- EVM
Expert Vitamin and Mineral Group
- LOAEL
lowest observed adverse effect level
- MPL
maximum permitted levels
- MSL
maximum safe levels
- NOAEL
no observed adverse effect level
- PSI
population safety index
- UL
upper levels
Food supplements are defined as concentrated sources of nutrients or other substances (e.g. fish oils) with a physiological or nutritional effect, marketed in dose forms such as tablets, capsules, powders and liquids, designed to be taken in unit quantities to supplement the diet(1). These products have become increasingly popular during recent decades. Surveys show that 40% of British adults take them(Reference Henderson, Irving, Gregory, Bates, Prentice, Perks, Swan and Farron2), and UK sales in ‘bricks and mortar’ shops amounted to £325·7×106 in 2005, multivitamins and fish oils being the most popular(3). Food supplements are taken for many reasons, mostly as an insurance policy to make up for a poor diet and/or to promote optimal health and fitness. Supplements are also used in an attempt to enhance sports and athletic performance and prevent or manage minor ailments such as colds and skin, hair and nail problems, as well as more distressing conditions such as premenstrual syndrome and menopausal symptoms and diseases such as arthritis, CVD, cataract and age-related macular degeneration.
One is okay: moderate multivitamins
A substantial proportion of multivitamins sold on the UK ‘high street’ contain amounts of vitamins and minerals approximating to the RDA. (The EU RDA (rather than the UK dietary reference values) is the reference value used to compare amounts of vitamins and minerals on the labels of food supplements in the UK and throughout the EU.) Such products are safe, and given that recent UK National Diet and Nutrition Surveys(Reference Henderson, Irving, Gregory, Bates, Prentice, Perks, Swan and Farron2, 4–6) have shown that some population groups are at risk from marginal intakes, a ‘moderate’ ‘one-a-day’ multivitamin–mineral supplement may be beneficial. Although supplements are not a substitute for a poor diet, evidence shows they can help to reduce nutritional gaps.
Studies in adults have shown that supplement use can make an important contribution to vitamin and mineral intake. The National Diet and Nutrition Survey in British adults has found that supplement users have higher intakes of vitamins and minerals and are less likely to have intakes below the reference nutrient intake than non-supplement users(Reference Bader, Bosy-Westphal, Koch and Mueller7). Similar findings have been reported for Ireland(Reference Kiely, Flynn, Harrington, Robson, O'Connor, Hannon, O'Brien, Bell and Strain8), Germany(Reference Beitz, Mensink, Fischer and Thamm9, Reference Schwarzpaul, Strassburg, Luhrmann and Neuhauser-Berthold10), the USA(Reference Archer, Stamler, Moag-Stahlberg, Van Horn, Garside, Chan, Buffington and Dyer11) and Canada(Reference Troppmann, Gray-Donald and Johns12). Food supplements have also been shown to make a substantial contribution to the intakes of vitamins and minerals in toddlers(Reference Briefel, Hanson, Fox, Novak and Ziegler13, Reference Fox, Reidy, Novak and Ziegler14) and teenagers(Reference Dwyer, Garcea, Evans, Li, Lytle, Hoelscher, Nicklas and Zive15–Reference Stang, Story, Harnack and Neumark-Sztainer18). Several studies(Reference Girodon, Blache, Monget, Lombart, Brunet-Lecompte, Arnaud, Richard and Galan19–Reference Wolters, Hermann and Hahn21) have also shown that supplementation with vitamins and minerals can improve plasma levels of micronutrients and reduce the prevalence of suboptimal plasma concentrations.
The use of multivitamins has also been associated with reduced risk of chronic disease in some, but not all, studies. Evidence from observational studies suggests a reduced risk of CVD in users of multivitamins(Reference Rimm, Willett, Hu, Sampson, Colditz, Manson, Hennekens and Stampfer22, Reference Holmquist, Larsson, Wolk and de Faire23). In the Cancer Prevention Study II cohort past multivitamin use (>10 years before enrolment), but not recent use (<10 years before enrolment) was found to be associated with modestly-reduced risk of colo-rectal cancer(Reference Jacobs, Connell, Chao, McCullough, Rodriguez, Thun and Calle24), but a small increase in prostate cancer(Reference Stevens, McCullough, Diver, Rodriguez, Jacobs, Thun and Calle25). In the Health Professionals’ Follow-up Study men who reported folate consumption from multivitamins for >10 years were found to have a 25% reduction in colon cancer risk(Reference Giovannucci, Rimm, Ascherio, Stamfer, Colditz and Willett26), and in the Nurses' Health Study women who reported multivitamin use (with folate) for ≥15 years were found to have a 75% reduction in colo-rectal cancer risk(Reference Giovannucci, Stampfer, Colditz, Hunter, Fuchs, Rosner, Speizer and Willett27). However, in a pooled analysis of eight prospective studies no significant association between the use of multivitamins and specific vitamin supplements and lung cancer risk was found(Reference Cho, Hunter, Spiegelman, Albanes, Beeson and van den Brandt28).
The use of multivitamin supplements has also been associated with reduced risk of cataract(Reference Kuzniarz, Mitchell, Cumming and Flood29, Reference Leske, Chylack, He, Wu, Schoenfeld, Friend and Wolfe30). However, evidence for a benefit of multivitamins in the prevention of infection is weak and conflicting, as confirmed by two systematic reviews–meta-analyses, one in the elderly(Reference El-Kadiki and Sutton31) and one in adults of all ages(Reference Stephen and Avenell32).
