Perhaps the two most currently emerging and challenging epidemiological trends in developing countries are the ageing of the population and the obesity epidemic(Reference Mokdad, Bowman and Ford1). Over the past 10 years, the population aged 65 years and over in the United States increased from 37⋅2 million in 2006 to 49⋅2 million in 2016 (a 33 % increase) and is projected to almost double to 98 million in 2060. Obesity trends are also rising, and the increasing prevalence of over the next 20–30 years will place a substantial strain on the finances and resources of the United States healthcare system(Reference Ard2). These trends have also been observed worldwide(Reference Malenfant and Batsis3). The prevalence of obesity in combination with sarcopenia, the age-related loss of muscle mass and strength, increases in adults aged 65 years and older(Reference Batsis, Barre and Mackenzie4). A major subset of adults over the age of 65 years is now classified as having sarcopenic obesity, a high-risk geriatric syndrome predominantly observed in an ageing population that is at risk of synergistic complications from both sarcopenia and obesity(Reference Batsis and Villareal5). In the present paper, we will discuss the definition of sarcopenic obesity, as well as the epidemiological and molecular pathways that link to adverse events in clinical populations. Understanding of these pathways will ultimately permit the development of interventions that can meaningfully address this syndrome and its profound implications in a high-risk population of older adults.
Age-related changes in body composition
The ageing process leads to considerable changes in body composition that affect one's physical and biological processes, specifically on the distribution of lean muscle and fat mass. Ageing promotes an adipogenic state from the third decade until the seventh decade due to multiple factors. These factors include a decrease in BMR(Reference Ruggiero, Metter and Melenovsky6), reduced physical activity and other proposed hormonal and inflammatory aetiologies(Reference Visser, Pahor and Taaffe7–Reference Cappola, Xue and Ferrucci9). The combination of these factors leads to increased fat mass deposition and reduction in fat-free mass. While fat mass peaks in the seventh decade, it drops significantly after this time. Such changes also affect the distribution of fat mass in older adults. Ageing leads to a loss of subcutaneous fat (peripherally first and then centrally), an accumulation of visceral fat, and ectopic fat deposition (e.g. muscle, liver and bone marrow)(Reference Cartwright, Tchkonia and Kirkland10). This is partly explained by the reduced capacity for lipid accumulation with age among other unknown aetiologies(Reference Sepe, Tchkonia and Thomou11).
Lean mass peaks in the fourth decade of life and its decline accelerates after the seventh decade(Reference Sayer, Syddall and Martin12). Grip strength follows such a pattern as well. In a large cohort study combining twelve population-based studies of 49 964 participants, males were stronger than females from adolescence onwards, with a peak median grip strength of 51 kg between ages 29 and 39, compared to 31 kg in females between ages 26 and 42(Reference Dodds, Syddall and Cooper13). By age 80, weak grip strength, defined as less than 2⋅5 standard deviations below the sex-specific mean, reached a prevalence of 23 % in males and 27 % in females. In a separate study using data from the Baltimore Longitudinal Study on Aging, muscle quality declined in both arms and legs with age in cross-sectional analyses, using cross-sectional area and fat-free mass(Reference Metter, Lynch and Conwit14). Importantly, the relationship between the muscle quality and age was dependent on how muscle mass was estimated and whether subjects are studied in a cross-sectional or longitudinal study design. This age-related reduction in lean muscle mass and strength(Reference Abizanda, Romero and Sanchez-Jurado15) accounts, in part, for the reduced RMR(Reference Gallagher, Belmonte and Deurenberg16). Skeletal muscle metabolism is a major determinant of resting energy expenditure. Other aetiological factors that cause a decline in RMR include reduced physical activity(Reference Wilson and Morley17), reduced mitochondrial volume and reduced oxidative capacity(Reference Conley, Esselman and Jubrias18,Reference Conley, Jubrias and Esselman19) . Age-related decreases in the components of total energy expenditure (e.g. RMR, thermic effect of food and physical activity) contribute largely to the gradual increase in body fat. The reduction in energy expenditure in ageing is typically not proportionally associated with a reduced drive to eat. The small yearly positive changes in energy balance might lead to weight gain(Reference Doucet, Imbeault and St-Pierre20).
