded mammary tumour detection within the AL, CER and IER groups(Reference Rogozina, Bonorden and Grande37).
The relevance of these IGF-1 mediated effects of IER in rodent models to the human situation is not resolved. CER does not reduce IGF-1 in human subjects unless there is concomitant protein restriction(Reference Musey, Goldstein and Farmer59, Reference Fontana, Weiss and Villareal60). In human subjects bioavailable IGF-1 is determined primarily by the amount bound to IGF binding protein (BP)-3 (a function of IGFBP-3 levels and the acid labile subunit that stabilises the IGF–IGFBP-3 complex), and is acutely regulated by IGFBP-2 and IGFBP-1. Short spells of ER (4 d of 80% ER) bring about acute reductions in free IGF-1 (−48%) mainly via increases in IGFBP-2 as well as increases in the acid labile subunit(Reference Rasmussen, Juul and Kjems61). We have similarly demonstrated an acute 17% increase in IGFBP-2 during the 2 d 70% ER in our IER; pre-restriction mean 158 (sd 9·3) μg/l compared to 177 (sd 7·0) μg/l after the 2 d of ER (P=0·12).
Effects of continuous energy restriction, intermittent energy restriction and intermittent fasting on adipokines
Breast cancer is associated with a reduced adiponectin:leptin ratio(Reference Grossmann, Ray and Dogan45). Dogan et al. found IER-fed animals to have a greater adiponectin:leptin ratio and up-regulation of mammary pad adiponectin and adipo R1 gene expression than CER-fed animals(Reference Dogan, Rogozina and Lokshin38). However, the adiponectin:leptin ratio did not consistently distinguish between tumour bearing and non-tumour bearing IER mice in this study(Reference Dogan, Rogozina and Lokshin38). In overweight human subjects, CER only increases adiponectin alongside large reductions in body fat and visceral fat (>10%)(Reference Klempel and Varady62). In our IER study, 6 months of a 2 d 70% ER per week increased adiponectin by 10% in association with a 7% weight loss, but there was no change during CER despite a comparable weight loss(Reference Harvie, Pegington and Mattson50). These data suggest IER may up regulate adiponectin, possibly linked to preferential changes in visceral fat stores described earlier.
Effects of continuous energy restriction, intermittent energy restriction and intermittent fasting on cell proliferation
Reduction in mammary epithelial cell proliferation could reduce cancer initiation and the subsequent promotion of initiated cells. CER is associated with 30% reductions in mammary epithelial cell proliferation. Comparable reductions have also been reported after 1 month of either alternate days of fasting or alternate days of a severe ER (85% ER) interspersed with days of hyperphagic feeding (175–185% of normal intake). Reduced proliferation with alternate day regimens occur in the absence of weight loss, even when assessed on the hyperphagic days of the regimens(Reference Varady, Roohk and McEvoy-Hein63), and importantly suggest alternate days of fasting or severe ER reduce proliferation in the absence of substantial weight loss. The reduced proliferation associated with CER in animal models may be driven, in part, by loss of oestrous cycle and reductions in reproductive hormone levels and IGF-1. These changes do not occur with the alternate day fed animals, in which proliferation is reduced through different and unspecified mechanisms dependent on very low energy intake(Reference Varady, Roohk and McEvoy-Hein63, Reference Hsieh, Chai and Hellerstein64). There are no human data on the effects of IER, IF and CER on breast cell proliferation.
Effects of continuous energy restriction, intermittent energy restriction and intermittent fasting on steroid hormones
In animal studies, IER, IF and CER are active in both oestrogen receptor positive and negative models suggesting that the effects of ER are independent of receptor activity (Table 2). However, Cleary et al. have consistently found IER to exert greater reductions in mammary tumours than CER in animals prone to develop oestrogen receptor positive, but equivalent effects to CER in an oestrogen receptor negative tumour model suggesting dual effects via steroid and other pathways (Table 2). Ovarian cycling or steroid hormones have not been assessed in these studies; however, other data suggest both the 25% CER and 7 d spells of 50% ER in the IER animals could interrupt cycling and cause significant reductions in hormones such as prolactin(Reference Sylvester, Aylsworth and Van Vugt43), and are likely to contribute to the anti-tumour effects of the CER and IER animals studied by Cleary et al. This does not appear to be the case for IF, which reduces tumour growth in the oestrogen unresponsive CH3 mice model(Reference Shankariah27, Reference Chen, Good and Engelman28). As mentioned earlier neither alternate days of fasting or severe restriction (85% restriction) affect cycling or reduce steroid hormone concentrations(Reference Varady, Roohk and McEvoy-Hein63, Reference Hsieh, Chai and Hellerstein64).