Such benefits observed for consumers of multivitamin supplements may result from their concerns about health and their attempts to live healthier lifestyles. It is well recognised that individuals who take supplements may be the ones who least need them. Intakes of fruit and vegetables(Reference Harrison, Holt, Pattison and Elton33) and micronutrients from food(Reference Kiely, Flynn, Harrington, Robson, O'Connor, Hannon, O'Brien, Bell and Strain8, Reference Dwyer, Garcea, Evans, Li, Lytle, Hoelscher, Nicklas and Zive15, Reference Stang, Story, Harnack and Neumark-Sztainer18) have been found to be higher in supplement users, although one study has found no difference(Reference Briefel, Hanson, Fox, Novak and Ziegler13). In a Canadian study Ca and vitamin D intakes from food were actually lower in supplement users than in non-supplement users(Reference Troppmann, Gray-Donald and Johns12), possibly because of lower dairy consumption in those taking supplements.
More is better? Higher levels of intake
Although a moderate-‘RDA’ multivitamin supplement is safe and also potentially beneficial for some population groups, some individuals choose to take combinations of several products (e.g. multivitamins, single vitamins or minerals, antioxidants, products marketed for specific periods of life, such as the menopause, and long-chain fatty acids) in an attempt to promote ‘optimal health’ and prevent or manage various conditions. In addition, the UK has traditionally had a liberal safety-based approach to the use and sale of vitamins and minerals, and several high-dose products are available on the UK market. Higher-dose products, defined as those with a daily dose at or above the doses recommended by the UK Expert Vitamin and Mineral Group (EVM)(34) have been estimated to represent 12–15% (£25–33×106 per annum) of the total UK vitamin and mineral supplement market(35).
So, what might be the benefits of higher intakes of vitamins and minerals? If one is okay, is more better? There is clear epidemiological evidence of links between micronutrient status and the risk of chronic disease(Reference Fletcher and Fairfield36), and indications that some nutrients (e.g. antioxidant substances) can beneficially influence biomarkers of chronic disease. These findings have prompted the search for evidence of efficacy for specific supplements from controlled intervention trials.
B-vitamins
Neural-tube defects
There is abundant evidence that folic acid protects against the development of neural-tube defects, specifically anencephaly and spina bifida, as described in a recent review(Reference Pitkin37). Supplementation of folic acid at amounts exceeding the RDA is recommended for all women capable of becoming pregnant and during the first 12 weeks of pregnancy. The recommended intakes are 4 mg/d for women at high risk (by virtue of a previous neural-tube defect pregnancy outcome or those with epilepsy) and 0·4 mg/d for all other women.
CVD
Hyperhomocysteinaemia is associated with increased risk of CVD, and supplementation with folic acid (with or without vitamins B6 and B12) results in reduced concentrations of homocysteine, as confirmed by meta-analyses(38, Reference Alfthan, Aro and Gey39). By contrast, results from trials with B-vitamin supplements that examined definitive clinical cardiovascular outcomes have been disappointing. However, supplementation trials have largely been conducted in patients with pre-existing CVD and results from secondary prevention studies may not reflect the outcome in healthy individuals taking supplements. For example, the two-year Vitamin Intervention for Stroke Prevention Trial in patients with non-disabling cerebral infarction has found no difference between high-dose B-vitamins (including 2·5 mg folic acid/d) and a low dose (including 20 μg folic acid/d) in relation to stroke, coronary event or death(Reference Toole, Malinow, Chambless, Spence, Pettigrew, Howard, Sides, Wang and Stampfer40). Furthermore, the Heart Outcomes Prevention Evaluation Trial has shown no benefit with folic acid (2·5 mg/d), vitamin B6 (50 mg/d) and vitamin B12 (1 mg/d) among patients with pre-existing CVD or diabetes(Reference Lonn, Yusuf, Arnold, Sheridan, Pogue and Micks41), while the Norwegian Vitamin Trial has found that the combination of vitamin B6, vitamin B12 and folic acid decreases plasma homocysteine but has no demonstrable benefit on other CVD-related outcomes in patients who have had a myocardial infarction(Reference Bonaa, Njolstad, Ueland, Schirmer, Tverdal and Steigen42). Data from the Women's Antioxidant and Folic Acid and Cardiovascular Study (CM Albert, NR Cook, JM Gaziano, SS Bassuk, E Zaharris, JG MacFayden, D Danielson, M Van Denburgh and JE Buring, unpublished results), are similar to those from the Heart Outcomes Prevention Evaluation Trial and the Norwegian Vitamin Trial in that among women with, or at increased risk for, CVD treatment with folic acid, vitamin B6 and vitamin B12 was not found to be associated with differences in cardiovascular-related death, myocardial infarction, stroke or revascularisation through a mean of a 7-year follow-up compared with placebo.
However, it may yet to be too early to abandon the idea that folic acid and other B-vitamins can reduce risk of CVD. Indeed, the B-Vitamin Treatment Trialists' Collaboration has recently reviewed the design and statistical power of twelve randomised trials assessing the effects of lowering homocysteine with B-vitamin supplements on risk of CVD(Reference Bairati, Meyer, Jobin, Gelinas, Fortin, Nabid, Brochet and Têtu43). They have concluded that the individual trials may not have involved a sufficient number of vascular events nor have been of sufficient duration to have produced a good chance on their own of detecting plausible effects of homocysteine lowering on risk of CVD. However, the combined analysis of these trials, which will be available within a few years, should have adequate power to determine whether lowering homocysteine reduces the risk of cardiovascular events.
