- QTL
quantitative trait loci
- Rubisco
ribulose 1,5 bisphosphate carboxylase/oxygenase
The concern for global food security results primarily from an imbalance between the supply and demand of the major food crops (wheat, rice and maize). Increasing production on a sustained basis is an essential component of ensuring food security; however, the wider issues of distribution and economics are also major challenges for the whole of society. Currently, at least 1 billion people are chronically malnourished and the situation is deteriorating; more people are hungrier now than at the start of the millennium. The United Nations Millennium Development Goal of substantially reducing the world's hungry by 2015 will not be met(Reference Lele1). Reliable food production and distribution determine the availability of food, and both are key factors in achieving food security. The demand for food is driven by the increase in world population, an estimated 8·2–11 billion people by 2050, and by increasing affluence coupled with an accompanied desire to eat meat. A major problem is the worldwide distribution of food availability, on the one hand resulting in poverty-stricken areas with major food shortages, while other areas have a problem of increasingly obese populations; part of a solution would be the achievement of equitable distribution. However, food production needs to increase 50% by 2030 and double by 2050 to meet projected demands. The projected increases in population appear to have been accepted as inevitable, but such increases are not sustainable and the viewpoint of acceptance of continued increases will need to be challenged to ensure future food security.
At the same time, that demand for food is increasing, production is progressively being limited by increased urbanisation, land degradation (erosion and salinisation), non-food uses of crops and cropland (e.g. bioenergy and leisure activities) and climate change. For example, in the UK, by 2015 more than a quarter of wheat grain may be destined for bioenergy production. Global climate change is projected to further decrease agricultural yields as a consequence of increasing temperatures and altered patterns of and more erratic rainfall.
To ensure global food security, a new green revolution in agricultural productivity is needed to dramatically increase crop yields and the supply of food. This requires an integrated, multifaceted and sustainable approach that will increase both production per unit area, and, at the same time, optimise the resource use efficiency of crops. The successful and acceptable application of biotechnology to crop breeding will be essential to provide the required stepwise increases in production. In recent years, reserve stocks of grain have been very low; the stock to use ratio in 2008 was at the lowest level in 50 years(2) and thus the situation needs to be addressed urgently.
This review summarises the key processes involved in plant growth and development and gives some examples of ways in which molecular technology, plant breeding and genetics may increase the yield and resource use efficiency of wheat which is a staple food in many countries and globally supplies about 20% of the food energies to the world's population (see Fig. 1).
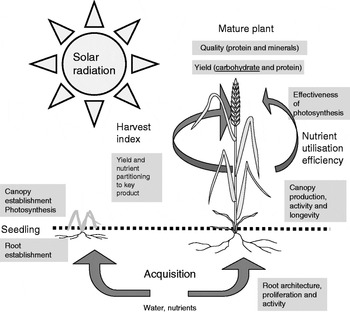
Fig. 1. Key processes contributing to yield and effective use of resources in a grain crop (wheat).
Carbon
In photosynthesis, plants convert light energy into chemical energy (ATP and NADPH), which is used in the assimilation of atmospheric CO2 and the formation of sugars that fuel growth and yield. Increasing photosynthesis has the potential to increase crop yields. Although wheat yields have increased, this was not due to an increase in total biomass but rather due to an increase in harvest index (i.e. the proportion of the total biomass devoted to grain at harvest) and to improvements in agronomic practice including the use of fertilisers, herbicides and pesticides. The harvest index for wheat is thought to be approaching a ceiling and any further increase in yield will need to involve an increase in total biomass and therefore more photosynthesis(Reference Sage and Sage3). CO2 enrichment experiments clearly demonstrate that provided that other constraints do not become limiting, increasing photosynthesis will increase yields(Reference Ainsworth and Long4). Thus, photosynthesis is a major target for improving crop productivity both via conventional breeding and biotechnology(Reference Richards5, Reference Dunwell6).
Total crop photosynthesis is dependent on (1) the ability of the crop canopy to intercept and capture light, (2) the period over which the canopy can capture light and (3) the photosynthetic rate per unit leaf area. All three are potential targets for crop improvement, although wheat breeding has already optimised canopy architecture and there may be a few further obvious opportunities for improvement(Reference Horton7).