It is not known whether IER or IF alter cycling in pre-menopausal women. Luteinising hormone pulsatility and menstrual cycling are disrupted by 5 d periods of reduced energy availability (defined as <126 kJ/kg FFM, i.e. intakes of <5·88 MJ)(Reference Loucks and Thuma65). Neither our 2 d 70% ER nor 25% CER altered serum androgens or prolactin; however, both regimens were associated with a 10–20% increase in sex hormone-binding globulin and decreased steroid hormone bioavailability after 6 months. The IER group did, however, report a greater average cycle length compared to the CER group (29·7 (sd 3·8) v. 27·4 (sd 2·7), P=0·002)(Reference Trentham-Dietz, Newcomb and Egan21) which may indicate greater follicular length and reduced breast cancer risk.
Breast cancer risk is linked to whole body and breast aromatase activity in post-menopausal women(Reference Morris, Hudis and Giri66). IER or IF-fed animals may have had reduced aromatase activity secondary to both decreased adiposity and the likely down-regulation of aromatase activity associated with reduced leptin and inflammatory mediators and the up-regulation of AMP kinase(Reference Brown and Simpson67), but this potential mechanism has not been assessed formally. Further studies are required to determine the effects of IER, IF and CER on sex steroid hormones in pre-menopausal women and the effect on aromatase in post-menopausal women and in animal models.
Possible problems with the use of intermittent energy restriction and intermittent fasting
Despite concerns of some researchers that IER or IF may have adverse health effects linked to spells of hyperphagia, the majority of IER and IF animal studies are associated with reduced tumour rates. An exception was seen in two carcinogen-induced models where ER was initiated close to the time of administration of carcinogen (see carcinogen induced mammary tumours, earlier). Also, the study of Tannenbaum failed to reduce rates or growth of spontaneous mammary tumours with two separate days of fasting per week since the animals were allowed AL feeding on non-fast days resulting in no overall ER(Reference Tannenbaum and Silverstone29). Most studies of IER and IF have shown reduced rates of tumour development despite overfeeding on non-restricted days, and often in the absence of weight loss or overall ER. The lack of benefit in the Tannenbaum study raises the possibility that overfeeding on non-restricted days may attenuate any beneficial effects of IER. The need for some restraint on non-restricted days requires further study. In human studies, there is little evidence of overfeeding. In our own studies of IER, there is a small reduction in energy intake on the non-restricted days(Reference Harvie, Pegington and Mattson50).
The severity of ER on restricted days needs consideration. There are concerns that spells of fasting or severe energy or carbohydrate restriction will cause surges in lipolysis and fat oxidation with large increases in circulating NEFA and ketone bodies, which could fuel tumour growth(Reference Martinez-Outschoorn, Pestell and Howell68–Reference Sauer, Nagel and Dauchy71). Alternate day fasting includes repeated 36 h fasts, which have been shown to increase circulating NEFA by 20–40%, and ketone bodies by four-fold in obese women and up to nine-fold in lean women(Reference Huda, Dovey and Wong72). Our IER (2 d of 70% ER) led to a minor (20%) increase in serum ketones after the two restricted days. Currently, we do not have data on NEFA(Reference Harvie, Pegington and Mattson50).
CER is well known to reduce oxidative stress. However, this reduction has not been consistently demonstrated in rodent studies of IER and alternate day fasting. Descamps et al. reported decreased mitochondrial generation of reactive oxidative species and reduced incidence of lymphoma in 8-month-old female mice following alternate day fasting (0 v. 33% in AL controls)(Reference Descamps, Riondel and Ducros73). However, two other studies linked IER and IF to increased reactive oxidative species production(Reference Descamps, Riondel and Ducros74, Reference Cerqueira, da Cunha and Caldeira da Silva75). These IER and alternate day fasting regimes did not lead to up-regulation of antioxidant enzymes which contrasts to other studies that have linked IER to increased cellular stress resistance, and resistance to oxidative stress by ‘hormesis’, whereby repeated spells of moderate stress with IER increases the production of restorative proteins and antioxidant enzymes(Reference Mattson76). Alternate day fasting has been linked to increased SIRT-1 gene expression in muscle of non-obese subjects(Reference Heilbronn, Civitarese and Bogacka77), and to greater neuronal resistance to injury compared with CER in C57BL/six mice(Reference Anson, Guo and de Cabo78). Thus, CER has consistent antioxidant effects that are seen in most but not all types of IER or IF. It is important to continue to test for potential adverse effects in ongoing and future IER and IF studies.
