Obesity is now the commonest antenatal co-morbidity and affects one in five pregnant women in the UK. The exponential rise in obesity across the developed world has prompted the World Health Organisation to describe it as one of the most important global health problems today(1). Maternal obesity is associated with increased morbidity and mortality for both mother and offspring. Antenatal risks include gestational diabetes, hypertensive disorders including pre-eclampsia and thromboembolic complications(Reference Bhattacharya, Campbell and Liston2–Reference Linne4). In the UK, the most recent Confidential Enquiry into Maternal and Child Health reported that 78% of women who died of a thromboembolism were overweight or obese (BMI>25 kg/m2)(Reference Cantwell, Clutton-Brock and Cooper5). Peripartum, women are more likely to face induction of labour, operative delivery and postpartum haemorrhage(Reference Denison, Price and Graham3, Reference Sebire, Jolly and Harris6). High pregravid BMI and excessive gestational weight gain are also important predictors of short-term postpartum morbidity(Reference Walker7) and higher postpartum weight retention(Reference Gunderson, Abrams and Selvin8) with the latter being associated with increased risks for future pregnancy and lifelong obesity(Reference Oken, Rifas-Shiman and Field9). Offspring of obese mothers tend to be large for gestational age at birth and are at higher risk of congenital anomaly, late fetal death and admission to the neonatal unit. Maternal obesity also increases the lifetime risk of obesity in offspring and a tendency to develop metabolic syndrome in childhood and adolescence(Reference Boney, Verma and Tucker10), thus perpetuating the cycle of obesity and its adverse consequences into the next generation. There is, therefore, an urgent need to develop interventions that improve pregnancy outcomes and long-term health for both mother and baby. This review will summarise the current evidence base for interventions aimed at improving pregnancy outcome for obese women.
Overview of interventions
The following key principles inform design, delivery and effectiveness of interventions aimed at improving pregnancy outcome for obese women. These will be outlined before considering the evidence for specific interventions.
What is (are) the aim(s) of the intervention and who is it aimed at?
Does the intervention target direct (e.g. maternal weight gain, dietary and/or cardiovascular fitness) or indirect (e.g. pre-eclampsia, gestational diabetes, caesarean section, fetal macrosomia and/or offspring obesity) consequences of obesity?
Many trials use limitation of maternal weight gain during pregnancy as their primary outcome(Reference Dodd, Grivell and Crowther11). Maternal obesity is a significant determinant of gestational weight gain, which if excessive is an independent predictor of adverse maternal and fetal outcome for pregnancy, with consequent lifelong health implications. However, the optimal gestational weight gain for women of different BMI categories remains to be established. Although guidelines, such as those generated by the Institute of Medicine(Reference Rasmussen and Yaktine12), now provide recommendations for gestational weight gain, dependent on maternal weight or BMI at antenatal booking, there are no recommendations to inform weight gain for very severely obese women with a BMI>40 kg/m2, who comprise an increasing proportion of the antenatal population. In addition, guidelines are only relevant for the population for whom they are generated. For example, the Institute of Medicine guidelines are intended for use in the USA. Although they may be relevant to other developed countries, they are not applicable to other countries where the antenatal population are either heavier or lighter, have different demographics or ethnic origin to women in the USA. If gestational weight gain is being used as a trial outcome, it is therefore, important that guidelines used are relevant to the study population. Furthermore, whether limiting maternal weight gain actually improves maternal and infant health remains to be established due to the lack of high-quality information from randomised trials(Reference Dodd, Grivell and Crowther11).
Intervention trials in pregnancy are also complicated because any intervention given during pregnancy affects both mother and fetus. Importantly, the outcomes for mother and fetus may differ and evolve over time. For example, the ORACLEII (Overview of the Role of Antibiotics in the Curtailment of Labour and Early delivery) trial randomised women at risk of preterm labour, with intact fetal membranes and no evidence of infection to receive erythromycin, co-amoxiclav or placebo daily until delivery. There was no difference in the primary outcome (composite of neonatal death, chronic lung disease or major cerebral abnormality on ultrasonography) before discharge from hospital between study groups(Reference Kenyon, Taylor and Tarnow-Mordi13). However, when babies were followed up at age 7, prescription of erythromycin for women in spontaneous preterm labour with intact membranes was associated with an increase in functional impairment among their children; and the risk of cerebral palsy was increased by either antibiotic, although the overall risk of this condition was low(Reference Kenyon, Pike and Jones14).