Age-specific physiological decline
The relationship between age and physiological function remains poorly defined and there are no unique physiological markers that reliably predict physiological ageing. This could be due to a variety of confounding genetic and lifestyle factors. The relationship between age and reduced physical activity has been well-established. For instance, the overwhelming majority of older people in the United Kingdom do not meet the minimum physical activity levels needed to maintain health(Reference McPhee, French and Jackson8). The sedentary lifestyles that predominate in older age result in premature onset of ill health, disease and frailty(Reference McPhee, French and Jackson8). The evidence demonstrates that regular physical activity is safe not only for healthy but even for frail older adults(Reference Pahor, Guralnik and Ambrosius21). Furthermore, the risks of developing major cardiovascular and metabolic diseases, obesity, falls, cognitive impairments, osteoporosis and muscular weakness are mitigated by regular exercise. For example, even short-term exercises (e.g. aerobic, resistance, balance and flexibility) have been shown to reduce the risk of falls(Reference Sherrington, Tiedemann and Fairhall22). Regular exercise has also been one of the few interventions to reduce the risk of osteoporotic fractures among older adults(Reference Giangregorio, Papaioannou and Macintyre23,Reference Thornley and Bhandari24) . What is defined as regular exercise includes completing activities ranging from low intensity walking through to more vigorous sports and resistance exercises. Among multiple physiological functions, VO2max was most closely associated with age. However, a high level of variance between the age and VO2max has also been observed(Reference Pollock, Carter and Velloso25). In conjunction with the reduction in type II muscle fibres, their loss leads may promote sarcopenia due to changes in oxidation and glycolytic energy expenditures(Reference Nilwik, Snijders and Leenders26). This is significant as type II muscle fibres are larger in size when compared to type I muscle fibres and thus are able to produce greater and quicker power, although for a shorter period of time, which may be an important consideration for activities of daily living.
Inflammatory and hormonal changes with ageing
The role of inflammation in the ageing process has been extensively studied and multiple pathways have been implicated. Higher plasma concentrations of cytokines such as IL-6 and TNF-α are associated with lower muscle mass and lower muscle strength in well-functioning older men and women(Reference Visser, Pahor and Taaffe7). It is also hypothesised that the rise in the aforementioned cytokines as well as in insulin-like growth factor-1, as often observed in healthy older persons, may contribute to the loss of muscle mass, strength and ultimately to progressive disability and death(Reference Cappola, Xue and Ferrucci9). Batsis outlined the interrelated pathways that link inflammatory and hormonal changes that may promote sarcopenic obesity (Fig. 1)(Reference Batsis and Villareal5). Diet and exercise interventions may alter these inflammatory cytokines and alter the relative ratio of adipokines. Such complex pathways are thought to lead to intramuscular fat deposition that may contribute to the impairment in functional status observed in this population. Importantly, Fig. 2 demonstrates MRI that reflects fat infiltration of one's quadriceps muscle. This deposition is the hallmark of the causative pathways that may lead to sarcopenic obesity.

Fig. 1. (Colour online) A proposed model of mechanisms leading to sarcopenic obesity. The proposed interplay between adipose and muscle tissue, which is believed to contribute to the development of sarcopenic obesity, is shown. The black lines are stimulatory, while red lines with flat ends indicate inhibition. IGF-1, insulin-like growth factor 1(Reference Batsis and Villareal5).