The period over which the canopy can capture light and thus produce sugars for growth and yield may be extended by establishing early ground cover and/or by delaying senescence. Early ground cover may be determined by a number of diverse component traits (e.g. grain size and low-temperature tolerance). Alternatively, stay green phenotypes can delay senescence and extend the period for light capture. However, in some environments, extending the period over which the canopy can capture light could be detrimental if other resources (e.g. water or temperature) are limiting.
There is some evidence from work on historic wheat genotypes which suggests that improvements in photosynthesis per unit leaf area have already occurred(Reference Fischer, Rees and Sayre8, Reference Reynolds, Maarten van Ginkel and Ribaut9). However, in wheat (and other C3 crops like rice), the enzyme that assimilates the CO2, ribulose 1,5 bisphosphate carboxylase/oxygenase (Rubisco), is not ideal for crop productivity: in addition to catalysing the productive carboxylation of CO2, Rubisco also catalyses a competing and wasteful reaction with O2. The extent of the wasteful reaction is dependent on the relative concentrations of CO2 and O2. The oxygenase reaction is favoured by high temperatures and conditions that promote stomatal closure and thus a lowering of the CO2 concentration within the leaf. The oxygenase reaction subsequently leads, in a mechanism called photorespiration, to the loss of previously fixed CO2 and NH3, and uses energy in the process. Between 10 and 60% of potential carbon assimilation is lost in photorespiration depending on the environmental conditions(Reference Long, Zhu and Naidu10).
In C4 plants, such as maize, the oxygenase reaction is greatly decreased. In C4 plants, photosynthetic biochemistry is segregated into two cell types. The atmospheric CO2 is initially fixed in the mesophyll cells, which are in contact with intercellular air spaces, into C4 acids (malate or oxaloacetate) by a high-affinity enzyme. The C4 acids are transported into gas tight bundle sheath cells where they are decarboxylated, and the CO2 released is recaptured by Rubisco. This process ensures that the CO2 concentration within the bundle sheath cells is high (10×atmospheric levels) and that the oxygenase reaction of Rubisco is negligible(Reference Furbank, von Caemmerer and Sheehy11, Reference Carmo-Silva, Powers and Keys12). C4 photosynthesis has evolved independently more than 50 times(Reference Sage and Sage3) and introducing a CO2 concentrating mechanism of this type into C3 plants such as grain cereals could be advantageous(Reference Sheehy, Mitchell and Hardy13). However, C4 photosynthesis is not advantageous in cool or light-limited environments, because the CO2 concentrating mechanism diverts precious light energy away from the Calvin cycle. Although introducing the pathway requires complex anatomical and biochemical changes, a large multinational project is underway to introduce C4 characteristics into rice by genetic transformation(Reference Furbank, von Caemmerer and Sheehy11, Reference Hibberd, Sheehy and Langdale14, 15). A similar approach is worthwhile for wheat.
Approaches to increase photosynthesis include:
1. Stay green phenotypes
2. Early vigour
3. Minimising stomatal and mesophyll resistance
4. Increasing photosynthetic capacity
5. Utilising best photosynthetic enzymes (C3/C4)
6. Minimising down regulation under stress
7. Decreasing photorespiratory losses
In some plants, a C4 system operates within a single cell where the C4-like cycle is separated between the cytoplasm and the chloroplast, rather than between two different cell types(Reference Edwards, Franceschi and Voznesenskaya16). Thus, so far, attempts to install a single cell C4 mechanism into rice have not been successful(Reference Taniguchi, Ohkawa and Masumoto17), and the leakage of CO2 from the chloroplast may require a great diversion of energy to attain high CO2 concentrations in the chloroplast. An alternate and simpler, and therefore potentially less technically challenging approach with inherently lower energy costs than that of the C4 pathway, would be introduced into C3 crop plants, an inorganic CO2 concentrating mechanism similar to that found in cyanobacteria and algae(Reference Price, Badger and Woodger18). This may only necessitate the introduction of the well-characterised cyanobacterial bicarbonate pumps (BicA and SbtA) into the chloroplast envelope of terrestrial mesophyll cells.