Thus, for any trial involving pregnancy, it is important that the primary outcomes are appropriate and consideration should be given for long-term follow-up studies for both mother and offspring to ensure that adverse long-term health outcomes are not missed.
What is the timing duration and intensity of the intervention?
Study design varies considerably, with some interventions being delivered before, some during and others after pregnancy. This makes comparison between studies difficult. For example, if the aim of the study is to reduce the incidence of a disease such as pre-eclampsia that only occurs during pregnancy, the intervention may be limited to the duration of pregnancy. However, for lifestyle interventions, which aim to alter maternal and/or offspring behaviour, the interventions may be longer term, potentially spanning the lifetime of both mother and offspring. Similarly, the intensity of an intervention may vary considerably, particularly if the intervention is aimed at changing maternal behaviour. For example, an exercise intervention may range from a single group session to a structured programme of exercises with goal setting delivered on a one-to-one basis by an exercise or personal trainer.
Lifestyle interventions
Pregnancy is a period in a woman's reproductive life when she may be more motivated to undertake lifestyle changes, such as altering the quantity or quality of the food that she eats or undertaking exercise. Below, the evidence for dietary, exercise and psychological interventions in improving pregnancy outcome are considered individually, before assessing the evidence for complex interventions involving all three lifestyle interventions.
Dietary advice
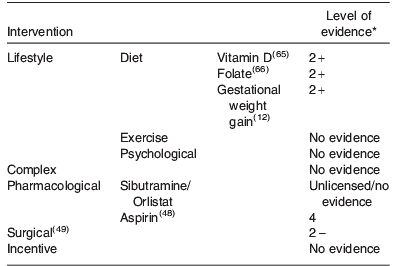
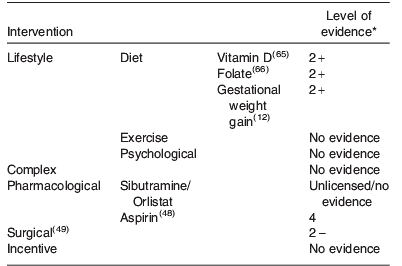
General dietary advice is part of routine antenatal care and is delivered by a wide range of health and allied health professionals. However, most of the written information currently available is generic, and is neither tailored to the individual needs of women nor takes into account their pre-pregnancy BMI. For example, the dietary information, food knowledge and nutritional intake required by a lean woman is very different from that required by an overweight or obese pregnant woman, who is likely to have a more unbalanced diet, to be consuming larger portion sizes and for whom excessive weight gain in pregnancy is to be avoided. However, whether tailored dietary advice alone translates to quantifiable changes in maternal behaviour, such as improving the quality of nutritional intake or pregnancy outcomes is not yet proven(Reference Dodd, Grivell and Crowther11). In addition, there is evidence that obese women are often unaware of the risk that obesity poses to their pregnancy and have a distorted view of the nutritional quality and quantity of the food they consume(Reference Keely, Gunning and Denison15). With regard to food quantity, it is important to educate women about appropriate energy intake and dispel the widely held myth that she must ‘eat for two’. In fact no extra energy intake is required in the first two trimesters of pregnancy, and only an extra energy intake of 837J (200 calories) per d in the third trimester(16).
