Gastrointestinal cancers
The term gastrointestinal cancer is used collectively to refer to cancers of the digestive tract and includes oesophageal, liver, gastric, gallbladder and biliary tract, pancreatic, gastrointestinal stromal tumours, small bowel, colorectal and anal cancers. Colorectal cancer is the most common being the fourth most common cancer in the UK accounting for 42 000 new cases every year, 12 % of all new cancers. Some gastrointestinal cancers, for example, gall bladder cancer are rare and account for 1000 new cases in the UK every year, accounting for less than 1 % of all new cancers. Diet and lifestyle plays a role in the aetiology of gastrointestinal cancers with increased body weight and lack of physical activity increasing risk. Specific components of diet also play a role, for example red and processed meat and alcohol increasing the risk of colorectal cancer whilst dairy foods and dietary fibre are protective(1).
Survival rates vary considerably between diagnoses with the best survival in colorectal cancer and anal cancer. In these diagnoses, 5 year survival is 54 and 57 % respectively for all stages of cancer. If cancer is diagnosed at an early stage then survival rates are higher, for example, 5 year survival for stage 1 colorectal cancer is 98 % and for stage 4 is 44 %. Oesophageal and pancreatic cancer patients have the worst outcomes with 12–13 and 1 % respectively being predicted to survive their disease for 10 years or more(2).
Early detection is therefore paramount in improving outcomes for people with cancer. Planned treatment pathways will vary depending on the stage of the disease with surgery being used more often in early stage disease.
Gastrointestinal cancer may be detected either by routine screening, for example, in the case of colorectal cancer or due to the presentation of local or systemic symptoms resulting in the person presenting to their general practitioner or to emergency healthcare. Unintentional weight loss, dysphagia, anorexia, early satiety and changes in bowel habits may be presenting features of the disease along with other less-specific symptoms including pain, fatigue or anaemia. In the UK, some of these may be identified as red-flag symptoms necessitating an urgent referral for suspected cancer under the ‘two week’ rule.
Treatment plans are formulated following appropriate investigations which allow the extent of the disease to be assessed. A multi-disciplinary team recommends the most appropriate treatment plan based on the staging and assessment which will either be potentially curative or aimed at extending life if the disease is deemed to not be curable. Treatments used are often multi-modal and may include a combination of systemic anti-cancer therapy (SACT), radiotherapy, surgery and more recently targeted treatments in the form of immunotherapy. An individualised treatment plan is developed and discussed with the person based on the knowledge of the most effective treatment, an assessment of the extent of the disease, co-morbidities and performance status (PS). In the UK, the recommendations for treatment are described in guidance produced by the National Institute for Health and Care Excellence(3).
Impact of gastrointestinal cancer on nutritional status
People with gastrointestinal cancer have a high rate of malnutrition compared to other types of cancers(Reference Bozzetti4). Upper gastrointestinal cancers pose the highest risk to the development of malnutrition and studies have identified that 22 % may be severely malnourished and 63 % being moderately malnourished or at risk of malnutrition. Lower gastrointestinal cancers pose a lower risk with 10–17 % being severely malnourished and 25–60 % being moderately malnourished or at risk of malnutrition(Reference Burden, Hill and Shaffer5).
The presence of cancer may influence nutritional status through a number of mechanisms. These can be due to changes in dietary intake due to a lack of appetite, physical obstruction of the gastrointestinal tract causing dysphagia or early satiety, changes in taste perception and the effect of the tumour on bowel symptoms. Psychological distress and anxiety are also instrumental in influencing food intake(Reference Wheelwright, Hopkinson and Darlington6). The resulting impact on dietary intake may be an overall reduction in food intake or an alteration in the habitual intake, both of which can influence the overall quantity of diet consumed or the quality of the diet. Additional symptoms such as gastrointestinal bleeding may influence particular nutrient status, for example iron, folate or vitamin B12(Reference Stein, Connor and Virgin7).
Changes in physical activity because of symptoms, such as fatigue, in addition to metabolic changes facilitated through inflammatory pathways, can impact body composition causing loss of lean muscle and adipose(Reference Cruz-Jentoft, Bahat and Bauer8). The resulting syndromes of cachexia and sarcopenia and their impact on the patient, tolerance to treatment and clinical outcomes are discussed in more detail.