A less elaborate approach would be to decrease the cost of photorespiration by increasing the probability that photorespiratory CO2 is recaptured. This could be achieved by introducing genes encoding proteins that can short-circuit the normal photorespiratory cycle(Reference Parry, Madgwick and Carvahlo19). Some success appears to have been achieved in Arabidopsis(Reference Kebeish, Niessen and Thiruveedhi20), but it will be important to prevent the accumulation of toxic intermediates which could occur if there was a high flux through the pathway.
Since Rubisco is the source of photorespiration and the catalytic properties of Rubisco are not optimal for current or projected environments, a direct approach to improve photosynthesis would be to replace the enzyme in wheat with another enzyme which had features more suited to high photosynthesis in current conditions(Reference Parry, Madgwick and Carvahlo19, Reference Parry, Andralojc and Mitchell21, Reference Reynolds, Foulkes and Slafer22). Rubisco is also such a slow catalyst that very large amounts are required in leaves to attain high photosynthetic rates. In wheat, more than 25% of the leaf N is invested Rubisco. Despite this huge investment in Rubisco, at CO2 concentrations less than ambient, net assimilation is generally limited by Rubisco amounts and kinetics, while at higher CO2 concentrations, the limitation shifts to the regeneration of the Rubisco substrate, ribulose 1,5 bisphosphate(Reference von Caemmerer23).
The weak affinity for CO2, and the competing reaction with oxygen, could be partially overcome by selecting for natural variants with greater affinity for CO2 or higher carboxylase capacity, relative to the competing oxygenase activity, such that the specificity factor remains unchanged or increases. Rubisco from diverse sources has a wide range of kinetic constants and the replacement of the Rubisco in wheat with that from other species, with a higher catalytic rate or selectivity for CO2 could be advantageous under some conditions. Such homologues already exist (e.g. in Limonium(Reference Galmes, Flexas and Keys24)) and could increase photosynthetic rates by 100% under some conditions(Reference Parry, Madgwick and Carvahlo19). However, although great progress has been made in introducing foreign Rubisco genes into model species, considerable technical advances, including the development of plastid transformation, are needed before this can be achieved in wheat or other cereals.
The balance between Rubisco and the other Calvin cycle enzymes is not optimised for wheat and other C3 crops, even under current conditions(Reference Mitchell, Theobald and Parry25). Models suggest that increasing the amounts of some Calvin cycle enzymes involved in ribulose 1,5 bisphosphate regeneration would be advantageous(Reference Zhu, Sturler and Long26). This confirms experimental evidence that increasing the activity of one Calvin cycle enzyme, sedoheptulose-1,7-bisphosphatase relaxes the limitation to assimilation caused by ribulose 1,5 bisphosphate regeneration and increases both photosynthetic rate and biomass(Reference Harrison, Willingham and Lloyd27–Reference Miyagawa, Tamoi and Shigeoka31). Under optimal conditions, increasing sedoheptulose-1,7-bisphosphatase activity in wheat should be advantageous and is easily testable.
Ensuring that the existing photosynthetic capacity is fully exploited could lead to significant increases in photosynthetic carbon assimilation. For example, at elevated temperatures photosynthetic rates are lower than models would predict. This is thought to result from the temperature-induced loss of Rubisco activity that is caused by the thermal inactivation of Rubisco activase. Species variation in the thermotolerances of Rubisco activases have been identified and new forms generated by directed evolution(Reference Salvucci and Crafts-Brandner32–Reference Kurek, Kai Chang and Bertain34). Their introduction into wheat plants could maintain Rubisco activity and therefore photosynthesis at elevated temperatures.
Efficient utilisation of mineral resources for food security
Plant growth, including canopy production for efficient photosynthesis (see earlier) is dependent on adequate nutrition, and optimised fertiliser inputs are an essential component of efficient crop production. The efficient use of both N and P is of particular concern for food security and sustainable production, and are key targets for crop breeding programmes which have previously often been ignored. However, the problems of efficient use of fertiliser inputs depend on circumstance: sustainability of food production must be underpinned by acceptable energy and environmental costs of the fertiliser employed, and will require appropriately individually tailored nutrient use efficient germplasm targeted, respectively, for highly managed intensive crop production or for low-input systems.
