Health is plastic and results from interactions between individual genotype and exposures plus the play of chance( Reference Mathers 1 ). The important exposures include environmental contaminants, smoking and other hazardous lifestyle habits, physical activity (PA) and diet. It has been estimated that up to 80 % of major cardiometabolic diseases and over one-third of cancers could be prevented by eliminating shared risk factors, including tobacco use, poor diet, physical inactivity and excess alcohol( Reference van Lerberghe 2 ). A cluster of health behaviours, including non-smoking, being physically active, moderate alcohol intake and consumption of fruit and vegetables, is associated with up to fourfold lower mortality risk, equivalent to 14 years in chronological age( Reference Khaw, Wareham and Bingham 3 , Reference van Dam, Li and Spiegelman 4 ). The risk of most common diseases increases with age as does the burden of disability and frailty. A recent analysis of the global burden of ill-health demonstrated that poor diet and physical inactivity accounted for about three quarters of the top twenty factors contributing to disability-adjusted life years in the UK( Reference Murray, Richards and Newton 5 ). This emphasises the primacy of changing lifestyle factors (notably smoking cessation, increasing PA and improving diet) in public health initiatives which are aimed at improving health throughout the life-course and, especially, in adulthood.
Despite considerable research on interventions to increase PA and to improve diet, in most cases the effect size achieved in such interventions is relatively modest, especially in the longer term. For example, our recent systematic review and meta-analysis of PA interventions in middle-age (55–70 years) with objective measures of PA outcomes showed that, at 12 months follow-up, the additional PA was equivalent to just over 2000 steps/d( Reference Hobbs, Godfrey and Lara 6 ). However, with longer-term follow-up, the interventions effects became non-significant( Reference Hobbs, Godfrey and Lara 6 ). A parallel systematic review and meta-analysis of randomised controlled trials (RCT) testing the effectiveness of dietary interventions in changing intakes of fruit and vegetables (F&V), indicators of a healthier eating pattern, at the same life stage was a little more encouraging( Reference Lara, Hobbs and Moynihan 7 ). The meta-analysis of twenty-two studies involving 63 189 participants found highly significant (P < 0·001) increases in F&V intake which equated to approximately one extra portion F&V/d( Reference Lara, Hobbs and Moynihan 7 ). Importantly, the mean improvement in F&V intake at 4–12 months was similar to that at 13–58 months suggesting that dietary behaviour change was sustainable in these studies( Reference Lara, Hobbs and Moynihan 7 ). Recent systematic reviews suggest important reductions in risk of several common non-communicable diseases (NCD) and age-related diseases associated with increased F&V consumption of the magnitude observed in our meta-analysis( Reference Lara, Hobbs and Moynihan 7 ) (Table 1). However, one should be cautious about the potential for bias in such studies since changes in dietary intake were measured by self-report and the well-known biases in dietary reporting may be amplified when assessing responses to interventions( Reference Adamson and Mathers 8 ).
Table 1. Evidence from systematic reviews and meta-analyses on the health effects of increased consumption of fruits and vegetables. Modified from Lara et al.( Reference Lara, Hobbs and Moynihan 7 )
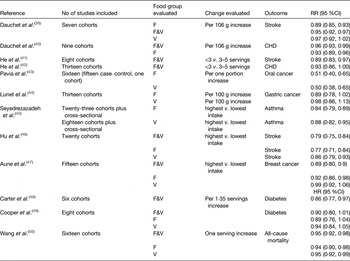
F&V, fruit and vegetables; F, fruit; V, vegetables.
To date, most strategies to reduce the NCD burden have been targeted at populations using ‘one size fits all’ public health recommendations, e.g. ‘eat at least five portions of fruit and vegetables daily’. However, the global burden of NCD worldwide continues to rise and this emphasises the need for more effective prevention strategies. In the present paper, we address three questions: (i) Is personalised nutritional advice more effective than the conventional ‘one size fits all’ approach?; (ii) What is the optimum basis for personalisation of dietary advice?; (iii) How can such personalised dietary interventions/services be delivered to enough people in cost-effective ways to make a significant improvement in public health?