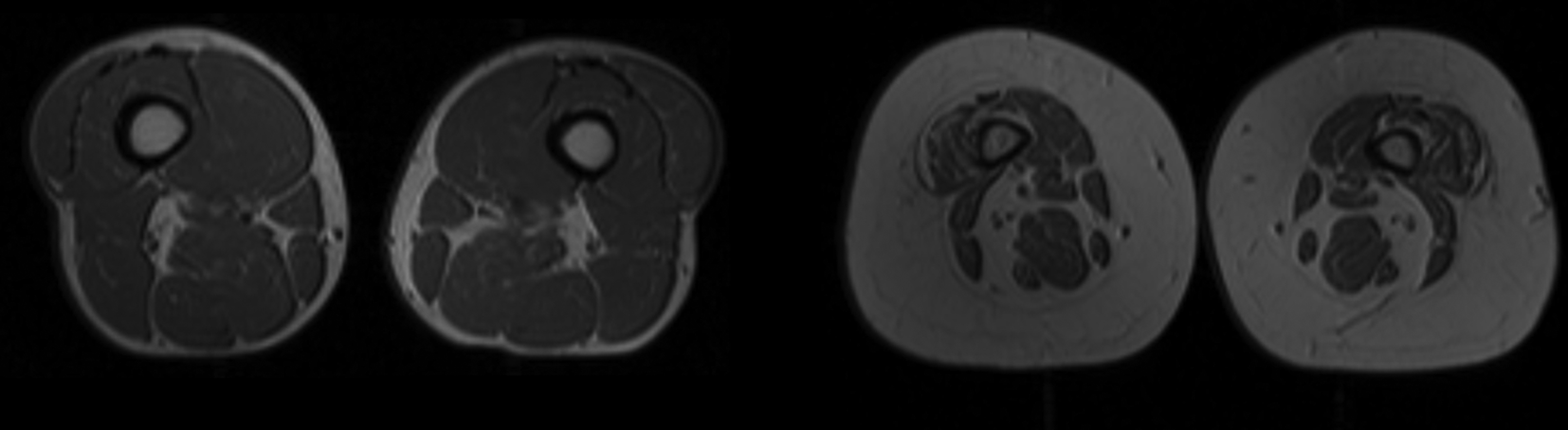
Fig. 2. MRI of individuals with and without obesity. Cross- sectional MRI of the quadriceps area of an individual without obesity with normal muscle characteristics (part a) and an individual with obesity with small muscles and infiltration by adipose tissue (part b) is shown. More muscle tissue is visible in part a than in part b.
Metabolic impairments observed in the InCHIANTI (Invecchiare in Chianti, Aging in the Chianti Area) and National Health and Nutrition Examination Survey (NHANES) datasets have consistently demonstrated the association with high BMI and low muscle strength or mass, and increased levels of the cytokine IL-6 and inflammatory marker, C-reactive protein(Reference Batsis, Mackenzie and Jones27–Reference Schrager, Metter and Simonsick29). Changes in oestrogen, testosterone and growth hormone are also implicated in inflammatory changes that promote phenotypic changes in fat and muscle mass. These hormones decline sharply with age(Reference Schrager, Metter and Simonsick29). Andropause and menopause are well defined with wide implications on ageing. For instance, the effect of oestrogen decreases during menopause whose effect extends past bone. Bone mass decreases with age after menopause in addition to increases in fat mass and decreases in lean mass(Reference Tremollieres, Pouilles and Ribot30). Total and bioavailable testosterone correlate with age and show the greatest decline after the age of 70, and appears to be central in the development of sarcopenia as it increases both muscle mass and activates satellite cells leading to increased muscle function(Reference Morley31). Finally, growth hormone deficiency has been implicated in the loss of muscle mass; however, it is unclear if growth hormone deficiency leads to muscle strength loss. A variety of other hormones as outlined in Fig. 1, including adipokines (adiponectin) and myokines (myostatin) appear to play minor roles in age-related alterations in muscle mass and function.