Increasing yield without additional inputs of mineral fertilisers is by definition an improvement in nutrient use efficiency. However, yields must be sustainable to provide food security. In many cropping systems, inputs are minimal and yields are sustainable while production is low, a situation which may be appropriate given other physical limitations to production. Small fertiliser inputs, for example, organic manures, can have a huge impact on yield and positive benefits for food security. Furthermore, soil improvement as a consequence of the introduction of organic matter can have subsequent positive impacts on water retention and mineralisation and/or the availability of other nutrients such as P. However, inappropriate unbalanced use of fertilisers together with increased productivity may lead to ‘mining’ of other mineral nutrients from the soil and a lack of sustainability. If the net balance of dynamics is such that take-off exceeds inputs, then the resulting imbalance is clearly not sustainable. Ultimately sustainability may be compromised by limited fertiliser availability or by the economics of fertiliser supply.
In any agricultural system, the inefficient use of fertilisers, and particularly nitrogen, contributes to the carbon footprint of agriculture and therefore potentially to climate change. On the other hand, climate change impacts on crop development and growth with concomitant implications for timing and amounts of fertilisers(Reference Parry, Hawkesford and Reynolds35). It has been estimated that for grain crops, globally, N use efficiency may be as low as 33%(Reference Raun and Johnson36). While not all agricultural systems are subject to such losses, worldwide this represents a huge waste of resources and a threat to food security due to the increased costs of fertiliser production and/or losses of non-renewable resources, which is specifically the case for phosphate (see below). Efficient utilisation requires both efficient capture and conversion into useable biomass: a crop plant will include vegetative and reproductive tissues and either may be croppable, although the major world staples are either reproductive tissues or storage organs (seeds, tubers etc.). Production of tissues such as the grain is dependent on the functioning (size, duration and activity) photosynthetic vegetative tissues (see previous section and later). Nutrient use efficiency may be defined in many ways; however, essentially there is a requirement for maximising outputs and not wasting inputs. In the case of many nutrients, the overall trait of efficiency is divided into two major components: efficient uptake (thus minimising fertiliser losses) and effective utilisation of the nutrients taken up to produce useful croppable biomass. In addition, post-harvest processing and utilisation will have a big contribution to the whole system nutrient budget. However, for the crop, uptake efficiency is primarily a set of root characteristics, principally architectural (density and depth of roots), but also related to function (uptake and translocation of resources). Prolific shallow roots may be required to capture applied fertiliser, particularly immobile species such as phosphate, and deeper roots are likely to be important for accessing water (see next section) and deeper N reserves. The second key trait involving efficient production of useable biomass will depend on canopy function (photosynthesis), architecture, longevity (as discussed above) and efficient remobilisation of nutrient from discarded/non-harvested material to the croppable biomass.
For the croppable biomass, both yield and quality are often desirable traits, and increasing one may not be compatible with the other, and therefore each needs to be targeted as appropriate for the market and end use. Increasing yield by improving photosynthesis and hence starch production, which is targeted to storage organs, without concomitant increased protein synthesis and/or essential nutrient uptake will lead to a dilution of the quality components. In wheat, as an example, at any given N input, grain yield is inversely proportional to grain percentage N(Reference Barraclough, Howarth and Jones37). Varieties that deviate from this rule(Reference Monaghan, Snape and Chojecki38) and have unusually high yield and grain N combinations are much sought after by breeders targeting bread-making varieties.
Sustainable agricultural production depends on many factors including, as discussed here, adequate supplies of water (see later), N and P. In addition, several other nutrients may limit productivity and are of importance to nutritional quality: K, S, Fe/Zn and other micronutrients are essential for healthy crops and may have important nutritional values (Zn/Fe/Se). Major projects are in place, such as HarvestPlus (funded by Gates, the World Bank and others) and Healthgrain (funded by the European Union), which are examining genetic variation for the ability to acquire mineral nutrients (particularly Fe and Zn) with the aim of introducing these key traits into key crop improvement programmes. Variations in Se availability for crops and the implications for the health of human subjects and livestock have been reviewed recently(Reference Hawkesford and Zhao39). Generally, there are either large reserves of appropriate raw materials for fertiliser production, or only relatively small quantities are required; however, economics and distribution problems result in shortages of these nutrients in many areas, worldwide. Partitioning of these nutritionally important elements between discarded and harvested crop fractions is again, as with overall biomass (harvest index) a major target for crop improvement and key genes which control remobilisation have been identified(Reference Uauy, Distelfeld and Fahima40).