Other conditions
Evidence exists of a link between low folate status and cancer, particularly colon cancer and cervical cancer(Reference Rampersaud, Bailey and Kauwell44). However, controlled trials are needed to determine whether supplementation reduces risk. B-vitamins have also been investigated in Alzheimer's disease and depression, and a meta-analysis of four randomised controlled intervention trials has provided no evidence that folic acid supplementation, with or without vitamin B12, has a beneficial effect on cognitive function or mood in cognitively-impaired older subjects(Reference Malouf, Grimley and Areosa45). More recently, in a trial that randomised 818 participants to 800 μg folic acid/d or placebo for 3 years the folic acid group were reported to show a better 3-year change in memory, sensorimotor speed and information processing speed than the placebo group(Reference Durga, van Boxtel, Schouten, Kok, Jolles, Katan and Verhoef46). A systematic review of three randomised controlled trials involving folate supplementation suggests that folate may have a potential role as a supplement to other treatment for depression(Reference Taylor, Carney, Goodwin and Geddes47), but has concluded that further trials are needed before supplementation can be recommended for this group of patients.
Vitamin C
Vitamin C is an antioxidant that also inhibits the formation of carcinogenic nitrosamines from dietary nitrates. It might therefore be expected to be protective against the development of CVD and cancer. Clinical trials have evaluated the effects of vitamin C supplements in the development of these diseases but evidence is inconsistent.
High doses of vitamin C are popularly recommended for the prevention and treatment of the common cold. A review of thirty trials by the Cochrane Collaboration has concluded that long-term daily supplementation with large doses of vitamin C does not appear to prevent colds, but there is a modest benefit in terms of reducing the duration of cold symptoms from the ingestion of high doses(Reference Douglas, Hemila, Chalker, D'Souza and Treacy48). An update of this review has added that vitamin C could be justified in individuals exposed to brief periods of severe physical exercise and/or cold environments and that regular vitamin C supplementation could reduce the duration and severity of colds. A more-recent randomised double-blind 5-year controlled trial has found that vitamin C supplementation at 500 mg/d reduces the frequency, but not the duration or severity, of the common cold in comparison with a dose of 50 mg/d(Reference Sasazuki, Sasaki, Tsubono, Okubo, Hayashi and Tsugane49). However, the authors urged caution in interpreting this study because of a variety of limitations, including a large number of subject drop-outs.
Vitamin D
Vitamin D deficiency continues to be more common than was thought some years ago, with a high prevalence in inner city areas among Afro-Caribbeans and Asians, particularly women(Reference Ford, Graham, Wall and Berg50). Individuals with inadequate sunlight exposure, especially older adults, young children and pregnant women may benefit from vitamin D supplementation to prevent deficiency. A suitable dose for adults in this category is generally 10 μg/d.
However, lack of vitamin D is increasingly associated with a range of conditions, such as osteoporosis, falls, cancer, CVD, diabetes mellitus and poor immune function, with the possibility that supplementation could be protective. Evidence from clinical intervention trials to date is strongest for benefit of vitamin D in protecting against fracture, but findings are conflicting. Two meta-analyses have found that vitamin D in a dose of approximately 20 μg/d prevents both vertebral and non-vertebral fractures in older adults(Reference Vieth51, Reference Bischoff-Ferrari, Willett, Wong, Giovannucci, Dietrich and Dawson-Hughes52). However, these effects have not been confirmed in more recent trials. An extension of the most-recent meta-analysis(Reference Bischoff-Ferrari, Willett, Wong, Giovannucci, Dietrich and Dawson-Hughes52) to include four further trials(Reference Izaks53) has concluded that high-dose, but not low-dose, vitamin D is effective in reducing fracture risk in the institutionalised elderly, but not in the general population. Two further meta-analyses have found that vitamin D reduces the risk of falls(Reference Bischoff-Ferrari, Dawson-Hughes, Willett, Staehelin, Bazemore, Zee and Wong54, Reference Jackson, Gaugris, Sen and Hosking55), which might be expected to reduce the risk of fall-related fractures.
Vitamin E
Vitamin E has both antioxidant and anti-inflammatory properties and has been one of the most-widely-studied vitamins in relation to the use of supplementary doses in excess of the RDA. Claims have been made for a wide variety of benefits of vitamin E supplementation, with most research attention given to CVD. The literature abounds with evidence that vitamin E inhibits smooth muscle proliferation, platelet aggregation, monocyte endothelial adhesion, LDL oxidation and improves vascular function, as exemplified in two recent reviews(Reference Jialal and Devaraj56, Reference Traber57). Unfortunately, many vitamin E studies have been carried out in tissue cultures rather than in supplemented human subjects, so the health benefits of these findings are unclear.
Several observational studies have examined the influence of vitamin E supplementation in CVD. In the Nurses’ Health Study consumption of vitamin E supplements for >2 years was found to be associated with a 41% lower relative risk of major coronary disease(Reference Stampfer, Hennekens, Manson, Colditz, Rosner and Willett58). Similar results were obtained in an observational study in male health professionals, for whom there was a 37% lower relative risk of CHD in those who took vitamin E supplements in doses of ≥100 mg daily for >2 years(Reference Rimm, Stampfer, Ascherio, Giovannucci, Colditz and Willett59).
However, clinical trials evaluating supplements of vitamin E have not consistently demonstrated protection against CVD. The first clinical trial to test the efficacy of vitamin E in heart-attack prevention was the Cambridge Heart Antioxidant Study, which has shown that vitamin E reduces the 1-year rate of non-fatal myocardial infarction, but causes no reduction in cardiovascular-related mortality(Reference Stephens, Parsons, Schofield, Kelly, Cheeseman and Mitchinson60). Extended follow up of the Heart Outcomes Prevention Evaluation Study has actually suggested an increased risk of heart failure in the vitamin E-supplemented group(Reference Lonn, Bosch, Yusuf, Sheridan, Pogue and Arnold61). More than 200 trials using vitamin E supplements have been carried out and a recent review and meta-analysis claims that vitamin E has neither benefit nor harm(Reference Shekelle, Morton, Jungvig, Udani, Spar and Tu62).
