- AA
amino acids
- ACP
acute-phase proteins
The National Institute for Health and Clinical Excellence (2006) has published guidelines on nutrition support in adults. The author, as chairman of the group that produced those guidelines, was not only involved in responding to the extensive comments on two earlier drafts but, since their publication, has discussed them widely. The author is therefore aware that although generally well received, the recommendation, ‘Nutrition support should be cautiously introduced in seriously ill or injured people, starting enteral tube feeding or parenteral nutrition at no more than 50% of the estimated target energy and protein needs’, has met with considerable adverse comment.
For some who have voiced criticism the fear is that if the National Institute for Health and Clinical Excellence (2006) recommendations are followed patients would receive an inadequate supply of both energy and N, but for the majority it is primarily fears about inadequate N that has caused concern. The National Institute for Health and Clinical Excellence (2006) approach leads to seriously-ill individuals receiving an initial provision of 0·12 g N/kg per d, a level close to the minimum maintenance needs for individuals who are not ill (Institute of Medicine, Food and Nutrition Board, 2005). This level is much lower than the recent recommendations from the European Society for Parenteral and Enteral Nutrition (2006) and the American Society for Parenteral and Enteral Nutrition (2002), which suggest provision of ≥0·25 g N/kg per d. While the concept of supplying only modest amounts of energy during the early phase of serious illness appears to have gained ground amongst experts in clinical nutrition (Elia, Reference Elia1995), most experts clearly believe that the supply of N should be generous (Hoffer, Reference Hoffer2003). The present paper examines the origins of that belief and questions its validity.
Catabolism, teleology and illness
There is no doubt that serious illness or injury leads to very high net N losses. These losses are predominantly a result of the catabolic response, although in many cases there are also contributions from impaired intake and absorption of nutrients and/or excess N losses via the gastrointestinal tract, open wounds, burns etc. Catabolism entails both an increase in protein synthesis and an even greater increase in protein breakdown. It is often thought of as being largely detrimental, but when looking at biology from a teleological viewpoint (according to The Concise Oxford Dictionary (Thompson, Reference Thompson1996) teleology is ‘the explanation of phenomena by the purpose they serve rather than by postulated causes’), it must in reality confer some survival advantage in the majority of circumstances. The only way that the operation of such an advantage can be envisaged is that the net tissue breakdown supplies the body with substrates needed to support the very-high nutrient demands of the acute-phase response, immunological defences and wound or tissue recovery.
Why, however, should anorexia and limitation of food intake accompany the catabolic response, when it would seem more logical that with the raised nutrient demands of sickness evolution would have ensured an increased appetite? There are several possible answers to this apparent paradox, including the possibility that lack of appetite limits the drive to move or forage, which might be dangerous when ill or injured. However, the most likely explanation would seem to be that the substrate requirements in the face of catabolism are not only larger than usual but different. If this explanation is valid, the link between catabolism and anorexia might be a means to ensure that the release of substrates specifically needed to cope with the illness or injury is coupled with a mechanism to limit intake of the substrates not currently required. By far the most likely candidates for such specific alterations in substrate requirements are the amino acids (AA) and there is evidence to suggest that such changes do occur.
Reeds et al. (Reference Reeds, Field and Jahoor1994) have pointed out that the acute-phase proteins (APC) have a relatively high content of phenylalanine, tryptophan and tyrosine compared with that seen in either transport proteins or muscle or the normal diet. For example, APC contain 105 g phenylalanine/kg whereas muscle protein contains only 40 g/kg. This disparity means that during the acute-phase response >2·5 g endogenous muscle protein (or protein derived from the diet or from conventional nutrition support) is needed to produce 1 g APC. These authors then went on to measure typical production rates of APC in patients undergoing uncomplicated surgical trauma. They have demonstrated that this imbalance in the AA content of endogenous or exogenous N sources compared with that of the APC could account for the entire net N loss seen in such patients, which amounts to about 0·13 g N/kg per d. Similar arguments can be made for unbalanced demands for other AA, including the S-containing group and glutamine (Reeds, Reference Reeds2000; Grimble, Reference Grimble2001).