N supply determines yield irrespective of the crop in question or the agricultural practice employed. N supply will determine the limit of vegetative growth, and in addition, other factors may limit productivity irrespective of nutrient availability, notably water availability and pest and diseases: optimising crops for efficient nutrient use requires the knowledge and management of these limiting factors. The importance of selecting varieties under reduced nutrient availability for low-input and organic systems has been highlighted(Reference Dawson, Huggins and Jones41). Indeed a detrimental impact on yield and quality was observed with the supply of organic N compared to conventional fertilisers to modern bread-making varieties of wheat(Reference Godfrey, Hawkesford and Powers42), and furthermore, for modern varieties generally variety performance at low and high inputs are highly correlated(Reference Barraclough, Howarth and Jones37). However, selection has seldom been made at low inputs.
It is questionable as to whether yield can be increased greatly without supplying more N, as N determines vegetative growth, e.g. canopy and therefore sets the potential for photosynthate production. In addition, storage organs would require N, and in cases where protein content is an essential quality attribute, optimised N supply is even more essential. However, when carbohydrate is the major component of yield, increased photosynthesis without an increased canopy biomass (and hence the requirement of N for canopy production), will enable an increase in yield without a concomitant increased crop N requirement. Ideally, N in the canopy will be either remobilised to the harvested organ as a useful protein or may be recycled as manure for future crop production.
Industrially manufactured (Haber process) N fertiliser supply is in theory unlimited and only dependent on energy inputs; however, there are substantial economic and environmental costs associated with fixation, distribution and application. Additional consequences of excessive fertilisation and inefficient capture or management are the negative effects on rivers, lakes and coastal waters. High inputs, particularly of N will accelerate soil acidification(Reference Guo, Liu and Zhang43), adding pressure for appropriate and efficient fertiliser application. Crop improvement relating to both capture and use efficiency of N (conversion into biomass) are key targets for sustainable food security with minimised environmental impact. Minimising losses may also be achieved by enhancing natural processes of exudation of biological nitrification inhibitors(Reference Subbarao, Tomohiro and Masahiro44). The ultimate solution to the supply of N for world food supply will be the incorporation of the N-fixation trait of the Rhizobia/legume symbiosis in cereals; however, this remains a prospect for the distant future.
P is a non-renewable reserve and in the long term a systems approach to conservation will be required. Estimates vary as to global reserves, however, agricultural use has reached a plateau or even decreased as management and regulation have controlled usage, while in some developed countries excess application still occurs. In many tropical countries, soil acidity places a severe restriction on P availability(Reference Ma and Ryan45) and selection or engineering of appropriate P-efficient varieties may be the most appropriate solution to this problem. Traits including root exudation of organic acids or phosphates combined with root morphology are likely contributors to improved P use efficiency(Reference Kochian, Hoekenga and Pineros46). Management of both fertiliser application and soil properties affecting availability are also important. Much P is subsequently lost as recycling and reclamation, for example, from animal wastes or sewage, are often not employed.
It is necessary to combine genetic improvement with resource management: major inefficiencies for N or P use are not uniformly distributed geographically or across farming systems or crops. In many cases, education and effective management can massively improve nutrient use efficiency. However, once the agronomy is optimised, the major gains are then to be made from genetic improvement of the crops. Historical selection for yield improvement has effectively selected for nutrient use improvement, because the definition of efficiency has been rightly related to yield, and as already noted this has usually been at high inputs. Due to the selection based on yield alone, and because nutrient use efficiency is a complex trait, optimal performance in the subtraits which include efficient capture may not have been combined in current elite varieties, and essential alleles may have even been lost from modern variety gene pools.