With hind sight, clinical trials of vitamin E, like those with other vitamin supplements, have been overly optimistic in their expectation that a vitamin could reduce the risk of multifactorial disease and provide benefit equal to or beyond that of pharmaceutical medication. However, it is noteworthy that in most clinical trials biomarkers were not used nor were oxidative stress and lipid peroxidation markers of plasma vitamin E concentrations measured.
Antioxidants
CVD
Clinical trials involving antioxidant supplements (e.g. combinations of β-carotene, vitamin C and vitamin E) and CVD have again mainly been disappointing. The Finnish Alpha-Tocopherol Beta-Carotene Cancer Prevention Study of heavy smokers has found no reduction in CHD morbidity or mortality during 5–8 years treatment with vitamin E (50 mg daily) and β-carotene (20 mg daily). Significantly more deaths were found in the β-carotene group than in the placebo group(Reference Hennekens, Buring, Manson, Stampfer, Rosner and Cook63) and during the 6-year post-trial follow up β-carotene increased the post-trial risk of first-ever non-fatal myocardial infarction(Reference Tornwall, Virtamo, Korhonen, Virtanen, Taylor, Albanes and Huttunen64). The UK Heart Protection Study involving adults with CVD or diabetes also found no significant differences in all-cause mortality, non-fatal myocardial infarction, coronary death or stroke with antioxidant supplementation (mg/d; vitamin E 600, vitamin C 250, β-carotene 20)(65). A trial in post-menopausal women with coronary disease has found that supplementation with vitamin E 266·8 mg twice daily and vitamin C 500 mg twice daily does not retard atherosclerosis(Reference Waters, Alderman, Hsia, Howard, Cobb and Rogers66). Results from the Supplementation en Vitamines et Mineraux Antioxydants Study have suggested that a combination of antioxidants (mg/d: vitamin C 120, vitamin E 30, β-carotene 6, Se 0·1, Zn 20) over an average of 7·2 years has no beneficial effects on carotid atherosclerosis and arterial stiffness(Reference Zureik, Galan, Bertrais, Mennen, Czernichow, Blacher, Ducimetière and Hercberg67) or risk of hypertension(Reference Czernichow, Bertrais, Blacher, Galan, Briançon, Favier, Safar and Hercberg68). A meta-analysis evaluating antioxidants has confirmed no benefit of vitamin E supplementation on all-cause mortality, cardiovascular mortality or cerebrovascular accident, and has shown that β-carotene is associated with a slight increase in all-cause mortality and cardiovascular death(Reference Vivekananthan, Penn, Sapp, Hsu and Topol69). An extensive US review has also suggested that there is no benefit of supplements containing vitamin E or vitamin C (either alone or in combination) on either CVD or all-cause mortality(Reference Shekelle, Morton, Hardy, Coulter, Udani and Spar70). More recently, a further meta-analysis that included sixty-eight randomised controlled trials with 232 606 participants has found that treatment with β-carotene, vitamin A and vitamin E singly or combined may increase mortality(Reference Bjelakovic, Nikolova, Gluud, Simonetti and Gluud71).
Cancer
In relation to cancer, intervention studies involving antioxidant supplements have again been conflicting. While the Chinese Linxian Trial has found that combined daily doses of β-carotene, vitamin E and Se over 5 years are linked with a 13% reduction in cancer deaths and a 9% reduction in all-cause mortality(Reference Blot, Li and Taylor72), the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study has found no reduction in the incidence of lung cancer among male smokers after 5–8 years of supplementation with vitamin E or β-carotene(73). Indeed, the incidence of lung cancer and overall death rate were found to be increased in the group receiving β-carotene. More recent data from this study has shown no reduction in the risk of either colo-rectal or gastric cancer. The Polyp Prevention Study has also found no evidence that antioxidant supplements reduce the risk of colo-rectal adenomas(Reference Greenberg, Baron, Tosteson, Freeman, Beck and Bond74). A 3-year trial among 1980 subjects in Venezuela (in a population with a high risk of gastric cancer) has found that supplementation with vitamin C, E and β-carotene does not influence progression rates of precancerous gastric lesions compared with placebo(Reference Plummer, Vivas, Lopez, Bravo, Peraza and Carillo75). However, other studies have shown that low-dose antioxidant supplementation is associated with reduced cancer incidence and all-cause mortality in men(Reference Hercberg, Galan, Preziosi, Bertrais, Mennen, Malvy, Roussel, Favier and Briançon76), and supplemental β-carotene and vitamin E is associated with reduced risk of prostate cancer(Reference Kirsch, Hayes, Mayne, Chatterjee, Subar and Dixon77). Two systematic reviews have found no evidence of benefit for antioxidant supplements in the prevention of cancer(Reference Coulter, Hardy, Shekelle, Morton, Udani and Spar78).
Eye disease
Antioxidant supplements have also been examined in relation to eye disease. Evidence for benefit of antioxidant supplements in cataract is limited, with no significant effect on cataract development and progression in the Age Related Eye Disease Study(79), although there was a small positive effect of supplements in the Roche European American Cataract Trial(Reference Chylack, Brown, Bron, Hurst, Köpcke, Thien and Schalch80, 81) The Age Related Eye Disease Study also evaluated the effect of antioxidant vitamins combined with Zn (80 mg/d) and Cu (2 mg/d) on age-related macular degeneration. Both Zn alone and antioxidants plus Zn were found to reduce the odds of developing advanced age-related macular degeneration in the higher-risk group, but showed no benefits at other stages of the disease.