Ageing and sarcopenia
As our understanding of sarcopenia improves, there is increasing evidence that muscle mass and muscle strength may not be synonymous or causally related. Recent longitudinal and intervention-based studies have clearly demonstrated that muscle atrophy is a relatively small contributor to the loss of muscle strength(Reference Clark and Manini32). More attention has been focused on the subclinical deficits in the structure and function of the nervous system and/or impairments of skeletal muscle to produce effective power as potential antecedents to reduced muscle strength(Reference Carson33). Therefore, ageing affects the muscular strength in more complex ways than previously perceived. More research is underway to further understand the neuromuscular complexity of reduced muscle strength.
Assessment of body composition
Many techniques are available to assess body composition, ranging from simple indirect measures to more sophisticated direct volumetric methods. Some of the more commonly used methods include anthropometry, tracer dilution, dual-energy X-ray absorptiometry, air displacement plethysmography and bioelectrical impedance analysis. Gold standard methods of assessing muscle and fat include computer tomography and MRI. Their precision and accuracy varies, and have been reviewed elsewhere(Reference Kuriyan34). In older adults, dual-energy X-ray absorptiometry scans offer the dual benefit of bone density measurement as well as measurement of visceral fat which can be of high clinical utility. Findings from epidemiological studies over the past 30 years have demonstrated that visceral adipose tissue is an independent risk marker of cardiovascular and metabolic morbidity and mortality(Reference Neeland, Ross and Despres35). This joint position statement from the International Atherosclerosis Society and the International Chair on Cardiometabolic Risk Working Group on Visceral Obesity summarises evidence for visceral adiposity and ectopic fat as emerging risk factors for type 2 diabetes, atherosclerosis and CVD(Reference Neeland, Ross and Despres35).
Commonly used anthropometric measures are often using in the definitions of sarcopenic obesity. However, BMI, while helpful from a population-based standpoint, has many limitations and exhibits poor sensitivity(Reference Batsis, Mackenzie and Bartels36). For instance, as individuals age, vertebral compression result in a reduction in height(Reference Xu, Perera and Medich37), and may affect the BMI ratio. The redistribution of body mass and loss of lean mass described earlier also interferes with the ability of BMI to accurately capture the extent of adiposity.
Assessment of sarcopenia
Muscle mass can be assessed using standard measures of body composition described earlier. Multiple methods can be used to assess muscular strength including grip strength(Reference Bohannon38), sit-to-stand testing(Reference Bohannon39) and short performance physical battery of lower extremity strength(Reference Guralnik, Ferrucci and Simonsick40). Grip strength has the advantage of ease of use in the clinical setting and its validation in prognostication(Reference Rantanen, Masaki and He41). Leg isometric strength has also been studied and is strongly correlated with fat-free mass(Reference Hulens, Vansant and Lysens42). The relationship between the muscle quality and age is dependent on how muscle mass is estimated and on whether subjects are studied cross-sectionally or longitudinally. In addition, creatine may measure a muscle property not accounted by other measures(Reference Metter, Lynch and Conwit14). It has been suggested that creatine (methyl-d3) dilution, may more accurately assess muscle mass(Reference Buehring, Siglinsky and Krueger43). At a cellular level, estimates of total body muscle can be obtained from endogenous metabolites of skeletal muscle, such as creatinine, 3-methylhistidine, urinary creatinine excretion and D3-creatine. D3-creatine is still in the early clinical phase of development, and the issue of normative data is lacking. A critical need to develop such standards to reliably identify homogeneous populations with sarcopenia is needed prior to its routine use in clinical practice and intervention studies(Reference Rubbieri, Mossello and Di Bari44).
Diagnosis of sarcopenic obesity
The diagnosis of sarcopenic obesity is highly debated and complex. In our opinion, the definition consists of separately defining sarcopenia and obesity. While we recognise that these have been highly variable within the literature, we present information that permit classification of participants using a given definition.