In addition to providing an explanation for much of the observed N losses seen during catabolism, the unusual pattern of AA demands during more severe illness could explain the teleological purpose of anorexia. As mentioned earlier, the AA pattern provided within the diet essentially matches that needed for the synthesis of ‘normal’ transport and structural proteins. During illness or injury food intake would therefore provide not only the AA needed for manufacture of APC, but a relative excess of those not required under such circumstances. This excess might be thought of as harmless, and certainly some authorities have suggested that AA toxicity is rare (Soeters et al. Reference Soeters, van de Poll, van Gemert and Dejong2004). However, animal studies of unbalanced AA feeding show that excess free AA are harmful (Harper & Peters, Reference Harper and Peters1983), and the anorexia seen in human subjects on high-protein diets also suggests that too many free AA are biologically unwelcome (Weigle et al. Reference Weigle, Breen, Matthys, Callahan, Meeuws, Burden and Purnell2005). Furthermore, there are complex interactions between AA levels and requirements. For example, three to four molecules of glycine would be required to metabolise one molecule of ‘unwanted’ methionine, and this requirement could be important if any excess of methionine precipitates relative glycine deficiency, since glycine is needed to maintain antioxidant defences (Meakins et al. Reference Meakins, Persaud and Jackson1998).
The potential danger posed by free AA means that the body must either metabolise them or incorporate them into proteins etc. Most can be oxidised to urea, which is usually relatively harmless, but seriously-ill individuals often have renal failure. Furthermore, even if they are not uraemic they are often prone to oedema, and a high production of urea reduces the capacity to excrete salt and water because of the additional glomerular solute load. Of even more concern is the possibility that the capacity of the urea pathway to oxidise any excess AA that are not needed for the acute-phase response might be exceeded if high levels are coming from food or nutrition support as well as from catabolic breakdown. If that situation does occur, the excess ‘unwanted’ AA would have to be incorporated into peptides and proteins and, since they are of the wrong pattern for the acute-phase response, protein synthesis would need to be pushed back towards ‘normal’ pathways. This process would improve overall N balance, but only by forcing metabolism away from pathways chosen by evolution to maximise survival. Furthermore, although there has been a long tradition of ignoring the contribution of protein metabolism to the energy delivery of nutrition support, it must be borne in mind that a patient in net negative N balance not only receives the energy equivalent of 100% of any protein given in diet or feeds, but also receives the energy equivalent of most of the net N loss. High-protein feeding will therefore add to any problems associated with high glucose or lipid provision.
There is one additional interesting conjecture when taking an evolutionary view of catabolism and AA supply. It is widely assumed that the ‘essential’ AA must be the most important, since they are the AA that are simply indispensable to the body. Teleological thinking turns this reasoning upside down. There are numerous examples amongst the mammals of animals that can produce every AA, and it therefore seems most likely that human ancestors lost the capacity to make those that are now essential in the diet. If true, those for which man has retained the capacity for synthesis (so that supply is maintained even when eating little) must really be the most important during periods of illness and catabolism. Indeed, catabolism could even be a mechanism to limit supplies of the so-called essential AA, whilst maximising the supplies of substrates truly needed for severe illness, which according to Reeds (Reference Reeds2000) might be glutamine, arginine and aspartate for lymphocyte proliferation and cysteine, glutamine and glycine for glutathione synthesis.
Nitrogen requirements in serious illness
The arguments described earlier lead to the conclusion that higher levels of exogenous protein provision might adversely affect seriously-ill individuals. This inference is clearly the opposite of currently-accepted practice, and it would certainly be unreasonable to challenge it on entirely hypothetical grounds. However, when the literature on the N requirements of the seriously ill is reviewed with such arguments in mind, it is easy to find evidence that appears to support a low-N approach.