A personalised (or stratified) approach to provision of dietary advice is predicated on knowledge of key characteristics of those to whom the intervention is being delivered. The more tailored the intervention is the more sophisticated, and potentially expensive, will be the process for acquiring, analysing and acting upon those participant characteristics. With conventional face-to-face interventions, the resource implications of the necessary information collection and processing could mean that such personalised nutrition (PN) interventions would limit their availability and that their use could be restricted to more affluent sections of society. Given that the prevalence of, and risk of death from, NCD are strongly socioeconomically patterned( Reference Di Cesare, Khang and Asaria 9 ), it is important that interventions aim to narrow, rather than to exacerbate, health inequalities. In that context, digital-based technologies for intervention delivery may offer several potential advantages including: (i) Convenience – the intervention could be available to the participant when and where she/he wishes to use it and be equally available on a number of devices, e.g. tablet, laptop or smart phone; (ii) Scalable – the intervention is not restricted to any geographical location and, in theory, could be available to unlimited numbers of participants over wide geographical areas with diverse socioeconomic circumstances; (iii) Personalised/stratified – suitable platforms could collect and process relevant participant characteristics (e.g. socioeconomic information, current diet and lifestyle, and dietary preferences) and use those characteristics to offer tailored feedback and advice; (iv) Sustainable – the platform could employ suitable behaviour change techniques (BCT)( Reference Michie, Ashford and Sniehotta 10 ) to maximise the likelihood that participants will maintain changes in eating patterns in the long-term and so maximise gains in health and wellbeing; (v) Reduced costs – while the development of suitable digital platforms may have greater upfront costs than conventionally delivered interventions, digital-based intervention platforms are likely to have lower costs per participant, especially when scaled up to the larger participant numbers for which they are well suited. In addition, maintenance and updating costs may be lower than for equivalent face-to-face systems and so such digital intervention platforms may be economically more sustainable.
However, is digital technology effective in achieving behaviour change? An earlier systematic review of the effectiveness of e-Learning approaches for improving dietary behaviours found that e-Learning interventions produced only modest improvements in diet( Reference Harris, Felix and Miners 11 ). For example, e-Learning interventions were associated with 0·24 serving/d increase in intake of F&V and reductions of 0·79 and 0·24 g/d in intakes of total fat and saturated fat respectively; data expressed as weighted mean differences( Reference Harris, Felix and Miners 11 ). Given these relatively small improvements in diet, it is not surprising that an economic analysis suggested that the use of e-Learning devices would be unlikely to be cost-effective( Reference Harris, Felix and Miners 11 ). Importantly, the authors of this systematic review noted that the estimates of cost-effectiveness were very sensitive to assumptions about the initial fixed cost of the digital device and that reducing the device costs produced dramatic increases in cost-effectiveness( Reference Harris, Felix and Miners 11 ). The retail prices of digital devices continue to fall, e.g. the average worldwide cost of a tablet is now less than half the cost in 2010( 12 ). As a consequence, ownership of such devices is expanding rapidly and it is estimated that >70 % of the UK population has a smartphone( 13 ). Although there is considerable digital illiteracy and lack of internet access in some sections of the UK population, the government is committed to improving UK-wide digital inclusion with the intention that by 2020 ‘everyone who can be digitally capable will be’( 14 ). These trends suggest that, within a few years, access to a reasonably priced digital device and training in basic digital skills are unlikely to limit the use of internet-based interventions to improve diet and other lifestyle behaviours.
Recently we completed a systematic review and meta-analysis of RCT that tested the effectiveness of personalised e-Health lifestyle-based interventions on weight loss( Reference Celis-Morales, Livingstone and Abraham 15 ) and on dietary and physical activity( Reference Celis-Morales, Abraham and Keenan 16 ). We found twenty-one articles that met our inclusion criteria and meta-analyses of the results showed that web-based personalised interventions were more effective in reducing body weight( Reference Celis-Morales, Livingstone and Abraham 15 ) (weighted mean differences: −1·83 kg 95 % CI −2·2, −1·4; P < 0·0001) and in increasing F&V consumption (weighted mean differences: 0·35 servings/d 95 % CI 0·28, 0·42; P < 0·0001) than non-personalised advice( Reference Celis-Morales, Abraham and Keenan 16 ) (Figs 1 and 2). In summarising the implications of their findings for research, Harris et al.( Reference Harris, Felix and Miners 11 ) called for more ‘theoretically informed work, which addresses the question of which characteristics of the target population, target behaviour, content and delivery of the intervention are likely to lead to positive results’. In the examples which follow, we illustrate how theoretically informed work, including the explicit application of BCT( Reference Michie, Ashford and Sniehotta 10 ), can be used in the development of internet-based interventions to improve diet and other lifestyle behaviours through the lifespan.