Calcium
Bone health
Many intervention trials have investigated the influence of Ca supplementation (with or without vitamin D) on bone density and fracture risk. A recent meta-analysis has found that children taking Ca supplements show only small improvements in bone density, which are unlikely to reduce the risk of fracture in either childhood or later adult life(Reference Winzenberg, Shaw, Fryer and Jones82). However, many of the included trials involved children who were Ca replete. In post-menopausal women there is evidence from meta-analysis that Ca supplementation can slow bone loss(Reference Shea, Wells, Cranney, Moher, Adachi, Treleaven, Peterson, Tugwell and Henry83). Moreover, there is strong evidence that Ca in combination with vitamin D decreases the risk for hip and non-vertebral fracture in post-menopausal women(Reference Chapuy, Arlot, Duboeuf, Brun, Crouzet, Arnaud, Delmas and Meunier84–Reference Dawson-Hughes, Harris, Krall and Dallal86). The National Institute for Health and Clinical Excellence is currently developing guidelines relating to osteoporosis that will look at prescribing vitamin D and Ca as an intervention.
Other conditions
Studies have demonstrated that Ca supplements may reduce blood pressure, protect against colon cancer and reduce menstrual pain. A Cochrane review has concluded that Ca supplementation is associated with small (2·5 (95% CI 4·5, 0·6) mmHg), but significant reduction in systolic but not diastolic blood pressure(Reference Dickinson, Nicolson, Cook, Campbell, Beyer, Ford and Mason87). In the Calcium Polyp Prevention Study Ca (1200 mg/d) was found to have a more pronounced anti-neoplastic effect on advanced colo-rectal lesions than on other types of polyps(Reference Wallace, Baron, Cole, Sandler, Karagas and Beach88). A recent Cochrane review has concluded that evidence from two randomised controlled trials suggests that Ca supplementation might contribute to a moderate extent to the prevention of colo-rectal adenomatous polyps, but that this outcome does not constitute sufficient evidence to recommend the general use of Ca supplements to prevent colo-rectal cancer(Reference Weingarten, Zalmanovici and Yaphe89). Two trials have shown a benefit of Ca supplementation (1000–1200 mg/d) in premenstrual and menstrual pain(Reference Thys-Jacobs, Ceccarelli, Bierman, Weisman, Cohen and Alvir90, Reference Thys-Jacobs, Starkey, Bernstein and Tian91). There is increasing interest in the possibility that Ca may have a role in the maintenance of body weight. However, a recent randomised controlled trial involving Ca supplementation (1000 mg daily) found no difference in body weight and fat-free mass between a supplemented group of women and the placebo group, but there was a trend towards loss of weight in the supplemented group, which the authors suggest could be consistent with a small effect(Reference Shapses, Heshka and Heymsfield92).
Selenium
Se supplementation has also been evaluated in relation to cancer end points. The US Nutritional Prevention of Cancer Trial was the first double-blind placebo-controlled trial in a Western population designed to test the hypothesis that Se supplementation could reduce the risk of cancer(Reference Clark, Combs and Turnbill93). Involving 1312 individuals with a history of non-melanoma skin cancer, the trial found that 200 μg Se/d was associated with a 37% reduction in total cancer incidence, 63% fewer cancers of the prostate, 58% fewer cancers of the colon and 46% fewer cancers of the lung. No significant differences were found in the incidence of basal-cell carcinoma or squamous-cell carcinoma, and there were more cases of breast cancer and leukaemia lymphoma in the Se group, but these differences were not significant. Further analysis has continued to show a protective effect of Se on the overall incidence of prostate cancer, although the effect was restricted to those with lower baseline prostate-specific antigen levels and plasma Se concentrations(Reference Duffield-Lillico, Dalkin, Reid, Turnbull, Slate, Jacobs, Marshall and Clark94). A systematic review and meta-analysis of sixteen studies has confirmed that Se supplementation may reduce the risk of prostate cancer. Further large randomised trials, which are ongoing, will help to throw more light on this issue(Reference Etminan, FitxGerald, Gleave and Chambers95).
Safety of high intakes
Some nutrients (e.g. vitamins A, D and B6 and Se) are well known to cause toxicity if consumed in excessive amounts. For vitamin A the intake at which toxic effects occur is about ten to twelve times the reference nutrient intake for adults, but only about three times that for infants and pregnant women. Vitamin A has received particular attention because of the possibility that high intakes of retinol could increase the risk of osteoporosis and fracture. Since 1998 four cross-sectional studies(Reference Melhus, Michaelsson, Kindmark, Bergstrom, Holmberg, Mallmin, Wolk and Ljunghall96–Reference Ballew, Galuska and Gillespie99) and seven cohort studies(Reference Promislow, Goodman-Gruen, Slymen and Barrett-Connor100–Reference Kawahara, Krueger, Engelke, Harke and Binkley106) have investigated the association between retinol and bone health. The cross-sectional studies have generally shown no association between vitamin A status or intake and risk to bone health. The cohort studies have reported mixed effects; some have found that excess vitamin A may increase the risk of hip fracture, while others have found no risk. One study has demonstrated benefit to bone density. All these studies are complicated by the presence of other nutrients in the diets of those studied, differences in vitamin A intake between study populations and difficulties in assessing vitamin A intake. Whether high intakes of retinol do have a deleterious influence on bone health is therefore unclear.
Concern about overdosing on dietary supplements is nothing new. However, the publicised benefits of taking supplements and the huge variety of products on the market, together with the knowledge that ⩽50% of the populations of Western countries take them has increased the safety concerns in more recent years. These concerns have encouraged several authorities worldwide to establish safe upper levels (UL) for vitamins and minerals.