Tachphylaxis of the beneficial metabolic effects to a prolonged stimulus with CER has been shown(Reference Henry, Scheaffer and Olefsky79). The repeated stimulus of IER or IF could also bring about a diminished beneficial response and desensitisation, but there are few data concerning this possibility. Rogozina found reductions in IGF-1 during restriction was attenuated with repeated cycles of IER(Reference Rogozina, Bonorden and Grande37). A recent rodent study showed metabolic adaption to twice weekly 24 h fasts with greater glucose uptake and reductions in ketone production by the seventh week of IER(Reference Thomas, Antonelli and Lloyd80).
The need for new studies of intermittent energy restriction and intermittent fasting
To date IER and IF studied regimens have arbitrary durations of restriction and refeeding. We have used a 2 d IER for convenience, an important consideration with respect to general applicability of this approach. However, IER and IF regimens will, potentially, have different effects depending on the extent of restriction (fasting or the degree of restriction), its duration, frequency and possibly the macronutrient composition of the diet on restricted periods and intervening days. For example, with alternate day restricted regimens reduced mammary epithelial proliferation is seen with alternate days of ≥85% restriction, reduced visceral fat with alternate days of ≥75% restriction and reduced fat cell size with alternate days of ≥50% restriction. The effect of less severe but longer spells of restriction is not known.
Restricted periods with IF and IER have varied between 36 h with alternate day fasting, 60 h with 2 d IER and several weeks of a 50% restriction. There is likely to be a minimum period of restriction which induces beneficial effects. For example, allowing just 1 meal/d and a fasting period of just 20 h has generally been linked to detrimental or neutral, rather than beneficial effects on glucose regulation and blood lipids(Reference Carlson, Martin and Stote81, Reference Stote, Baer and Spears82). The optimum duration of restriction needs to strike a pragmatic balance of being achievable while being long enough to deliver beneficial physiological effects. Numerous potential permutations of IER or IF which could be studied. A sensible starting point to define best regimes could be to conduct animal studies testing the anticancer effects of less onerous regimens known to be achievable in human subjects, i.e. the 2 d/week 70% ER we are testing in the clinic. To date the beneficial effects of IER for mammary tumorigenesis have mainly been shown with regimes of 3 weeks ER and 3 weeks AL which is unlikely to be a viable public-health intervention.
Randomised trials of IER or IF v. CER for preventing breast cancer would need to be large and thus not feasible. Testing one ER approach v. a non-intervention control group is estimated to require about 55 000 subjects studied over 5 years assuming a 15% risk reduction and 80% power(Reference Ballard-Barbash, Hunsberger and Alciati83). The comprehensive effects of IER, IF and CER on tumorigenesis could instead be elucidated using emerging technologies of nutrigenomics, i.e. transcriptomics, proteomics and metabolomics, during both the restricted and unrestricted phases of IER. Most studies have reported the effects of IER and CER on fasting metabolism, while their relative effects on postprandial metabolism could better inform their potential for disease prevention(Reference Hsieh, Hayashi and Webb84).
Conclusions
Animal studies of CER have consistently shown reductions in mammary tumourogenesis, which has recently been corroborated by human studies(Reference Harvie, Howell and Vierkant4–Reference Ostlund, Lu and Lagergren9). Interest in IER and IF as alternative breast cancer prevention strategies to CER has arisen from the hope that intermittent restriction will be easier to adhere to and could also deliver greater cancer protective effects than CER.
The feasibility of administration of IER and IF has been demonstrated in human subjects. Currently, tested IER regimens have been found to be equivalent but not better for weight loss compared to CER, but could provide an alternative approach for weight loss better suited to some individuals.
Animal studies summarised in the paper have shown IER and IF can reduce mammary tumour rates, but their superiority to CER has only been reliably demonstrated in one animal model (oestrogen responsive MMTV–TGF-α) receiving one specific IER regimen (3-week cycles of 50% ER and 3 weeks of AL feeding). Important questions thus remain of whether IER or IF can provide additional cancer protective effects compared with CER in other tumour models and with other regimens. Animal studies and our own data have highlighted possible mechanistic differences between IER or IF and CER which might lead to preferential tumour reduction or prevention including reduced hepatic and visceral fat stores, improved insulin sensitivity, reduced IGF-1 activity, increased adiponectin and reduced cellular proliferation. The likelihood of these mechanistic differences also occurring in human subjects is suggested and needs future study.
The potential long-term safety of IER or IF approaches are required, particularly if restricted days are interspersed with periods of hyperphagia. These data will inform whether IER or IF can be recommended for weight control, and whether these approaches could also offer cancer protective effects among healthy weight subjects.
Acknowledgements
M. H. was funded by the Genesis Breast Cancer Prevention Appeal. The authors declare no conflict of interest. M. H. drafted the original manuscript. M. H and A. H. edited the manuscript. Both authors read and approved the final manuscript.