Identification of the presence of disease-related malnutrition or the risk of malnutrition should initially be through the use of thorough and systematic nutrition screening. Nutrition screening is mandated by the National Institute for Health and Care Excellence and should be used for all people with a cancer diagnosis as early as possible(9). It should be undertaken during the process of investigations, particularly if the person is presenting with nutrition impact symptoms or unintentional weight loss. A nutrition screening tool that has been validated in cancer should be used as these have been demonstrated to have better sensitivity than generic tools(Reference Zhang, Pang and Sharma10,Reference Shaw, Fleuret and Pickard11) .
Identifying this early is crucial to ensure that they can be referred for appropriate advice and support from a registered dietitian as required(Reference Bozzetti4,12) . Often referrals to a dietitian are late in the treatment pathway making optimal management of dietary intake more challenging(Reference Lorton, Griffin and Higgins13). Nutrition impact symptoms are a significant barrier to dietary intake and patients often experience more than one symptom. In a cohort of patients referred for dietary advice, of which half were gastrointestinal or biliary cancer patients, 70 % had two nutritional barriers. The most common cited were anorexia, nausea and early satiety(Reference Lorton, Griffin and Higgins13). An assessment by the dietitian as to whether the patient would have benefited from an earlier referral identified that referrals should have occurred earlier in nearly half of cases.
Cancer cachexia
Cancer cachexia is a syndrome characterised by weight loss, loss of muscle and fat mass. It contributes to impaired function, decreased physical performance, decreased tolerance to cancer treatment and poorer survival(Reference Martin, Senesse and Gioulbasanis14). The contributory factors are reduced dietary intake, weight loss and metabolic changes which create a proinflammatory environment that may drive an increased energy expenditure(Reference Dunne, Loh and Williams15,Reference Fearon, Strasser and Anker16) . Systemic inflammation is further exacerbated by proinflammatory cytokines produced by the tumour. Tumour-derived cytokines including IL-1, IL-6 and TNF-α act as signals resulting in the disruption of metabolism of carbohydrate, fat and protein(Reference Arends, Baracos and Bertz17).
A consensus view on the definition of cachexia was published by Professor Ken Fearon and this identified that there are potential three stages of cachexia, that is precachexia, cachexia and refractory cachexia(Reference Fearon, Strasser and Anker16) (Fig. 1). Each stage is defined by varying degrees of weight loss, anorexia and metabolic changes which include reduced albumin and raised markers of systemic inflammation, for example C-reactive protein. There is no universal consensus on the definition of cancer cachexia and more recent studies aiming to classify cancer cachexia have used a scoring system using additional assessments including weight loss, a screening questionnaire for sarcopenia (SARC-F), appetite loss and abnormal biochemistry(Reference Martin, Senesse and Gioulbasanis14,Reference Zhou, Wang and Liu18) .