Establishment of upper levels
In the UK the food supplement industry began researching the concept of safe UL in the mid 1980s, and in October 1997 the European Federation of Health Product Manufacturers and the Council for Responsible Nutrition established safe UL for both long-term and short-term consumption of twenty-five vitamins and minerals(Reference Shrimpton107). The approach they used was to base the UL well below the level at which a significant adverse effect had been reported in the literature. Subsequently, several other authorities in the USA(108–111), Europe(112), the UK(34) and Australia and New Zealand(113) have set safe UL for vitamins and minerals.
Nutrient risk assessment
The model for estimation of the UL recommended in a recent joint report of the WHO and FAO(114) is based on nutrient risk assessment, which is illustrated in Fig. 1. The UL set by the USA, Europe, the UK and Australia and New Zealand have also been based on this model (albeit with some differences in methodological detail).

Fig. 1. Model for nutrient risk assessment and estimation of upper safe levels (UL) as recommended in the joint WHO/FAO report(114). NOAEL, no observed adverse effect level; LOAEL, lowest observed adverse effect level.
Hazard identification and hazard characterisation
The process begins with the identification of adverse health effects associated with the nutrient concerned and makes use of human, animal and in vitro data. Studies are rated according to quality and tabulated to summarise the data. A key point in the assessment process is the selection of the critical adverse health effect, which focuses on identifying the effect associated with a level of intake most likely to provide public health protection. In practice, this effect would usually be the adverse health effect that occurs at the lowest level of intake within the population or subpopulation of interest.
Dose–response relationship
The next stage is to assess the dose–response relationship for the critical adverse health effect, which includes the determination of a no observed adverse effect level (NOAEL; (i.e. the highest intake of a nutrient at which no adverse effects have been observed) or, alternatively, a lowest observed adverse effect level (LOAEL; i.e. the lowest intake of a nutrient at which an adverse effect has been demonstrated; Fig. 2). For example, the US Food and Nutrition Board set a NOAEL of 200 mg for vitamin B6 because according to its interpretation of the literature this level is the highest intake of vitamin B6 at which no peripheral neuritis has been observed(109). For β-carotene the UK EVM set a LOAEL of 20 mg because it considered this level to be the lowest intake of β-carotene at which an increased risk of cancer has been observed in smokers(34). In some instances authorities have considered that it was not possible to set a NOAEL or a LOAEL. For example, the UK EVM has not set either a NOAEL or a LOAEL for Mg(34). Studies reporting mild diarrhoea in a small percentage of healthy subjects at doses of 384–470 mg/d were used as evidence to set a guidance UL of 400 mg supplemental Mg/d.

Fig. 2. Relationship between no observed adverse effect level (NOAEL), lowest observed adverse effect level (LOAEL) and upper level for a nutrient.
Uncertainty
Following the determination of a NOAEL or LOAEL, account is taken of uncertainties (e.g. those associated with extrapolating data from a small number of subjects, a short-term study, subjects with clinical disease or animal studies to the general healthy population). Estimation of UL is associated with a large extent of uncertainty because of the paucity of well-designed studies intended to determine the risk of nutrient intake. However, if the available data allow, a quantitative adjustment for uncertainties is made to the value (i.e. NOAEL or LOAEL) derived from the dose–response assessment. As a LOAEL is a less-robust value than a NOAEL, a larger uncertainty value tends to be ascribed to a LOAEL. These uncertainty considerations are also checked against the level of recommended intake relative to biological essentiality or the levels of intake associated with demonstrable health benefits. After uncertainties have been taken into account, the resulting value is the UL for the nutrient concerned in the specified population or subpopulation.
Dietary risk assessment
The next stage of nutrient risk assessment is dietary intake assessment (i.e. habitual dietary intake among individuals within the population), which is then combined with the outcomes of the hazard characterisation to describe the overall nature of the risk and its magnitude. Characterisation of risk includes a description of the scientific uncertainties and calculation of the margin between the RDA or actual intake and UL. Subgroups of the population with distinct sensitivities to certain nutrients (e.g. patients with haemochromatosis may be sensitive to high intakes of vitamin C) should also be considered.
Differences in upper levels
Table 1 shows that there are differences in the current UL set by the different authorities. There are two main reasons for this variation. First, some countries (e.g. the EU, USA and Australia and New Zealand) have set UL to cover the total intake from food and food supplements while others (e.g. the UK) have set UL for intake from supplements only, although the UK EVM has also set separate levels for some nutrients to include intake from food and supplements. Moreover, the UK EVM distinguishes between nutrients for which it considered there was sufficient evidence to set a UL and those for which it considered evidence was less robust and therefore set guidance levels only.
Table 1. Upper safety limits for vitamins and minerals set by various authorities

EU RDA, the RDA considered sufficient to prevent deficiency in most individuals in the population; CRN/EHPM, upper safe level defined by the European Federation of Health Product Manufacturers Association and the UK Council for Responsible Nutrition as daily intakes from supplements that could be consumed on a long-term basis; EVM UK, values produced by the UK Expert Vitamin and Mineral Group; FNB USA, tolerable upper intake levels defined by the Food and Nutrition Board of the US National Academy of Sciences as the highest total level of a nutrient (diet plus supplements) that could be consumed safely on a daily basis that is unlikely to cause adverse health effects to almost all individuals in the general population. As intakes rise above the upper level, the risk of adverse effects increases. The upper level describes long-term intakes, so that an isolated dose above the upper level need not necessarily cause adverse effects. The upper level defines safety limits and is not a recommended intake for most of the population most of the time; SCF EU, tolerable upper intake levels defined by the EC Scientific Committee on Food as the maximum level of chronic daily intake of a nutrient (from all sources) judged to be unlikely to pose a risk of adverse effects to human subjects; AUS/NZ, upper levels of intake for vitamins and minerals in adult men and women.
* Likely safe total daily intake from supplements alone.
† Safe upper level from supplements alone.