The N requirements of serious illness have been the subject of research over many decades, but nearly all the studies have been small and have used N balance or preservation of lean tissue mass as the primary end point. In healthy individuals a protein intake equivalent to about 0·12 g N/kg per d is more than adequate to maintain N balance (Institute of Medicine, Food and Nutrition Board, 2005), and much lower levels will do so after a period of adaptation, but in illness these levels change dramatically. Studies in the critically ill show that provision at normal maintenance levels is associated with a marked negative N balance and that levels of approximately 0·25 g N/kg per d are needed to minimise net losses (Shaw et al. Reference Shaw, Wildbore and Wolfe1987; Larsson et al. Reference Larsson, Lennmarken, Martensson, Sandstedt and Vinnars1990; Ishibashi et al. Reference Ishibashi, Plank, Sando and Hill1998). Nevertheless, the same studies and others, such as those by Wolfe et al. (Reference Wolfe, Goodenough, Burke and Wolfe1983), show that patients are still in net negative balance and that higher provision still does not help (Table 1).
Table 1. Studies on nitrogen balance in different groups of critically-ill patients

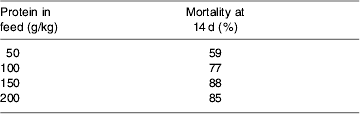
Since most experts assume that the maintenance of best-possible N balance in seriously-ill patients must be associated with better maintenance of organ function, wound healing and immune defence, the data from studies such as those in Table 1 have been used to underpin the common recommendation that the seriously ill or injured should receive ≥0·25 g N/kg per d. However, Koretz (Reference Koretz2005) has forcefully pointed out the dangers of assuming that studies on intermediate end points cannot be substituted for studies on clinical outcomes, and the teleological arguments presented earlier also suggest that best N balance may not equate with best clinical outcome. Forcing N accretion in the face of the catabolic process may compromise evolutionary survival responses, and some animal experiments appear to support this notion. For example, Peck et al. (Reference Peck, Alexander, Gonce and Maskell1989) have shown that high levels of protein provision in a guinea-pig model of peritonitis lead to increased mortality, even though N retention was found to be best in the groups fed more protein (Table 2). Furthermore, it is now well recognised that the improvement in N balance that is achieved in critically-ill patients by using growth hormone is associated with significantly higher mortality (39% deaths in patients receiving the hormone v. 20% in controls (relative risk 1·9 (95% CI 1·3, 2·9); P<0·001); Ruokonen & Takala, Reference Ruokonen and Takala2000).
Table 2. Mortality in a guinea-pig model of peritonitis with differing levels of nitrogen provision (Peck et al. Reference Peck, Alexander, Gonce and Maskell1989)
Clinical-outcome studies
If maintenance of best N balance cannot be used as a surrogate for best practice, analysis of clinical-outcome studies is needed. As in all areas of nutrition support, however, results are difficult to interpret for several reasons, including:
(1) optimal levels and balance of nutrition must vary between different groups of patients (e.g. post surgery and those with sepsis, trauma, crush injury, head injury and burns), as well as varying within groups depending on pre-existing malnourishment and the seriousness of the insult;
(2) optimal levels and balance of nutrition probably vary within individual patients depending on the timing of planned support in relation to the onset of the problem and stage of its resolution;
(3) results from studies are often confused by non-achievement of planned feeding levels, especially in the critically ill if limited gastrointestinal function reduces the effectiveness of any enteral feeding (a problem that invalidates nearly all comparisons of enteral tube feeding v. intravenous nutrition in the critically ill);
(4) benefits of nutrition support may not always be related to the provision of macronutrients per se, but to mechanisms such as correction of micronutrient deficits, stimulation of cell metabolism or preservation of gut integrity;
(5) in most studies of sick individuals deliberately or inadvertently being given low levels of N, those who receive low levels often receive different levels of energy.
It should also be recognised that in most patients receiving critical care it is highly unlikely that optimising nutrition will have more than modest effects on final outcomes, and hence very large studies would be needed to show benefit. The converse, however, that too much nutrition support could actually be detrimental, could be demonstrated in smaller studies.