There is also a paucity of evidence about the beneficial health effects (or otherwise) of specific micronutrient supplementation in obese women. Exposure of the skin to sunlight is the main source of vitamin D synthesis. In countries where there is limited sunlight of appropriate wavelength, for example, the UK, or where, for cultural reasons skin is covered thus preventing exposure to sunlight, skin exposure alone may not be sufficient to achieve optimal vitamin D status for pregnancy(Reference Swan17). This deficiency is accentuated in obesity. Women with a BMI>30 kg/m2 are at increased risk of vitamin D deficiency compared to healthy weight controls(Reference Wortsman, Matsuoka and Chen18), possibly because of sequestering of vitamin D in adipose tissue, with high pre-pregnancy BMI being associated with low serum vitamin D levels during pregnancy(Reference Bodnar, Catov and Roberts19). The UK Royal College of Obstetricians and Gynaecologists recommend vitamin D supplementation of 10 μg/d for women with a BMI>30 kg/m2 (Centre for Maternal and Child Enquiries/Royal College of Obstetricians and Gynaecologists joint guideline). However, there are no randomised clinical trials to support these recommendations, and limited evidence regarding the safety of higher dose antenatal vitamin D regimes(Reference Roth20).
The current recommendation by the Royal College of Obstetricians and Gynaecologists in the UK(Reference Modder and Fitzsimons21) that obese women should take a high dose pre-conceptual folic acid to reduce their increased risk of neural tube defects is similarly based on a paucity of evidence. In the general obstetric population, the use of peri-conceptual folic acid to reduce the risk of neural tube defects is well established. Thus, all women trying to conceive are encouraged to take peri-conceptual folic acid at the standard 400 μg/d dose(Reference De-Regil, Fernandez-Gaxiola and Dowswell22). A higher dose of folate supplementation is recommended for those women identified as being at higher risk, for example, women with a previously affected pregnancy. In these high-risk women, this higher dose of folic acid reduces the risk of having a fetus affected by a neural tube defect in a subsequent pregnancy(23). However, the protective effects of peri-conceptual folic acid do not appear to benefit obese women. Following introduction of flour fortification with folic acid in women with increased BMI, a Canadian study demonstrated no benefit, in terms of reduction in incidence of neural tube defects(Reference Ray, Wyatt and Vermeulen24). Whether higher dose supplementation of folic acid effects a reduction in risk of neural tube defects in obese women remains to be established in clinical trials, thus current recommendations should be viewed with caution.
Exercise
In non-pregnant individuals, regular exercise is associated with maintenance of healthy weight and improved cardiovascular fitness. Many women who are accustomed to regular exercise outside pregnancy, therefore, wish to continue exercising during pregnancy. In healthy pregnant women, regular aerobic exercise during pregnancy maintains or improves physical fitness with studies demonstrating improvements in functional aerobic capacity and cardiorespiratory capacity(Reference Collings, Curet and Mullin25–Reference Kramer and McDonald28). Short bouts of maternal exercise are also associated with fetal physiological responses, in particular, an increase in fetal heart rate(Reference Brenner, Wolfe and Monga29, Reference Webb, Wolfe and McGrath30). Whether these beneficial effects extend to obese pregnant women is not known.
In non-pregnant women, the benefits of exercise extend beyond weight maintenance, to include lowering of blood pressure(Reference Cocco and Pandolfi31), improved insulin sensitivity(Reference Sigal, Kenny and Wasserman32), reduced risk of CHD(Reference Manson, Hu and Rich-Edwards33) and Type II diabetes(Reference Jeon, Lokken and Hu34) and improved psychological well being. Whether these beneficial effects extend to pregnancy and are associated with improved maternal and offspring health outcomes are, however, less clear. A recent Cochrane review that evaluated the role of exercise or other physical activity in lean women to prevent pre-eclampsia and its complications concluded that ‘There is insufficient evidence for reliable conclusions about the effects of exercise on prevention of pre-eclampsia and its complications’(Reference Meher and Duley35). In obese women, although some studies suggest that regular exercise either before or during pregnancy may improve cardiovascular fitness(Reference Melzer, Schutz and Boulvain36), reduce the risk of gestational diabetes(Reference Tobias, Zhang and van Dam37) and attenuate the gestational increase in blood pressure in obese women(Reference Stutzman, Brown and Hains38), other studies are not supportive. Similarly, whether regular exercise influences the rate of other pregnancy complications such as preterm birth is not clear. Although a recent Cochrane review demonstrated that increasing exercise in non-obese sedentary women does not result in a clinically important shortening of gestation (mean difference+0·10, 95% CI −0·11, +0·30 weeks), it also showed that increasing exercise was associated with a non-significant increase in the risk of preterm birth (risk ratio 1·82, 95% CI 0·35, 9·57)28. The optimal combination of exercise with/without other lifestyle interventions to improve clinical outcome, therefore, remains to be established by adequately powered clinical trials.