In addition to traditional breeding methods and the selection of varieties for nutrient use efficiency, whether for yield and high nutrient use efficiency under intensive conditions, or for effective nutrient scavenging under nutrient-limited conditions, a complementary approach is the targeted identification of underpinning processes contributing to nutrient use efficiency, for example, and the constituent genes controlling these processes. These genes would be involved in nutrient acquisition as well as efficient utilisation of the nutrients taken up, including appropriate partitioning between harvested/non-harvested plant parts. A number of approaches are being followed, including traditional quantitative trait loci (QTL) analysis(Reference Habash, Bernard and Schondelmaier47) and mapping of underpinning genes as well as target gene manipulation, with candidates identified either through biochemical or genetic approaches. Examples would be genes enhancing nutrient remobilisation from the canopy to the grain in wheat, albeit at the expense of yield(Reference Uauy, Distelfeld and Fahima40) or the enhancement of N acquisition, possibly by alleviating negative feedback regulation, by a transgenic expression of genes affecting local N pools, such as alanine amino transferase(Reference Good, Johnson and De Pauw48, Reference Shrawat, Carroll and DePauw49).
In summary, each crop and each specific agricultural situation will have specific requirements and targets for optimising nutrient use efficiency. Nutrient imbalances resulting in the huge gap between low input, but unsustainable agriculture in many developing countries compared to excess (and also unsustainable) inputs in many developed and rapidly growing economies(Reference Vitousek, Naylor and Crews50) require different and unique approaches, combining both management and appropriate germplasm. In low-/no-input systems, increasing capture may exacerbate nutrient mining problems and not provide food security. In high-/excessive-input systems, both managed inputs and appropriate germplasm will contribute to maximum nutrient capture. The clear targets are early developing and extensive root systems. These may be combined with specific attributes to maximise biological availability (phosphorus) or minimise losses (nitrification), as described earlier. Example traits to optimise nutrient use in wheat were identified as root density to aid capture, stem storage, low leaf N, efficient remobilisation to grain and customised grain attributes (protein v. carbohydrate) suitable for specific markets(Reference Foulkes, Hawkesford and Barraclough51).
Water
The availability of water is the major constraint on world crop productivity(Reference Baldocchi, Valentini, Field and Raupach52). Global climate change is predicted to alter patterns of rainfall and the overall availability will decrease. By 2050, it is estimated that more than 65% of the global population will live where water is scarce(Reference Wallace53).
Since more than 80% of the available water is used for agricultural production(Reference Morison, Baker and Mullineaux54), there is little opportunity to use additional water for crop production, especially because as populations increase, the demand to use water for other activities also increases(Reference Parry, Flexas and Medrano55).
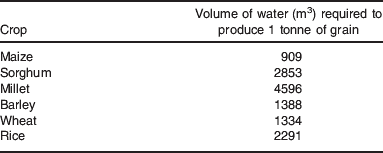
A real and immediate challenge for agriculture is to increase crop production with less available water(Reference Marris56). This requires an increased water productivity (i.e. the amount of water required per unit biomass production). Water is essential for plant growth and cell expansion, but often only 10% of the water available to crops is used productively in transpiration(Reference Morison, Baker and Mullineaux54). This means that there are significant opportunities to improve water productivity both by increasing the water allocated to transpiration and the efficiency with which transpired water produces biomass(Reference Wallace53). The amount of water required per unit of yield varies greatly from crop to crop. Although C4 species like maize have inherently greater water use efficiency under well-watered conditions compared to C3 crops such as wheat, this is not always reflected in these figures. The global average water productivities for various crops are shown in Table 1, but there is considerable variation in water productivity between individual crops growing in different regions(Reference Gleick and Gleick58).
Table 1. The global average water productivity of various C3 and C4 cereals.(57)
The constraint that water availability imposes on plant productivity is complex, because it is not constant and varies within and between environments. Thus specific strategies to improve water productivity are often not applicable to all crops or all environments. For example, traits related to coleoptile length may not be valuable in an environment only subjected to terminal drought(Reference Wang, Chapman and Bonnett59).