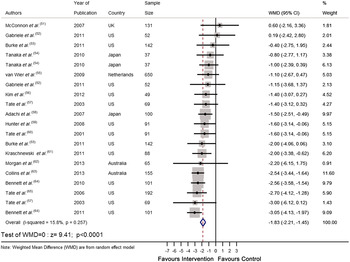
Fig. 1. (colour online) Forest plot of the effect of e-Health personalised randomised controlled trials on body weight change (kg) in 2414 adults.
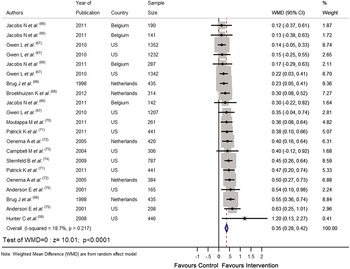
Fig. 2. (colour online) Forest plot of the effect of e-Health personalised randomised controlled trials on change in fruit and vegetable intake (portions/d) in 10 936 adults.
Lifestyle-based intervention to enhance healthy ageing: The LiveWell Programme
Nutrition and other lifestyle factors, including smoking behaviour and PA are major determinants of the ageing trajectory( Reference Stanner, Thompson and Buttriss 17 , Reference Langie, Lara and Mathers 18 ). While intakes of individual foods and nutrients may influence ageing, the strongest evidence for positive effects of diet on ageing in human subjects comes from studies of the Mediterranean diet( Reference Trichopoulou, Martinez-Gonzalez and Tong 19 ). Observational studies have shown that greater adherence to this dietary pattern is associated with lower mortality risk and lower risk of many age-related NCD including CVD, cancers and neurodegenerative diseases( Reference Sofi, Abbate and Gensini 20 ). Importantly, results from the European Prospective Investigation into Cancer and Nutrition study indicate that such health benefits can be achieved by adopting these dietary patterns in different latitudes and thus are not exclusive to residents of Mediterranean countries( Reference Trichopoulou, Orfanos and Norat 21 , Reference Couto, Boffetta and Lagiou 22 ). In addition, there is accumulating evidence that interventions aiming to shift dietary patterns towards the Mediterranean diet pattern are effective in both primary( Reference Estruch, Ros and Salas-Salvado 23 ) and secondary( Reference de Lorgeril, Salen and Martin 24 ) prevention of age-related NCD. The LiveWell Programme, an initiative by our multidisciplinary team based at Newcastle University, aims to develop pragmatic lifestyle-based interventions to enhance healthy ageing (http://research.ncl.ac.uk/livewell/). These interventions are targeted at those in the peri-retirement period because life events, such as retirement from full-time work, represent windows of opportunity in which behaviour change interventions may be more effective( Reference Schäfer, Jaeger-Erben and Bamberg 25 ). Initially, these interventions will be delivered via the web for use on laptops and tablets but they have been designed to be potentially deployable on other digital platforms such as smartphones. We built our interventions using: (i) Evidence from systematic reviews of the literature on interventions to improve diet( Reference Lara, Hobbs and Moynihan 7 ) and physical activity( Reference Hobbs, Godfrey and Lara 6 ) in those aged 55–70 years and evidence that social role interventions may improve health and wellbeing for people in the retirement transition( Reference Heaven, Brown and White 26 ); (ii) Suitable behaviour change theories and BCT( Reference Michie, Ashford and Sniehotta 10 ); (iii) A participatory design approach involving workshops with older people.