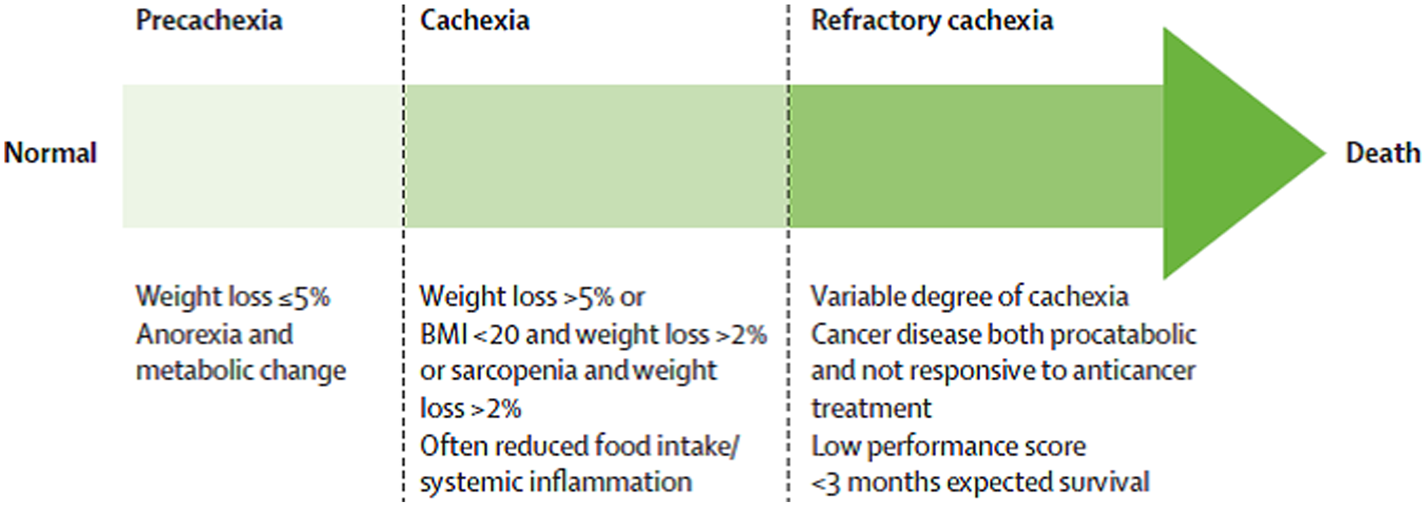
Fig. 1. Stages of cancer cachexia(Reference Fearon, Strasser and Anker16).
Documentation of PS is common in cancer care. It is a score that estimates the patient's ability to perform certain activities of daily living(Reference West19). People who have a worse PS and limited functional capacity tend to have more difficulty tolerating rigorous cancer treatments and have less favourable outcomes than those with a better PS. Formal assessment and categorising of cancer cachexia, which is also associated with poorer outcomes, is rare in the clinical setting.
Sarcopenia
The definition of sarcopenia is low muscle strength, low muscle quantity or quality and low physical performance(Reference Cruz-Jentoft, Bahat and Bauer20). Sarcopenia is part of the ageing process and has been well categorised in older people and its presence increases the risk of falls, fractures, physical disability and mortality(Reference Cruz-Jentoft, Bahat and Bauer8). Studies have recognised that sarcopenia may begin earlier in life and there is overlap between the physiological changes that occur in cancer cachexia and sarcopenia(Reference Ryan, Power and Daly21).
The European Working Group on Sarcopenia in Older People recommends the use of SARC-F questionnaire to elicit self-reports from patients on indicators that are characteristic of sarcopenia(Reference Cruz-Jentoft, Bahat and Bauer20). The self-report questionnaire asks about limitations in strength, walking ability, rising from a chair, stair climbing and experience with falls. Additional tests such as hand grip strength, chair stand test may be used to assess strength and muscle mass may be assessed using dual-energy X-ray absorptiometry, bioelectrical impedance analysis, whole body MRI or lumbar muscle cross-sectional area by computed tomography. Tests for physical performance may include gait speed and the timed-up-and-go test.
Impact of cachexia and sarcopenia on clinical outcomes
Cachexia and sarcopenia have a negative clinical impact on outcomes in cancer(Reference Naumann, Eberlein and Farnia22). A systematic grading system of percentage of involuntary weight loss and BMI developed by Martin et al. demonstrated that both of these factors predicted survival independently of conventional prognostic factors including cancer site, stage and PS(Reference Martin, Senesse and Gioulbasanis23). Observational studies that have linked unintentional weight loss with poorer outcomes during SACT have suggested that this may be due to reduced dose of drugs which is based on body weight and surface area estimations and increase toxicity of drugs requiring breaks in treatment(Reference Andreyev, Norman and Oates24). The consequences of cancer cachexia are both clinical and financial. They include reduced immune competence with increased risk of infections, psychosocial stress, lower quality of life, treatment toxicity and greater risk of mortality(Reference Arends, Baracos and Bertz17). Financial implications include higher costs of care and increased length of hospital stay(Reference Arends, Baracos and Bertz17).