Psychological interventions
Cognitive behavioural therapy for obesity uses cognitive restructuring, stimulus control and self-monitoring to promote weight loss(Reference Vetter, Faulconbridge and Webb39). Cognitive restructuring is based on the theory that behaviour can be controlled by conscious thought. Stimulus control techniques teach patients to understand, control and potentially avoid triggers associated with eating and self-monitoring, teaching patients to become aware of their eating patterns, and to keep records of their food intake to enable them to assess energy intake(Reference Wadden and Foster40). Studies consistently demonstrate that interventions based on the theory of cognitive behavioural therapy increase the desire to control weight, boost self-esteem, and increase self-efficacy and satisfaction with body areas and appearance(Reference Poobalan, Aucott and Precious41). However, although behavioural interventions are generally effective in promoting weight loss in the short term, they are less effective in maintaining weight loss in the long term(Reference Van Dorsten and Lindley42). There are no studies using cognitive behavioural approaches as a method of controlling weight in pregnancy.
Complex interventions
Complex intervention clinical trials, comprising dietary advice, exercise and psychological interventions are widely used as a method of effecting weight reduction in non-pregnant participants. In non-pregnant obese participants, a comprehensive programme of lifestyle modification induces a mean weight loss of 7–10%(Reference Skouteris, Hartley-Clark and McCabe43).
In pregnancy, there are currently several clinical trials ongoing which are evaluating the use of a complex intervention to improve pregnancy outcome in obese women. To date, evidence is conflicting about the effectiveness of such interventions in pregnancy. A recent systematic review assessed the role of complex interventions in limiting gestational weight gain or reducing the risk of macrosomia(Reference Dodd, Grivell and Crowther11). There was significant heterogeneity across the studies related to the intensity of the intervention provided, ranging from additional dietetic sessions at each antenatal visit(Reference Polley, Wing and Sims44, Reference Wolff, Legarth and Vangsgaard45) to a single dietetic visit at the start of pregnancy(Reference Thornton, Smarkola and Kopacz46), making direct comparisons between studies difficult. However, the review found no statistically significant differences between women who received the antenatal intervention and those who did not for mean gestational weight gain (four studies; 416 women; weighted mean difference 3·10 kg; 95% CI 8·32, 2·13 (random effects model)) or large-for-gestational-age infant outcome (three studies; 366 women; risk ratio 2·02; 95% CI 0·84, 4·86), and concluded that there is currently ‘Little high quality evidence available from randomised controlled trials to guide practitioners of the effect of limiting gestational weight gain in terms of important maternal and infant health outcomes’.
Drug interventions
Weight-loss drugs are recommended as an adjunct to lifestyle intervention in non-pregnant participants who are unable to loose sufficient weight using a combination of exercise and diet alone(Reference Vetter, Faulconbridge and Webb39). Currently only sibutramine (a selective serotonin and norepinephrine reuptake inhibitor, which acts centrally to reduce food intake) and orlistat (a pancreatic lipase inhibitor) are licensed for long-term weight loss by the Food and Drug Administration in the USA. In Europe, only orlistat is licensed for weight loss. Neither of the drugs is licensed for use in pregnancy, and their use is, therefore, not recommended as an adjunct to lifestyle intervention to promote weight reduction or maintenance in pregnancy.
Pharmacotherapy may have some benefits if used in a targeted approach to reduce the risk of specific complications, which are increased with obesity, such as pre-eclampsia. Low dose of the antiplatelet aspirin is of mild to moderate benefit in the prevention of pre-eclampsia in women at high risk of developing the disease(Reference Duley, Henderson-Smart and Knight47). However, ‘moderate risk factors’ are ill defined and data for the benefits of aspirin in obese women are lacking. Despite this, it is the opinion of the National Institute for Health and Clinical Excellence guideline development group in the UK that women with more than one moderate risk factor, which potentially includes obesity, may benefit from 75 mg aspirin from 12 weeks gestation(48).