The Foundation for the National Institutes of Health Biomarkers Consortium Sarcopenia Project used an evidence-based approach to develop these criteria(Reference Studenski, Peters and Alley45). The final recommended cut-points for weakness established in 2014 are grip strength <26 kg for men and <16 kg for women, and for low lean mass, appendicular lean mass adjusted for BMI <0⋅789 for men and <0⋅512 for women(Reference Studenski, Peters and Alley45). In an updated consensus by the same group, the Sarcopenia Definitions and Outcomes Consortium was formed to develop evidence-based diagnostic cut-points for lean mass and/or muscle strength that identify people at increased risk of mobility disability. The conclusion was that grip strength, absolute or adjusted for BMI, is an important discriminator of mobility disability and other endpoints(Reference Cawthon, Travison and Manini46).
An additional effort to include gait speed in conjunction with grip strength in a clinical algorithm was developed by the European Working Group on Sarcopenia in Older People. This group developed a consensus diagnostic criteria for age-related sarcopenia(Reference Cruz-Jentoft, Baeyens and Bauer47). In 2019, the European Working Group on Sarcopenia in Older People revised the algorithm in which the grip strength or the chair test was used to assess the probability of sarcopenia. The diagnosis was then further confirmed by muscle quality or quantity imaging tests such as dual-energy X-ray absorptiometry, MRI, bioelectrical impedance analysis or computer tomography scan. The severity of sarcopenia was then determined by physical performance testing such as timed up and go test, 400 m walk test, short physical performance battery or gait speed(Reference Cruz-Jentoft, Bahat and Bauer48).
The differences in defining sarcopenia and sarcopenic obesity has led to significant differences in estimating the prevalence of this condition in older adults and markedly hampers the scientific advancement of the field. Prevalence of sarcopenic obesity in older adults varies up to 19–26-fold depending on current research definitions when applied to NHANES 1999–2004 data(Reference Batsis, Barre and Mackenzie4). Despite the large variation in the prevalence of sarcopenic obesity across different obesity definitions(Reference Kim, Yang and Yoo49–Reference Khor, Lim and Tay52), recent evidence suggests that waist circumference as a surrogate for central obesity, is best associated with poorer muscle function(Reference Khor, Lim and Tay52). Grip strength, absolute or adjusted for BMI, is an important discriminator of mobility disability and other endpoints. Additional research is needed to develop a predictive risk model that takes into account sarcopenia components as well as age, sex, race and comorbidities(Reference Cawthon, Travison and Manini46). Unfortunately, the few studies that have evaluated the concordance of definitions demonstrate minimal overlap. Specifically, the concordance rates are 2⋅5 % between the European Working Group on Sarcopenia in Older People and the International Working Group on Sarcopenia, 1⋅8 % between the European Working Group on Sarcopenia in Older People and the Foundation for the National Institutes of Health and 1⋅0 % between the International Working Group on Sarcopenia and Foundation for the National Institutes of Health among community-dwelling older adults in Bavaria, Germany(Reference Kemmler, Teschler and Weissenfels53). An international consensus on an evidence-based definition of sarcopenia is needed and will facilitate more effective research and interventions. Ethnic/racial and country-specific definitions may be necessary that reflect a study population. Alternatively, worldwide consensus aggregating large-scale, longitudinal, epidemiological cohorts to increase sample sizes to ascertain its impact on long-term functional impairment are critically needed.