The presence of sarcopenia has been shown to confer poorer outcomes in both surgical gastrointestinal cancer patients and those receiving SACT(Reference Peng, Hyder and Firoozmand25–Reference Tan, Brammer and Randhawa27). Reduced muscle mass, as measured by total psoas area from cross-sectional imaging, was identified as an independent risk factor for mortality following pancreatic resection for adenocarcinoma(Reference Peng, Hyder and Firoozmand25). Studies of the impact of sarcopenia in different cancer diagnoses have indicated varying results. Some have indicated that the loss of muscle mass influences tolerance to SACT with low muscle mass, in people with normal or increased body weight, increases the risk of toxicity often resulting in dose reduction of drugs(Reference Prado, Lieffers and McCargar28,Reference van den Berg, Kok and Posthuma29) . Other studies have suggested that myosteatosis, fatty infiltration of muscle, defined as attenuated mean skeletal muscle Hounsfield units observed on computed tomography scanning, has a greater bearing on survival rather than reduced muscle mass alone(Reference Rollins, Tewari and Ackner26). These observational studies have raised important questions as to whether interventions can reverse or prevent such changes and what effect this may have on clinical outcomes.
Management of cachexia by drugs or nutrients has yet to produce effective solutions(Reference Arends, Bachmann and Baracos30). Approaches have included appetite stimulants such as progestins, corticosteroids and cannabinoids, drugs and nutrients to influence body weight and the inflammatory response in cachexia including non-steroidal anti-inflammatory drugs and fish oils high in n-3 fatty acids along with nutrients such as specific amino acids aimed at increasing fat-free mass.
Diet following diagnosis of cancer
Optimisation of nutritional status before, during and after cancer treatment is recommended with the aim of improving tolerance to treatment and improving quality of life(Reference Arends, Bachmann and Baracos30). The dietary advice or suitable nutritional support will vary according to the patient, their nutritional status, nutrition impact symptoms, planned treatment, toxicity of treatment, co-morbidities and PS. These factors should be assessed at the beginning of treatment and at regular intervals through the treatment pathway to ensure that nutrition impact symptoms are well managed and nutritional needs addressed accordingly.
Prehabilitation
Prehabilitation is the optimisation of the individual through diet, exercise, symptom management, medicines management and optimal management of co-morbidities prior to cancer treatment. It requires expert screening and intervention along with appropriate support to enable people to change their behaviour with respect to diet and exercise(Reference Ferreira, Agnihotram and Bergdahl31). Early studies have demonstrated that this approach confers benefit in some patients in terms of improvements in post-operative body composition, more rapid recovery following surgery and an improved quality of life(32–Reference Gillis, Fenton and Sajobi35). Pooled data from three prehabilitation trials in colorectal cancer patients detected an improvement in 5-year disease-free survival in those with stage 3 disease(Reference Trepanier, Minnella and Paradis36). The author of the present paper calls for further trials to confirm this which is also supported by publication of principles and guidance for prehabilitation within the management and support of people with cancer(32). A systematic review of any prehabilitation, nutrition only or nutrition with exercise, significantly shortened length of hospital stay by 2 d after colorectal surgery(Reference Gillis, Buhler and Bresee37). Currently there are varying definitions of what constitutes a prehabilitation intervention; however, the recent review by Gillis et al. suggested that it should be at a minimum defined by its nutritional and exercise components with nutrition adding complementary and functional benefits for the colorectal patient(Reference Gillis, Buhler and Bresee37).
Dietary advice and nutritional support during treatment
The importance of identifying the risk of disease-related malnutrition has been identified in European guidance(Reference Arends, Baracos and Bertz17,Reference Arends, Bachmann and Baracos30) . Regular screening through treatment should feed into a robust system that gives the person access to an appropriate nutritional assessment and management plan. A full nutritional assessment should be carried out by a registered dietitian with the relevant expertise in oncology. This should include a full assessment of weight, height, weight history, measures of body composition and energy expenditure, nutrition impact symptoms including appetite, biochemical parameters including cancer-related systemic inflammation such as C-reactive protein and albumin and physical function(Reference Arends, Bachmann and Baracos30). Additional factors which may impact the advice given include psychosocial factors, co-morbidities and anticipated treatment. An important role of the registered dietitian may be to address questions about nutrition and dispel some of the myths that arise from testimonials and opinions, particularly those voiced on social media. Such dietary regimens may include changes in macronutrient intake, for example restriction of sugar intake, or promotion of individual foods or dietary supplements with the aim of specifically influencing the growth of cancer. These dietary changes have no proven efficacy and may increase the risk of malnutrition through the restriction of macronutrient or micronutrient intake(Reference Arends, Bachmann and Baracos30). Their use should be discussed along with the use of any dietary supplements that can potentially interact with cancer treatment.