Bariatric/surgical interventions
In the USA, the incidence of bariatric surgery has increased by 800% from 1998 to 2005, with more than 50 000 women aged 18–45 currently having bariatric surgery per annum(Reference Maggard, Yermilov and Li49). The rate of bariatric surgery is similarly increasing in other developed countries(Reference Buchwald and Oien50). A wide range of different surgical procedures are performed including laparoscopic adjustable gastric banding, vertical-banded gastroplasty, Roux-en-Y gastric bypass (gastric bypass) and biliopancreatic diversion/duodenal switch, with laparoscopic adjustable gastric banding being the most commonly performed procedure.
A systematic review by Maggard et al.(Reference Maggard, Yermilov and Li49) evaluated the effect of bariatric surgery on pregnancy outcomes. To date, there are no randomised controlled trials comparing pregnancy outcome after bariatric surgery. Current evidence is, therefore, dependent on observational cohort or case–control studies, and case reports. Matched cohort studies demonstrated that the rates of maternal complications were substantially lower in obese women after bariatric surgery when compared to obese women without bariatric surgery, and approached rates found in non-obese controls. One cohort study that compared rates of complications in thirteen consecutive deliveries following laparoscopic adjustable gastric banding surgery with outcomes of 414 consecutive patients who were obese (BMI ≥30 kg/m2) who delivered at the same practice between 2004 and 2006 found a significant reduction in rates of pre-eclampsia (0% v. 3·1%, P<0·05) and gestational diabetes (0% v. 22·1%, P<0·05)(Reference Ducarme, Revaux and Rodrigues51). Neonatal outcomes were similarly better after laparoscopic adjustable gastric banding surgery (7·7% v. 14·6% for macrosomia, 7·7% v. 10·6% for low birth weight and 7·7% v. 7·1% for premature delivery; all P<0·05). However, when gastric bypass was compared to non-obese controls, the studies are conflicting regarding maternal outcomes and there were no differences in neonatal outcomes for premature delivery, low birth weight and macrosomia. Currently, evidence is insufficient to assess the effect of bariatric surgery on mode of delivery, nutritional status and fertility. Similarly, there are few data available to inform timing of surgery with respect to pregnancy, with successful pregnancies being achieved within 1–2 years of the procedure. Finally, bariatric surgery is not without its complications, which can include bowel obstruction, preterm delivery and ultimately maternal and fetal death(Reference Moore, Ouyang and Whang52).
Incentives and social marketing
Incentives are effective in effecting simple, time-limited behavioural change, for example attending hospital appointments(Reference Marteau, Ashcroft and Oliver53). Offering financial or other incentives to encourage lifestyle modification is becoming increasingly common for promoting complex behavioural change, including weight reduction. However, despite their rising popularity, there is a paucity of high-quality evidence about the effectiveness of such interventions, their mechanisms of action, and effects at an individual or societal level. Hence the type, level and mode of delivery of incentivisation are essentially arbitrary. Whether incentives have a role in improving pregnancy outcome in obese women is not known.
A summary of all the intervention strategies discussed is illustrated in Table 1. The level of evidence (defined in Table 3) to support these strategies is also shown, highlighting the relative lack of good quality evidence currently available.
Antenatal care
In the absence of an intervention(s) proven to improve pregnancy outcome in obese women, clinicians are left with optimising maternal health pre-pregnancy and providing appropriate high-risk (often non evidence-based) antenatal care for obese pregnant women.
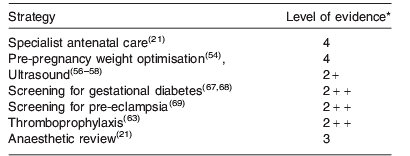
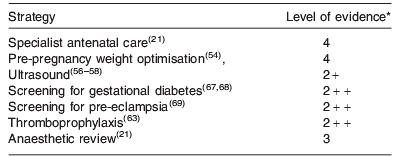
Pre-pregnancy, obese women planning a pregnancy should be given the opportunity to optimise their weight and treatment of common conditions associated with maternal obesity such as essential hypertension. Losing 5–10% of their body weight prior to conception has significant health benefits(54), and rationalising drug therapy can reduce the risk of drug-induced teratogenicity(Reference Friedman55).