Epidemiologic of sarcopenic obesity and adverse events
Despite such challenges, epidemiological evidence using well-established datasets has demonstrated a relationship between sarcopenic obesity and impaired long-term outcomes. Both sarcopenia and sarcopenic obesity are related to incident functional decline and disability in cross-sectional and longitudinal studies(Reference Baumgartner, Wayne and Waters54). Cross-sectional data using NHANES 1999–2004 demonstrated significantly higher rates of physical limitations in both males and females(Reference Batsis, Mackenzie and Lopez-Jimenez55). Low muscle strength, defined as the lowest sex-specific tertile of knee extensor strength, and BMI ≥ 30 kg/m2 had significantly lower walking speeds and steeper declines and higher risk of developing new mobility disability over a 6-year follow-up(Reference Stenholm, Alley and Bandinelli56). There may be sex-specific differences in mortality risk in those with sarcopenia and sarcopenic obesity, as older women with sarcopenia have an increased all-cause mortality risk independent of obesity(Reference Batsis, Mackenzie and Barre57). When different body composition measures and muscle strength measures are used to determine the functional decline in older men and women, low muscle mass was not significantly associated with such functional decline(Reference Schaap, Koster and Visser58). Low muscle strength co-existent with obesity, but not muscle mass, was predictive of increased falls risk score in middle-aged and older adults. In clinical settings, muscle function assessments may be useful for predicting falls risk in participants with obesity.
Falls are an important cause of mortality and morbidity in older adults(Reference Tinetti59). In a study of falls risk in middle-aged and older adults, sarcopenic obesity defined used muscle mass had no significant relationship with falls among any categories irrespective of obesity type (global v. central)(Reference Scott, Sanders and Aitken60). In contrast, in the same study, the multivariable linear regression analyses revealed mild but significantly increased falls risk scores for low muscle strength with obesity(Reference Scott, Sanders and Aitken60). In a multiethnic cohort of postmenopausal women, sarcopenic obesity-related fall risk was high in women younger than 65 and those aged 65 and older when defined using body fat percentage greater than 42 %(Reference Follis, Cook and Bea61).
Increasingly, epidemiological studies using the Korean NHANES, the British Regional Heart Study, the English Longitudinal Study of Aging and a Japanese cohort have demonstrated marked associations between the sarcopenic obesity and risk of medical co-morbidity. Using the Korean NHANES, sarcopenic obesity defined using appendicular skeletal mass normalised for body weight below 2 standard deviations and with BMI ≥ 27 kg/m2 demonstrated an OR 3⋅51 (95% CI 2⋅15, 5⋅75) in the development of radiographic knee osteoarthritis(Reference Lee, Kim and Kim62). Depression was strongly associated in a cross-sectional study using appendicular skeletal mass, grip strength and gait speed for sarcopenia, and percent body fat for obesity(Reference Ishii, Chang and Tanaka63). This was also observed longitudinally with low grip strength and obesity defined using BMI ≥ 30 kg/m2, with an OR 1⋅79 (95% CI 1⋅10, 2⋅89) over 6 year follow-up(Reference Hamer, Batty and Kivimaki64). Psychological stress was also highest in individuals with sarcopenic obesity, with a higher degree of psychological health (OR 1⋅79 (95% CI 1⋅10, 2⋅89)) and higher stress (OR 6⋅05 (95% CI 1⋅89, 19⋅38))(Reference Cho, Shin and Shin65). Lastly, the risk of type 2 diabetes was markedly higher (hazard ratio (HR) 3⋅57 (95% CI 2⋅04, 6⋅24)) in the English Longitudinal Study of Aging in individuals with sarcopenic obesity defined using grip strength and BMI(Reference Cuthbertson, Bell and Ng66).
Sarcopenia and visceral obesity have been suggested to aggravate each other, resulting in a vicious, bi-directional cycle. In a study of 379 Korean men and women (mean age 51⋅9 (sd 14⋅6) years) from the Korean Sarcopenic Obesity Study, visceral fat area was an independent negative predictor of the changes in appendicular lean soft tissue after adjusting for confounders(Reference Kim, Park and Ryu67). Participants with visceral obesity and low muscle strength had significantly higher risk of mortality, worsening disability and hospitalisation(Reference Rossi, Bianchi and Volpato68).