Then we worked with a web design company to use this information to develop a web-delivered intervention platform which enables older people to make appropriate behaviour changes in the areas of diet (with a focus on the Mediterranean diet as a model of healthy eating), PA and social engagement aimed at enhancing healthy ageing. Advice on these areas is tailored to the needs, preferences and circumstances of individual participants. At present, we are piloting this intervention at locations in the North East of England. In addition, we have developed a panel of measurements aimed at capturing key aspects of the Healthy Ageing Phenotype( Reference Lara, Godfrey and Evans 27 ) which we are using as outcomes measures to assess the efficacy of the intervention. Very recently, using data from an online survey among older people, we have identified clusters of perceived barriers to healthy eating which may form the basis for an additional tool to tailor dietary interventions for those of peri-retirement age( Reference Lara, McCrum and Mathers 28 ).
Using genetic information for more effective dietary behaviour change
Sequencing of the human genome combined with the recognition that interactions between genotype and diet influence health has opened opportunities for personalisation of dietary advice based upon individual genotype. An early review of the concept concluded that genotype-based PN had potential but that the evidence base was insufficiently developed( Reference Joost, Gibney and Cashman 29 ). Recent steep reductions in the cost and time for genomic sequencing and an increasing ability to extract information of interest, e.g. disease risk and ancestry, from sequence data have fuelled interest in personal genetics among the public. For example, a survey of 1588 readers of the journal Nature revealed that 18 % had had a genome analysis and 54 % would do so if given the opportunity( Reference Maher 30 ). However, the effectiveness of genetics-based information in facilitating behaviour change is poorly understood. In a systematic review, Marteau et al. reported that the evidence was weak because of the small number of studies of limited quality available and concluded that ‘claims that receiving DNA-based test results motivates people to change their behaviour are not supported by the evidence’( Reference Marteau, French and Griffin 31 ).
In a test of the utility of genomic data in motivating behaviour change, 3639 employees of health and technology companies received subsidised access to a commercial genotyping service, which provided estimates of lifetime risk of common health conditions( Reference Bloss, Schork and Topol 32 ). This showed no evidence that such genomewide profiling produced any measurable change in psychological health, diet or exercise behaviour in the 2037 participants who provided follow-up data at 5·6 (sd 2·4) months( Reference Bloss, Schork and Topol 32 ). However, a Canadian RCT in which young adults were offered genotype-based dietary advice or conventional dietary advice reported that the genotype-based advice was more understandable and more useful than general dietary advice( Reference Nielsen and El-Sohemy 33 ). However, whether the genotype-based advice produced significantly greater changes in dietary behaviour is not known. A more recent scientific and ethical analysis of the basis for genetic-based PN showed that there are still divergent views on the robustness of the evidence base( Reference Görman, Mathers and Grimaldi 34 ).
Internet-delivered personalised nutrition advice: The Food4Me Proof of Principle study
In conclusions from their systematic review, Marteau et al. called for larger and better quality RCT investigating the utility of DNA-based information in promoting behaviour change( Reference Marteau, French and Griffin 31 ). The Food4Me Proof of Principle (PoP) study is a relatively large RCT which was designed to: (i) determine whether it is possible to deliver PN across seven European countries; (ii) ascertain whether PN advice is more effective than general healthy eating guidelines in changing eating patterns; (iii) investigate the utility of the internet as a delivery platform (www.food4me.org). Importantly, the Food4Me PoP Study investigated the basis for personalisation of dietary advice by randomising participants to one of four treatment arms:
-
Level 0: Control – conventional (non-personalised) dietary advice;
-
Level 1: PN advice based on current diet alone;
-
Level 2: PN advice based on current diet plus phenotypic information (adiposity and blood metabolites);
-
Level 3: PN advice based on current diet plus phenotypic and genotypic information.
To maximise the integrity of intervention delivery, all aspects of the study were supported by Standardised Operating Procedures, pre-training of all researchers delivering the intervention and frequent internet-based conference calls to share best practice and to resolve issues. In addition, eighteen BCT were embedded in the intervention design to help facilitate appropriate behaviour change. The protocol for the present study has been submitted for publication (C Celis-Morales, K Livingstone, JC Mathers et al. unpublished results). Reliable, and quantitative, assessment of dietary intake was an essential feature of the present study and presented some challenges because participants to the Food4Me PoP Study were recruited via the internet and self-recorded, and uploaded, dietary and other information through the Food4Me website. For this purpose, an online FFQ was developed which recorded and quantified (using online standardised colour photographs of portion sizes) consumption of 157 food items( Reference Forster, Fallaize and Gallagher 35 ). This online FFQ was validated against a 4-d-weighed food record in 100 participants( Reference Fallaize, Forster and Macready 36 ). Potential participants for the study consented to join the study after registering through the website. Analysis of data from 5571 screeneres showed that those interested in PN delivered via the internet broadly representative of the wider European adult population, most of whom would benefit from improved diet and/or greater PA( Reference Celis-Morales, Livingstone and Marsaux 37 ). Of the 1607 participants randomised to the Food4Me PoP Study RCT, 1285 completed 6 months follow up and the primary outcome data are being analysed at present.