Once pregnant, obese women should be recognised as a high-risk group and should be referred for appropriate antenatal care(Reference Cantwell, Clutton-Brock and Cooper5). Ultrasound is widely used for pregnancy dating, detection of fetal anomalies and assessment of fetal growth. However, ultrasound assessment is less accurate in obese women(Reference Phatak and Ramsay56). Women should, therefore, be informed about the reduced sensitivity of ultrasound with increasing maternal size(Reference Colman, Maharaj and Hutton57, Reference Dudley58). To ensure accurate diagnosis of hypertensive complications of pregnancy including pre-eclampsia and pregnancy-induced hypertension, it is important to use appropriate sized blood pressure cuffs. Too small a cuff will overestimate blood pressure, too large a cuff is associated with less error(Reference Maxwell, Waks and Schroth59). Gestational and pre-existing diabetes are also more common in obese women. While there is no debate that appropriate treatment of diabetes (pre-existing or gestational) in pregnancy significantly reduces the risk of serious adverse perinatal outcome (e.g. death, shoulder dystocia, bone fracture and nerve palsy)(Reference Crowther, Hiller and Moss60), there is no consensus about how and when to screen for gestational diabetes, and what diagnostic criteria should be used. Once gestational diabetes has been diagnosed, glycaemic control should be optimised using dietary modification first, and if that is insufficient, then the oral hypoglycaemic drug metformin(Reference Rowan, Hague and Gao61) or insulin should be used.
The antenatal period is also a time of increased thrombotic risk, particularly for obese women(Reference Knight62) with thromboembolism remaining a leading cause of maternal mortality worldwide. To reduce the risk of thromboembolism, low molecular weight heparins are used, dependent on a risk-based scoring system for antenatal thromboprophylaxis. The Royal College of Obstetricians and Gynaecologists in the UK recommends that antenatal thromboprophylaxis with low molecular weight heparin be considered for any woman who has a BMI greater than 30 kg/m2 plus two or more additional risk factors and this should commence as early as practically possible in pregnancy(Reference Nelson Piercy, MacCallum and Mackillop63). Similar scoring systems are recommended for use in the USA. The optimal dose of low molecular weight heparin required to achieve effective thromboprophylaxis in lean and obese women, however, remains to be established in clinical trials(Reference Stock, Walker and Edelshain64).
Maternal obesity poses particular risks at the time of delivery with the risks of instrumental delivery, caesarean section, shoulder dystocia and postpartum haemorrhage all being increased with maternal BMI(Reference Denison, Price and Graham3). Anaesthetic procedures such as siting an epidural or spinal anaesthetic and performing a general anaesthetic are also more difficult with maternal obesity. It is, therefore, helpful if women, particularly those with Class III obesity (BMI>40 kg/m2), are reviewed by an anaesthetist antenatally and deliver in a medically led delivery suite so that intrapartum problems can be recognised early and managed appropriately with staff who have experience in managing the care of these high-risk women intrapartum.
Table 2 provides a summary of the antenatal care strategies discussed and the level of evidence (defined in Table 3) currently available upon which these strategies are based.
Table 3. Levels of evidence

Summary
The global pandemic presents a major challenge for healthcare providers, and has significant short- and long-term implications for both maternal and fetal health. At present, the evidence-base underpinning many of the interventions either currently in use or recommended to improve pregnancy outcome in obese women is limited. Until the outcomes of ongoing current trials are reported and provide firm evidence base on which to base future intervention strategies and guide evidence-based care for obese pregnant women, pregnancy outcome is best optimised by high-risk antenatal care delivered by healthcare providers who are experienced in supporting these high-risk women.
Acknowledgements
The authors have no conflicts of interest. F.C.D. and C.C. are supported by Tommy's, the Baby Charity, and PiggyBankKids. F.C.D. was the primary author of the manuscript. C.C. contributed to the manuscript.