Having acknowledged these difficulties, there is also the problem that, as far as the author is aware, there actually have been no studies on the deliberate provision of low-N feeds to any groups of patients in hospital settings. However, some studies undertaken in the context of severe malnourishment in the field may have relevance. In investigations performed in relief camps in Somalia during the famine of 1992–3 (Collins et al. Reference Collins, Myatt and Golden1998) 573 severely-malnourished adults (mean body weight 35 kg, BMI 13·1 kg/m2) were identified, of whom eighty-three were oedematous and 377 non-oedematous. These famine victims were allocated to either high (16·4%)-protein or low (8·5%)-protein refeeding regimens. Overall mortality rates were reported to be 21%, but in the oedematous patients they were found to be much higher (37%), with striking differences between those receiving low-protein v. high-protein feeds (Table 3). The high-protein feeds were also shown to be associated with increasing oedema, whilst low-protein feeds led to oedema resolution.
Table 3. Mortality and oedema in Somalian famine victims given high- and low-protein diets (Collins et al. Reference Collins, Myatt and Golden1998)

In explanation, it has been pointed out that the high-protein regimen was also higher in carbohydrate than the low-protein regimen, and hence the differences in outcome may have been ascribable to well-recognised carbohydrate-triggered refeeding-type problems. However, persistently poor appetite was observed in the group offered the high-protein feeds as opposed to rapidly-improving appetites seen in the low-protein group. As a consequence, the actual intakes of carbohydrate were probably similar in both groups. The study therefore suggests that high levels of N provision do increase mortality, possibly by the promotion of continued salt and water retention. Similar findings have also been reported in malnourished children (Golden, Reference Golden1982; Bredow & Jackson, Reference Bredow and Jackson1994).
Although, as already mentioned, there are no low-N–clinical-outcome studies in seriously-ill hospitalised patients, other studies on general levels of feeding can also provide clues. It is now accepted that very high levels of nutrition support, hyperalimentation, are definitely harmful (Cerra, Reference Cerra1987; Elia, Reference Elia1995) and, although it is easy to ascribe all harm to the high levels of fat or carbohydrate given, it is not known whether the high levels of N also contribute. It is known, however, that the small studies examining N balance with variable levels of provision (e.g. those shown in Table 1) have not shown clinical benefit when N losses are minimised, but neither do they suggest obvious harm with higher N provision. Nevertheless, some retrospective studies of general levels of feeding in patients receiving intensive care suggest that until it has been established that lower-energy higher-N provision really does make a clinical difference, low levels of both might be optimal.
For example, Krishnan et al. (Reference Krishnan, Parce, Martinez, Diette and Brower2003) undertook a retrospective analysis of outcomes v. achieved feeding levels in 187 patients in the intensive care unit who had not undergone surgery and showed that, overall, patients receiving between one-third and two-thirds of their estimated needs had the best levels of survival compared with those receiving less than one-third or more than two-thirds of their estimated needs (Table 4). These results might be explained by inequalities in the severity of illness represented by patients in each group, since sicker patients would have poorer gastrointestinal function and so would achieve lower levels of feeding, except for the very sickest patients whose gastrointestinal function would be so poor that they would need intravenous nutrition. However, this interpretation cannot explain why among the sicker patients with higher acute physiology scores the group receiving less than one-third of their estimated nutrient needs have the best survival, the group receiving between one-third and two-thirds of their estimated nutrient needs have poorer outcomes and the group receiving more than two-thirds of their estimated nutrient needs have the worst outcomes (Table 4). These results suggest that least is best for the really ill.
Table 4. Chances of survival in patients fed 33–65 or >65% of their estimated nutritional needs compared with those receiving <33% of their needs for 187 patients in the intensive care unit who had not undergone surgery and had differing acute physiology scores (SAPSII; Krishnan et al. Reference Krishnan, Parce, Martinez, Diette and Brower2003)

RR, relative risk.