Both obesity and low handgrip strength, independent of each other, predict the risk of death in adult men and women with additive pattern. The predictive value of obesity varies by age, whereas low muscle strength predicts mortality in all age groups aged >50 years and across all BMI categories. Data from adults between ages 50 and 91 years in a Finnish cohort demonstrated that the highest mortality was among those with low handgrip strength and obesity (HR 1⋅23 (1⋅04–1⋅46))(Reference Stenholm, Mehta and Elo69). The NHANES III data demonstrated sex-specific differences in mortality using body fat and muscle mass cut-offs(Reference Batsis, Mackenzie and Barre57,Reference Batsis, Mackenzie and Emeny70) . A meta-analysis of all sarcopenic obesity definitions demonstrated differential mortality estimates for muscle mass (HR 1⋅06 (95% CI 0⋅91, 1⋅22)) as compared to muscle strength (HR 1⋅23 (95% CI 1⋅09, 1⋅38))(Reference Tian and Xu71). When promoting health among older adults, more attention should be paid to physical fitness in addition to body weight and adiposity(Reference Stenholm, Mehta and Elo69). The LIFE study is a key study that reduced mobility disability in older adults through a walking and resistance programme(Reference Pahor, Guralnik and Ambrosius21). Other health promotion studies have demonstrated the importance of physical fitness as a surrogate for better health, yet future agreement among experts is needed before implementation into clinical practice(Reference Kitzman, Brubaker and Morgan72–Reference Sui, LaMonte and Laditka75). These are important effects that go beyond cardiometabolic factors and changes in cognition(Reference Edwards and Loprinzi76–Reference Rheaume, Arsenault and Dumas78).
What did the epidemiological data show us?
Long-term associations observed in the epidemiological literature are strongly related to definitions of sarcopenia and obesity. Despite the lack of consensus on the definition of sarcopenic obesity, the detrimental clinical implications of this syndrome are unequivocal. Muscle strength is likely a stronger predictor of decline than muscle mass. This correlation can put more emphasis on the clinical screening for sarcopenic obesity with simple tests such as grip strength. The synergy of low muscle mass/strength and obesity (body fat or elevated BMI) is associated with significantly higher morbidity/mortality than either one alone. Therefore, treatments should address both obesity and reduced muscle mass/strength.
Energy restriction
Energy restriction is the hallmark of any intentional weight loss programme. Energy restriction triggers a complex series of intricate events, including activation of cellular stress response elements, improved autophagy, modification of apoptosis and alteration in hormonal balance(Reference Golbidi, Daiber and Korac79). Energy restriction aids with healthy ageing through weight loss. In the CALERIE (Comprehensive Assessment of Long term Effects of Reducing Intake of Energy) study, weight-loss was strongly associated with improvements in VO2 and knee strength at 2-year follow-up(Reference Racette, Rochon and Uhrich74). Conversely, energy restriction on its own, which in most cases can entail a 20–40 % reduction of food consumption relative to normal intake, is a significant intervention that can also result in detrimental effects(Reference Lee and Longo80). Caution should be exercised with energy restriction to avoid loss of lean muscle mass and strength. Generally, loss of weight is ¼ muscle and ¾ fat(Reference Heymsfield, Gonzalez and Shen81). Combining energy restriction with aerobic and resistance exercises can mitigate this potential detrimental effect and can provide greater improvement in physical function(Reference Villareal, Chode and Parimi82). Also of concern during weight-loss efforts is loss of bone density which, without a resistance exercise component, can result in a decrease in hip bone density; resistance exercises can potentially lead to the corresponding increased hip bone mineral density(Reference Soltani, Hunter and Kazemi83).
Aerobic and resistance exercise
Multiple modalities have investigated the effects of resistance training, aerobic training or combination training on sarcopenic obesity. Older adults with sarcopenic obesity who engaged in the resistance training, aerobic training and combination training demonstrated increased muscle mass and reduced total fat mass and visceral fat area compared with those without training(Reference Chen, Chung and Chen84). The muscle strength performance and serum insulin-like growth factor-1 level improvements were most pronounced in a resistance/aerobic training group over an 8-week period. Compared with resistance training alone, protein supplementation combined with resistance training may have a stronger effect in preventing ageing-related muscle mass attenuation and leg strength loss in older people(Reference Liao, Tsauo and Wu85).