The optimal time for initiating nutrition support has yet to be defined, however, the consensus view is that this should occur prior to malnutrition developing and ideally should be in the pre-cachexia stage(Reference Fearon, Strasser and Anker16,Reference Martin, Senesse and Gioulbasanis23) .
In pre-cachexia and cachexia nutritional support aims to lower or alleviate the burden of cachexia by the provision of adequate nutrients. European Society for Clinical Nutrition and Metabolism guidelines recommend the measurement of resting energy expenditure through the use of indirect calorimetry for all cancer patients(Reference Arends, Baracos and Bertz17). In clinical practice, this may not be possible and in the absence of this measurement nutritional requirements can be estimated as 105–126 kJ/kg/d with 1⋅2–1⋅5 g protein/kg/d(Reference Arends, Baracos and Bertz17) as a target.
Dietary advice is individualised to take into account personal preferences, nutrition impact symptoms, avoidance of foods which confer a high risk of food borne pathogens and ability to cook and shop whilst experiencing symptoms such as fatigue. Attention should be given to individual symptoms that are influencing food intake, particularly dysphagia, anorexia, taste changes and mucositis. Dietary advice encompasses food and fluid choice, fortification of food, meal pattern, snacks and advice to help with symptom management. Oral nutritional supplements may be used to help meet requirements when dietary advice alone is ineffective. Nutritional supplementation has been demonstrated to be effective at improving dietary intake and body weight when used in conjunction with dietary counselling in malnourished patients, as described in a systematic review published by Baldwin and Weekes(Reference Baldwin and Weekes38). Individualised dietary advice during radiotherapy treatment for colorectal cancer was demonstrated to have a positive effect on late radiation toxicity and mortality in a group of normally nourished colorectal patients(Reference Ravasco, Monteiro-Grillo and Camilo39).
Artificial nutrition support
Artificial nutrition support may be considered when estimated requirements are unable to be met with dietary intake alone. In practice, for those people receiving SACT, it is likely that their dietary intake will be highly variable on a daily basis due to the side effects of treatment resulting in short-term fluctuations in nutritional status. Overall an aim to resume dietary intake and regain any lost weight prior to the next course of SACT would be optimal. Consideration of artificial nutrition support should be made when a person has a deterioration in nutritional status despite dietary advice and oral nutritional supplements or where it is anticipated that they will be unable to meet requirements. The use of nutritional support have been shown to improve body weight and energy intake but not survival(Reference Arends, Bachmann and Baracos30). European guidance does not recommend the use of routine artificial nutrition support for people undergoing SACT or radiotherapy(Reference Arends, Bachmann and Baracos30). In the case of surgery when it is anticipated that dietary intake will not resume within a period of up to 5 d then appropriate nutritional support should be planned and implemented to prevent a deterioration in the nutritional status. Enteral nutrition should be instigated as the first choice with parenteral nutrition being used when the gastrointestinal tract is not accessible or in cases of intestinal failure(12,Reference Arends, Bachmann and Baracos30) .
Refractory cachexia
The term refractory cachexia is used to characterise patients with a low PS, who are not responsive to anticancer treatments, and have an expected survival time of less than 3 months(Reference Fearon, Strasser and Anker16,Reference Zhou, Wang and Liu18) .
The use of artificial nutrition support in advanced gastrointestinal cancer can be controversial. If SACT is being offered to the patient, even though the intention of treatment is palliative rather than curative, then ongoing screening and assessment of nutrition should be provided as part of holistic care. If nutritional status deteriorates then this should be discussed with the patient and within the multi-disciplinary setting to ascertain whether artificial nutritional support is acceptable and will be thought to be beneficial(Reference Druml, Ballmer and Druml40). As artificial nutrition support is a medical intervention it therefore requires an indication for achieving a treatment goal and the informed consent of the competent patient(Reference Druml, Ballmer and Druml40). Cancer cachexia may progress with advancing disease to become refractory cachexia(Reference Fearon, Strasser and Anker16). It is in this advanced state that the provision of nutrition is unable to reverse the changes in body composition despite the provision of adequate energy, protein and micronutrients.