Second, different data, or different interpretations of the same data, have in some cases been used to set UL. For some nutrients this disparity occurred only because the values were based on the published studies available when the reports were being prepared. Thus, the UL for vitamin A set by the EU and the USA are higher than that set by the UK EVM because some studies showing a link between high vitamin A intakes and risk of fracture had not been published when the EU and USA set their safety limits. In the case of vitamin B6 the UK EVM considered the available human data to be inadequate so it set a LOAEL of 50 mg/kg body weight per d and an uncertainty factor of 300 based on animal studies that produced a UL of 10 mg/d. By contrast, both the EU and USA used human data, but even their authorities also came to different conclusions. The EU set a LOAEL of 100 mg, an uncertainty factor of 4 and a UL of 25 mg/d, while the USA set a NOAEL of 200 mg, an uncertainty factor of 2 and a UL of 100 mg/d.
UL should be reviewed regularly in line with emerging data. An argument is already being made to increase the current US UL for vitamin D from 50 μg to 250 μg (and also increase the dietary reference intake from 10 μg/d to 25 or 50 μg) on the basis of its emerging public health benefits(Reference Hathcock, Shao, Vieth and Heaney115).
Maximum permitted levels of vitamins and minerals in food supplements
The European Commission Food Supplements Directive(1) recognises that consistently-high total intakes of some vitamins and minerals may result in adverse effects and makes provisions for setting maximum permitted levels (MPL) of vitamins and minerals in food supplements. MPL should be distinguished from UL, which are in some cases upper levels for total intake and have no legal bearing on the vitamin and mineral content of food supplements.
The Directive also states that in setting MPL, ‘account should be taken of ULs, as established by scientific risk assessment, based on generally acceptable scientific data, and of intakes of these nutrients from the normal diet’. The UL for vitamins and minerals determined by the EVM and in the EU are based on scientific risk assessment and not dietary need, and could therefore be used to establish MPL for supplements across the EU. The Directive also states that ‘due account should be taken of reference intake amounts, which is the amount considered necessary to ensure nutritional sufficiency’. However, intakes of vitamins and minerals from the diet vary across member states, and between communities within a member state, because of differing food habits, which will lead to problems in agreeing MPL for supplements intended to apply across the EU.
Models for setting maximum permitted levels in supplements
Several models have been put forward for setting MPL in food supplements, including the risk-management model of the European Responsible Nutrition Alliance/European Federation Association of Health Product Manufacturers(116), the Danish(Reference Rasmussen, Andersen, Dragsted and Larsen117), French(118), German(Reference Domke, Grossklaus, Niemann, Przyrembel, Richter and Schmidt119, Reference Domke, Grossklaus, Niemann, Przyrembel, Richter and Schmidt120) and International Life Science Institute(Reference Flynn, Moreiras, Stehle, Fletcher, Muller and Rolland121) models. The Danish, French and International Life Science Institute models focus on amounts of nutrients for addition to conventional foods (i.e. food fortification) and will not be considered further here.
European Responsible Nutrition Alliance/European Federation Association of Health Product Manufacturers model
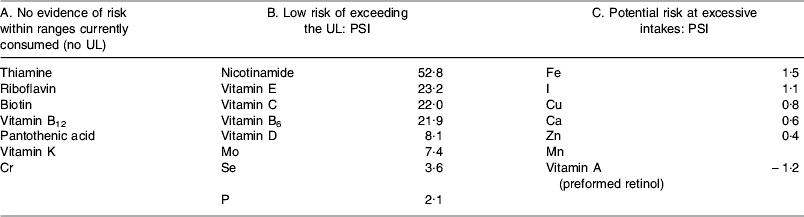
This model works by dividing vitamins and minerals into three categories of risk by estimating the potential of population groups with higher levels of intake to exceed the UL. This potential is known as the population safety index (PSI), which is characterised as follows:
where MHI is the mean highest intake from dietary sources (97·5th percentile of the average male adult intake from studies undertaken in Ireland, Italy, The Netherlands and the UK), IW is the potential intake from water, RLV is the reference labelling value (developed by the EU Scientific Committee on Food and equivalent to the RDA).
This model has produced cut-off values for PSI, such that where the PSI is >1·5 (i.e. where the difference between the current highest intake from food and the UL is >150%×RLV) the chance of exceeding the UL through supplementation is considered to be extremely low. Where PSI is ⩽1·5 (i.e. where the difference between the current highest intake from food and the UL is <150%×RLV) supplementation may potentially lead to intakes that approach the UL. The cut-off of 1·5 is based on nutrient intake data from the UK and Germany that indicate that supplements contribute a maximum of 141% of the RLV to total nutrient intake.
On the basis of the evaluation of PSI for nutrients where a UL exists, and taking into account qualitative characteristics for nutrients where no UL exists, three categories of vitamins and minerals emerge with this model (see Table 2).
Table 2. Categorisation of vitamins and minerals based on risk according to the model developed by European Responsible Nutrition Alliance and European Federation Association of Health Product Manufacturers
UL, upper intake level; PSI, population safety index.
MPL, or maximum safe levels (MSL) as they are termed in this model, are then set as follows (see Table 3):
group A nutrients: this model suggests no evidence of risk within ranges currently consumed and therefore no rationale for setting an MSL;
group B nutrients: the assumption is made that the risk of exceeding the UL on the basis of current intake is small. Taking into account intake from dietary sources (including fortified foods) and water, the model has developed equations for calculating the MSL for vitamins and minerals in this group:

group C nutrients: there is a narrow range of safety, so MSL are calculated on a case-by-case basis.
Table 3. Examples of maximum supplement levels proposed by different models

ERNA, European Responsible Nutrition Alliance; EHPM, European Federation Association of Health Product Manufacturers; BfR, German Federal Institute for Risk Assessment, proposed maximum levels in food supplements.