The ability of a crop to yield well with limited water is determined by multiple genes. Improvement of water productivity, as noted above for C or nutrient resources, also requires a multifaceted and integrated approach that considers both agronomic practices and germplasm and the effective transfer of ‘best practice’ to individual farmers. This must include, for example, the management of soils to conserve water and the management of nutrition to control the development of the crop canopy in addition to the development of improved genotypes with high water productivity. Existing natural variation or induced variation (mutagenesis and transgenesis) can be used in multi-site, multi-environment field studies to identify key traits associated with water productivity in different environments (e.g. phenology, architecture and metabolism); often these traits may themselves be determined by a number of component traits(Reference Richards5). Furthermore, there are strong genotype–environment interactions; component traits relevant to an environment in which water was limited at germination will be very different from those where water is limited during grain filling. Some important traits may not themselves be directly linked to water use efficiency, but to avoidance by allowing a crop to escape periods of limited water availability by having a shorter life cycle. For annual crops like wheat, these traits must enable the crop to thrive and produce grain with a limited water supply, rather than to merely survive(Reference Sinclair and Purcell60). Survival traits are, however, important for perennial crops(Reference Parry, Flexas and Medrano55).
An example of a success story is the Australian wheat variety ‘Drysdale’ which was selected by C isotope discrimination, because it uses water more efficiently; this is achieved by slightly restricting stomatal aperture and thereby the loss of water from the leaves(Reference Condon, Richards and Rebetzke61). While this reduces photosynthetic performance slightly under ideal conditions, the plants have access to water later in the growing season thereby increasing total photosynthesis over the life of the crop.
Association genetics and mapping populations can be used to identify genetic loci (QTL) in the genomic regions underlying individual component traits(Reference Wang, Chapman and Bonnett59). Although QTL have been identified, the available genetic maps are at too low resolution, and it is difficult, even when exploiting the synteny between species, to identify the genes for the underlying traits; very few of the genes responsible for QTL have been identified(Reference Salvi and Tuberosa62). However, in the near future, the availability of a complete wheat genome sequence and of high-resolution maps saturated with markers should enable the genes for the underlying traits to be more easily de-convoluted. In contrast, there are numerous examples where the genes underlying QTL in model species have already been identified. Such candidate genes can be validated in crop plants by functional genomics approaches such as transformation and TILLING (targeting induced local lesions in genomes)(Reference Pastori, Wilkinson and Steele63–Reference He, Jones and Chen65).
Much research effort has focused on the identification and manipulation of drought responsive genes that relate to a wide range of biological processes. This includes genes involved in the biosynthesis of osmolytes, scavenging active oxygen, molecular chaperones, signalling molecules, transporters and transcription factors (see review(Reference Shinozaki and Yamaguchi-Shinoza66)). While the results are interesting and have provided detailed understanding of the mechanisms involved in response to drought stress, many of the reports of increased drought tolerance relate to survival rather than sustained growth under limited water. Since performance under all but the most severe drought is closely related to yield potential, a shift in emphasis to those constitutive traits related to yield potential in any environment is most likely to be beneficial; for example, traits related to architecture(Reference Zhang, Li and Cao67) or, as described above, photosynthetic performance. Similarly, the potential benefit of indirect effects such as the introduction of herbicide tolerant transgenics that permit the introduction of water conserving minimum tillage systems should not be ignored.
Conclusions and prospects
Food security will be a major issue for the increasing world population. The problem will be almost certainly increased by climate change. The green revolution of the last century was achieved through the adoption of both new germplasm and agricultural practice and led to a several-fold increase in yields. In recent times, yield increases for most crops have been more modest and incremental. The immediate future will see further such incremental increases for most crops, with longer-term possibilities of greater improvements. Major yield increases may come about as varieties are developed which are able to exploit inhospitable environments, thus increasing agricultural land use. In the best agricultural land, economic demand will always favour high yields and production; however, in addition, efficient use of resources in agricultural and consumer systems will be a priority.
Acknowledgements
Rothamsted Research is an institute of the Biotechnology and Biological Sciences Research Council of the UK. The authors research was supported by DEFRA (as a component of the WGIN project (Wheat Genetic Improvement Network: www.wgin.org.uk) and ‘An integrated approach to increasing water use efficiency and drought tolerance of wheat production in UK’) and the European Commission (OPTIWHEAT – Improving the Yield Stability of Durum Wheat under Mediterranean Conditions (EC Contract Number: INCO-CT-2006-015460)). The authors contributed equally to the writing of the review and have no conflicts of interest.