Nutrient ingestion and digestion
Gastrointestinal cancer may have a significant impact on the ingestion and digestion of nutrients in the diet. Of particular influence are the upper gastrointestinal cancers where oesophageal and gastric cancer may impact the ability to eat and drink. This is managed with the use of texture modification and fluids to ensure nutritional adequacy of the diet. Artificial nutrition support may be required if nutritional status has deteriorated or nutritional requirements cannot be met through oral intake. Gastrointestinal cancers or adhesions from previous surgery may influence gastrointestinal function. Malignant bowel obstruction has a profound effect on the ability to eat, drink and digest nutrients. Management depends on the site of the obstruction, potential treatments including surgery, stenting and SACT(Reference Franke, Iqbal and Starr41). Adequate nutrition continues to be a goal of treatment and may require the use of parenteral nutrition if the gastrointestinal tract is not functioning. A deterioration in nutritional status is associated with prolonged hospitalisation, reduced tolerance to treatment and poor overall survival(Reference Franke, Iqbal and Starr41).
Pancreatic cancer can be a cause of steatorrhoea or diarrhoea, abdominal pain, bloating and weight loss through the systemic effects on reduced appetite in addition to the specific influence on pancreatic exocrine function. Digestion requires pancreatic stimulation, synthesis and release of pancreatic enzymes and the synchronisation of secretions to mix with ingested food(Reference Nikfarjam, Wilson and Smith42). The presence of cancer in the pancreas may disrupt the delivery of pancreatic enzymes into the lumen of the gut. Alternatively, surgery to the upper gastrointestinal tract may influence small intestinal function resulting in poor synchronisation of enzymes and food.
Nutrient absorption
Some types of gastrointestinal cancer can influence nutrient absorption necessitating specific medical or nutritional support. Nutrients necessary for the formation of erythrocytes are subject to high losses, for example, when bleeding occurs within the gastrointestinal tract. Absorption of iron may be reduced when gastric acid is suppressed following partial gastrectomy or through the use of acid suppressing drugs(Reference Stein, Connor and Virgin7,Reference Kim and Kim43,Reference Lam, Schneider and Quesenberry44) . Vitamin B12 absorption may be influenced by loss of intrinsic factor due to the presence of disease or removal of the stomach as part of the treatment of gastric cancer(Reference Kornerup, Hvas and Abild45,Reference Dawson, Sawers and Sharma46) . Removal of any part of the small intestine or formation of a stoma, for example, an ileostomy may influence the ability to absorb macronutrients, electrolytes, fluids and trace elements(Reference Pironi, Arends and Bozzetti47). This can be due to loss of total surface area of the gastrointestinal tract that is available for absorption or the effect on a specific site of the gut such as the terminal ileum responsible for the absorption of vitamin B12 and the reabsorption of bile acids or may be due to increased losses through the stoma site. Optimal nutritional and fluid management requires a multi-professional team approach including gastroenterology and dietetics along with an assessment of the residual part of functioning bowel(Reference Mountford, Manas and Thompson48). Continued SACT, immunotherapy or radiotherapy may further exacerbate nutritional losses through toxicities affecting the integrity of the gut or gut motility.