* Level not set because ERNA/EHPM model considers no evidence of risk at current intakes.
† 200 μg for children aged between 4 and 10 years.
‡ 10 μg for adults >65 years of age.
§ No use of nicotinic acid.
‖ No supplements for children or adolescents aged <18 years.
¶ Maximum level not suitable for children aged <11 years.
The German model
Germany's Federal Institute for Risk Assessment has made proposals for maximum levels of vitamins and minerals in food supplements and also for maximum levels for the fortification of conventional foods(Reference Domke, Grossklaus, Niemann, Przyrembel, Richter and Schmidt119, Reference Domke, Grossklaus, Niemann, Przyrembel, Richter and Schmidt120) (Table 3). In this model the amount of each nutrient that can be added to the diet as a whole with no appreciable risk of adverse health effects is determined as the difference between the UL and the current estimated intake of the respective micronutrients from non-fortified foods at percentile 95 or 97·5.
where R is the residual amount available for addition to supplements or fortified foods, DINF is the current estimated level of intake of a micronutrient from non-fortified food at percentile 95 or 97·5. R constitutes the tolerable intake of a vitamin or mineral via food supplements plus the tolerable intake via fortified foods. The percentage of R available for addition to supplements or fortified foods is selectable and may vary between 0 and 100%, but the sum of the two percentages may not exceed 100%.
Considering that individuals may take more than one supplement or consume several portions of fortified food daily, a multiple-exposure factor has been introduced, and maximum levels for single-portion supplements or foods calculated. For nutrients with large margins between the UL and the 95th or 97·5th percentile of intake (e.g. folic acid) this large residual amount is divided into equal parts between food supplements and fortified foods. In the case of nutrients with small margins (e.g. Zn) the available (small) residual amount is allocated to food supplements only with no fortification of conventional foods permitted. Maximum amounts calculated for addition to supplements are shown in Table 3. In assuming the daily consumption of two food supplements and two fortified foods containing nutrients at the maximum level, this model derives maximum amounts of food supplements that are lower than those of the European Responsible Nutrition Alliance/European Federation Association of Health Product Manufacturers model.
European Commission discussion paper
In 2006 the European Commission published a discussion paper on the setting of maximum and minimum amounts for vitamins and minerals in foodstuffs, including food supplements(122). Responses from EU member states and various stakeholders have been published on the Europa website(123). These responses represent a range of viewpoints on questions such as whether MPL should be set for vitamins and minerals for which risk of adverse effects seems to be low or non-existent even at high levels of intake, with some suggesting there is no need for the setting of MPL while others maintain that MPL should be set for all vitamins and minerals. Views also differ on whether separate levels are needed for food supplements and fortified foods and whether different MPL should be set for different population groups. The question of whether RDA should be taken into account when setting MPL is also being discussed, but the majority view is that RDA should not play a major role and that MPL should be based on risk assessment. The European Commission plans to put forward proposed levels for agreement by the member states in the Standing Committee on the Food Chain, but it is unlikely to happen before 2008.
When the levels are set, they will apply across the EU. For countries such as the UK, the Republic of Ireland and The Netherlands, which have traditionally had a liberal policy on the sale of supplements and the doses they contain, there is a concern that products would disappear from the market, limiting consumer choice. What may be possible, although it is as yet unclear, is that individual member states may be permitted to set higher MPL for products sold only in that member state.
Thus, the UK Food Standards Agency has suggested three options:
option 1 would be to establish one maximum level for each vitamin and mineral in supplements throughout the EU, taking into account available data on the highest intake from dietary sources for each vitamin and mineral across member states. This approach would be in line with the single market intention of the Directive but would be excessively precautionary and restrict consumer choice;
option 2 would be to establish common MSL for vitamins and minerals as in option 1, but with higher national maximum levels where there is evidence that dietary levels at a national level are lower than the amount used in option 1 or a national expert opinion supported safe supplemental intakes. This option would protect consumer safety throughout the EU, trade would be allowed across the EU for vitamins and minerals at an agreed level and UK consumer choice would be largely maintained. However, the Directive makes no mention of member states being able to set levels on a national basis so there is considerable uncertainty as to whether it would be allowable without an amendment being made;
option 3 would follow the same approach as option 2 except that national MPL would be replaced by national guidance levels. Like option 2, this approach would allow common MSL for the purposes of intra-community trade across the EU but would also allow single-dose supplements that exceeded these levels to be sold at the discretion of national governments provided they carried warning labels. This option is the one recommended by the Food Standards Agency for the UK.
Conclusions
Food supplements have become increasingly popular and almost half the UK population takes them. Moderate-RDA multivitamins are amongst the most-frequently-consumed supplements, and although they are not a substitute for a poor diet, they can help to bridge nutritional gaps. Epidemiological evidence also suggests that users of multivitamins may be at lower risk of developing some conditions, e.g. CVD and cancer, than individuals who do not take such supplements. Evidence for benefit for single or combination nutrients at higher doses is inconsistent apart from some exceptions, e.g. folic acid in the prevention of neural-tube defects and Ca with vitamin D in the prevention of fracture in the institutionalised elderly. However, many intervention trials have not evaluated biomarkers, and it is difficult to be certain that appropriate combinations and doses of nutrients and appropriate durations of study have been used in these trials. With this increased popularity of food supplements and the likelihood of taking more than one product and/or high-dose products, the possibility of overdosing on supplements has become an area of concern. Several authorities have set safe UL for vitamins and minerals and the EU is in the process of setting maximum levels for vitamins and minerals in food supplements, probably by the end of 2008. Such measures should help to ensure consumer safety.