What is the best basis for personalisation of dietary advice?
Both example studies outlined earlier, the LiveWell Programme and the Food4Me PoP Study, made explicit use of BCT in designing and delivering web-based interventions but they used different approaches to personalisation of dietary advice. The dietary intervention developed within LiveWell Programme aims to shift current eating patterns towards that of the Mediterranean diet and personalisation is based on individual preferences for foods and meals. Having expressed particular preferences, participants are offered advice and support although features such as recipes, meal planning and tips for choosing healthier snacks. In the Food4Me PoP Study, an individual participant's diet was assessed at baseline and then advice was offered targeting the top three nutrients which diverged most from currently recommended healthy intakes. Well-designed, theory- and evidence-based interventions, in particular those which deploy recognised BCT, are expected to be more effective than interventions which do not employ such features. However, the efficacy of particular BCT in improving diet among any section of the population is poorly understood. We have undertaken further analysis of data from RCT testing the effectiveness of dietary interventions in changing intakes of F&V (reported by Lara et al.( Reference Lara, Hobbs and Moynihan 7 )) to identify the BCT used in complex dietary behaviour change interventions and to explore associations between BCT utilised and intervention effectiveness. We have found that studies using the techniques ‘barrier identification/problem solving’, ‘plan social support/social change’, ‘goal setting (outcome)’, ‘use of follow-up prompts’ and ‘provide feedback on performance’( Reference Michie, Ashford and Sniehotta 10 ) were associated with greater effects of interventions on F&V consumption compared with studies not using these BCT( Reference Lara, Evans and Hobbs 38 ). Outcomes from recent systematic reviews identifying BCT which improve the effectiveness of lifestyle-based interventions targeting diet, PA and weight loss are summarised in Table 2.
Table 2. Behaviour change techniques (BCT) associated with intervention effectiveness. Modified from Lara et al.( Reference Lara, Evans and Hobbs 38 ).

PA, physical activity.
Conclusions
Improving diet and other lifestyle behaviours has considerable potential for reducing the global burden of NCD, promoting better health across the life-course and increasing wellbeing. However, realising this potential will require the development, testing and implementation of much more effective behaviour change interventions than are used conventionally. Evidence-based, personalised interventions which incorporate effective BCT and which are delivered digitally are likely to be an important route to scalable and sustainable interventions. Future research should focus on such interventions aiming to reduce health inequalities and to improve overall public health.
Financial Support
J. L. and J. C. M. acknowledge support from the LiveWell Programme which is funded through a collaborative grant from the Lifelong Health and Wellbeing initiative, managed by the Medical Research Council on behalf of the funders: Biotechnology and Biological Sciences Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, Medical Research Council, Chief Scientist Office of the Scottish Government Health Directorates, National Institute for Health Research/The Department of Health, The Health and Social Care Research & Development of the Public Health Agency (Northern Ireland) and Wales Office of Research and Development for Health and Social Care and the Welsh Assembly Government (grant number G0900686). C. C. M. and J. C. M. acknowledge support from the Food4Me Study which is funded by the European Commission under the Food, Agriculture, Fisheries and Biotechnology Theme of the Seventh Framework Programme for Research and Technological Development (grant number 265494).
Conflicts of Interest
None.
Authorship
J. C. M., C. C. M. and J. L. drafted this manuscript based on the lecture given by J. C. M. at the Irish Section of the Nutrition Society Meeting ‘Changing dietary behaviour: from physiology through to practice’. University of Ulster, Coleraine, June 2014. All authors approved the final manuscript.