Similar results, also favouring very low levels of feeding, have been reported in a randomised controlled trial by Ibrahim et al. (Reference Ibrahim, Mehringer, Prentice, Sherman, Schaiff, Fraser and Kollef2002) that examined clinical outcomes in patients who were mechanically ventilated and received early v. late enteral feeding (Table 5). Although other commentators (Powell-Tuck, Reference Powell-Tuck2007; Taylor, Reference Taylor2007) seem happy to ascribe such observations to the benefits of a low-energy approach, while still stating confidently that higher N must be given, it may be argued that the studies actually support the National Institute for Health and Clinical Excellence (2006) low-energy and low-N approach.
Table 5. Clinical outcomes in patients who were mechanically ventilated and received either 27 or 7·5% of their needs over the first 4 d (Ibrahim et al. Reference Ibrahim, Mehringer, Prentice, Sherman, Schaiff, Fraser and Kollef2002)

ICU, intensive care unit.
Some of the critics of the ‘start at 50%’ approach from the National Institute for Health and Clinical Excellence (2006) have cited studies of the benefits of early enteral feeding in intensive care as evidence that the recommendation must be wrong. However, although Heyland et al. (Reference Heyland, Dhaliwal, Drover, Gramlich and Dodek2003) have shown possible benefits in mortality and complications when initiating early enteral feeding in patients receiving intensive care, as did Taylor et al. (Reference Taylor, Fettes, Jewkes and Nelson1999) for head injury and Lewis et al. (Reference Lewis, Egger, Sylvester and Thomas2001) after general surgery, the benefits of such early nutritional intervention almost certainly have no relevance to levels of N provision. There are many reasons why early enteral intake might grant benefit that has nothing to do with levels of macronutrient provision. Furthermore, it was pointed out many years ago by Jeejeebhoy (Reference Jeejeebhoy1988) that across all feeding settings the ‘benefits of nutritional support are evident when too little nutrition is given for too short a time to have any noticeable influence on lean body mass or circulating proteins’. In addition, in most studies of early enteral feeding in the seriously ill or injured the levels of nutrition actually achieved in the first 48 h are <50% of the estimated needs (McClave et al. Reference McClave, Sexton, Spain, Adams, Owens, Sullins, Blandford and Snider1999; Marik & Zaloga, Reference Marik and Zaloga2001). Hence, they are closer to the levels recommended by the National Institute for Health and Clinical Excellence (2006) than to any recommendations suggesting higher levels of N. Indeed, the reason why early enteral feeding may be of benefit in the seriously ill may actually be because the levels of feeding achieved are usually limited.
Finally, even if the National Institute for Health and Clinical Excellence (2006) recommendation to introduce feeding at a maximum of 50% of the estimated needs is followed for 1 week (the National Institute for Health and Clinical Excellence (2006) actually recommends building up to meet full target levels in approximately 48 h, depending on metabolic and gastrointestinal tolerance), a 70 kg seriously-ill patient given 0·12 g N/kg per d would receive a total of about 60 g N, compared with 120 g N in the same period if given 0·25 g N/kg per d. While at first sight this disparity might appear to be important, the difference of 60 g N must be compared with the total catabolic N turnover in such a patient over 1 week, which is likely to equate to >1500 g N, with a total negative N balance of approximately 320 g. It is therefore difficult to conceive of a mechanism whereby, within this context, the higher input might really grant clinical benefit.
Conclusions
The answer to the question, protein and the critically ill: do we know what to give is an emphatic no. Although there is little doubt that high N intakes reduce net N losses in this context, there are no studies to suggest that this approach grants any clinical benefits and several considerations that raise the suspicion that it might do harm. Certainly, current evidence strongly suggests best survival occurs in critically-ill patients who are given small amounts of both energy and N, started as soon as possible, using the enteral route rather than the parenteral route. Further research is required to determine whether modest-energy higher-N feeding would prove better or worse than this approach in terms of clinical benefit and not just N retention. Investigations to explore the use of feeds that are better designed to match the AA needs of illness are also required.