Dietary management of gastrointestinal toxicity of cancer treatment has been a subject of debate for many years. Transient malabsorption of specific nutrients such as lactose as a result of chemotherapy was identified in the 1990s yet no intervention studies have confirmed that its restriction is effective at influencing symptoms(Reference Parnes, Fung and Schiffer49,Reference Andreyev, Ross and Donnellan50) . Pelvic radiotherapy causes damage to non-cancer gastrointestinal mucosa and has an effect on secretory and absorptive functions(Reference Wedlake, Shaw and Whelan51,Reference Andreyev52) . A review of dietary interventions to manage symptoms highlighted weak evidence for some dietary changes including elemental, low or modified fat and low lactose interventions(Reference Wedlake53). The use of probiotics was more promising with four out of five studies suggesting that some symptoms benefit from their use although variations in the strength and strains and study methodologies make firm recommendations difficult. It is also important that there are additional data on the safety of probiotic use in people receiving SACT and immunotherapy. Higher intakes of dietary fibre intake during pelvic radiotherapy may impact the severity of acute and long-term symptoms(Reference Wedlake, Shaw and McNair54). The introduction of immunotherapy has created a different spectrum of toxicities with colitis being a significant feature which impacts dietary intake(Reference Haanen, Carbonnel and Robert55). No specific dietary interventions are known to be effective for these and the medical management is often the use of corticosteroids increasing the risk of steroid induced diabetes. Initial recommendations based on professional consensus suggest some dietary modifications in the initial stages to assist in symptom management, however, these have not been tested in the clinical setting(Reference Haanen, Carbonnel and Robert55). There is much interest in the link between the gut microbiome and the efficacy of immunotherapies in cancer treatment(Reference Helmink, Khan and Hermann56). The underlying mechanisms of interactions are poorly understood but may, in the future, provide a means of influencing the response to treatment.
Diet after treatment for gastrointestinal cancer
People who have completed treatment for cancer have significant health concerns compared to the general population(Reference Tan, Turner and Kerin-Ayres57). This is generally centred on management of symptoms following treatment including fatigue, insomnia, anxiety and concern about the risk of recurrence. Health and well-being events aim to provide information for people to help them manage symptoms, consider lifestyle behaviours including diet and physical activity and manage psychological aspects of living with a diagnosis of cancer. Diet may continue to be a focus through management of malnutrition, symptom management or being considered along with lifestyle with the aim of influencing recurrence. Intervention studies to date in people following treatment for gastrointestinal cancer have failed to demonstrate that changes to body composition, dietary intake or physical activity can influence the risk of recurrence or overall survival(Reference Aubrey, Hon and Shaw58). Observational studies have suggested that body increased waist circumference, higher BMI and low adherence to the World Cancer Research Fund; Dietary Guidelines for Primary Prevention are associated with poorer global health status in people following treatment for colorectal cancer(Reference Vissers, Martucci and Mols59,Reference van Veen, Mols and Bours60) . Ongoing dietary intervention studies aim to provide stronger guidance for the optimal lifestyle, including dietary changes that should be adopted at the end of treatment. Advice should be tailored to the individual depending on their diagnosis, stage of disease, any future long-term treatment, nutritional status and growing evidence base.
Conclusion
Diet has an integral role in the development and management of gastrointestinal cancer. Its role in aetiology is potentially through specific dietary components such as dietary fibre, dairy products, red and processed meat and alcohol or the overall influence on body composition, body weight and obesity.
Following diagnosis of cancer, dietary intake, along with metabolic changes can contribute to the development of cancer cachexia and sarcopenia. These changes can influence body composition with accelerated loss of body weight, muscle mass and changes in physical function. Such changes can negatively impact PS, tolerance to cancer treatment and quality of life. European and UK recommendations highlight the importance of nutrition screening about the time of diagnosis, especially for people who are experiencing nutrition impact symptoms and weight loss. Once nutrition risk has been identified then a full nutrition assessment from a registered dietitian with a nutrition care plan should be developed alongside medical management and planned cancer treatment. Increasingly the optimisation of people through diet and exercise, known as prehabilitation, is recommended before cancer treatment commences although the evidence base to demonstrate improved outcomes needs to be strengthened. Treatment for gastrointestinal cancer can have a major impact on dietary intake and absorption of nutrients and requires appropriate nutritional support alongside optimal symptom management.
Appropriate individualised lifestyle advice on diet and exercise at the end of treatment should be offered to optimise health or to assist in the management of gastrointestinal late effects of treatment.
Financial Support
Clare Shaw is partially funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at The Royal Marsden NHS Foundation Trust and the Institute of Cancer Research, London.
The views expressed are those of the author and not necessarily those of the NIHR or the Department of Health and Social Care.
Conflict of Interest
None.
Authorship
The author had sole responsibility for all aspects of preparation of this paper.