The improvement in VO2max and other marker has been shown to be possible in older adults using exercise programmes to improve measures of physical function and preclinical disability with impairments in physical performance(Reference Binder, Schechtman and Ehsani86). Binder et al. randomised 150 older adults (mean age 83 (sd 4) years) with mild to moderate frailty who participated in a 9-month low-intensity home exercise v. an exercise-training programme. The latter programme had improvements of 1⋅0 v. 5⋅2 points for the modified physical performance score, 0⋅9–3⋅6 ml/kg/min for VO2 peak, and 1⋅6–4⋅9 points for the functional status questionnaire. The aforementioned findings and increasingly emerging evidence confirm the continued need to advocate for more physical activity in the elderly.
Aerobic and resistance exercise, but not weight-loss, though, has a marked effect on the molecular and cellular level of the type II muscle fibres. Significant reductions in markers of muscle inflammation and anabolism, including Toll-like receptor 4, insulin growth factors and type II fibre sizes were observed with exercise in frail older adults with obesity(Reference Nilwik, Snijders and Leenders26,Reference Lambert, Wright and Finck87) . Yet, a combined aerobic/resistance programme was found to be more effective than either alone in improving muscle protein synthesis and myocellular quality during weight-loss interventions(Reference Colleluori, Aguirre and Phadnis88).
Energy restriction with exercise
Few studies specifically evaluate individuals with sarcopenic obesity. A randomised controlled trial in older female participants (aged 70 years and over) with sarcopenic obesity were enrolled in a 3-month study of a 60-min exercise class twice weekly, nutrition (consisting of essential amino acid supplementation and tea fortified with catechins), both, or health education classes. The combined intervention led to significant declines of total body fat (OR 4⋅42 (95% CI 1⋅21, 16⋅19)) and improved walking speed (OR 3⋅05 (95% CI 1⋅01, 9⋅19))(Reference Kim, Kim and Kojima89). A pivotal trial by Villareal et al. randomised four groups (aerobic, resistance, combined and control) and found that the combined aerobic/resistance group had a mean 9 % weight-loss, but marked improvements of 21 % v. 14 % in the aerobic or resistance groups in physical performance status(Reference Villareal, Aguirre and Gurney90). Changes were also observed in undercarboxylated osteocalcin, sclerostin and improvements in pancreatic insulin secretion(Reference Colleluori, Napoli and Phadnis91). Although, weight loss from lifestyle interventions results in significant decreases in total and free oestradiol levels in frail, older men with obesity, this did not result in a clinically important increase in total testosterone nor a significant increase in free testosterone. The combination of diet and exercise in frail, older adults with obesity leads to increases in testosterone and reduced oestradiol(Reference Armamento-Villareal, Aguirre and Qualls92). The implications of these findings are currently unclear and require further investigation.
Conclusions
Sarcopenic obesity is prevalent and increases the risk of decline in the older adults. We propose a shift to translate research-based findings to clinical environments from muscle mass to muscle strength. Sarcopenic obesity has detrimental effects on quality of life, independence and mortality. We advocate a focus on physical function and quality of life. Many evidence-based interventions involve dietary and changes in physical activity. Gaining an understanding of the underlying behavioural and biological mechanisms is critical in targeting interventions. More research is needed to harmonise the definitions and clarify mechanisms contributing to this geriatric syndrome which could then lead to personalised targeting of interventions to the individual participant.
Financial Support
Dr Batsis receives funding from the National Institute on Aging of the National Institutes of Health under Award Number K23AG051681. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interests
None.
Authorship
Both Drs. El Bizri and Batsis were involved in the design, concept, draft and approval of the paper.





