- NTD
neural-tube defects
- RCF
erythrocyte folate
Rationale for a mandatory folic acid food fortification programme in Ireland
Since 1991 it has been known that ⩽70% of neural-tube defects (NTD) can be prevented by the consumption of folic acid before and at the time of conception(1). From the mid 1990s Ireland, in common with many other developed countries, had a policy of advising women of child-bearing age to take folic acid supplements. However, experience has shown that this policy has only had a marginal impact on NTD rates in Ireland and elsewhere(Reference Botto, Lisi and Robert-Gnansia2). This outcome is mainly the result of:
1. the impromptu nature of pregnancy. Despite advances in contraception less than half all pregnancies are planned in Ireland; a rate that is comparable with other developed countries;
2. it is only in the very early stages of pregnancy that folic acid can be protective. Taking folic acid when pregnancy is suspected is generally too late to prevent NTD because the neural tube is completely developed 21–28 d post conception.
As a result of this situation mandatory folic acid fortification of cereal grains and flour was introduced in North America in 1998. Research has shown that such food fortification has resulted in a 20–70% reduction in rates of pregnancies affected by NTD(Reference Honein, Paulozzi, Mathews, Erickson and Wong3–Reference Persad, Van den Hof, Dube and Zimmer5).
By 2006 all available evidence had indicated that a mandatory folic acid food fortification programme would contribute substantially to a reduction in the numbers of babies born with NTD in Ireland(6). For >30 years Ireland had been recognised as having one of the highest rates of pregnancies affected by NTD in the world(Reference Botto, Lisi and Robert-Gnansia2). The cause of this particular vulnerability to the development of NTD in Ireland has a genetic basis. Recent studies have shown that approximately 50% of the Irish population have variations in the gene coding for an enzyme involved in folate metabolism, 5,10-methylenetetrahydrofolate reductase(Reference Molloy, Daly, Mills, Kirke, Whitehead, Ramsbottom, Conley, Weir and Scott7–Reference Whitehead, Gallagher, Mills, Kirke, Burke, Molloy, Weir, Shields and Scott9). Such genetic variations may account for ⩽26% of NTD-affected births in Ireland(Reference Kirke, Mills and Molloy10). The situation in other countries is different in that the overall incidence of NTD is lower and most of the affected pregnancies are terminated. Termination of pregnancy is illegal in Ireland and as a consequence Ireland bears a much higher burden of disease. Research carried out by the Health Research Board shows that the percentage of pregnancies affected by NTD that are delivered as live births is much higher in Dublin (81) compared with other centres (e.g. Paris, France 11 and North Thames, UK 13; PN Kirke, personal communication). This position places a greater onus on Ireland to maximise the primary prevention of these serious birth defects.
In July 2006 the Minister for Health and Children in Ireland accepted the recommendation of the National Committee on Folic Acid Food Fortification to introduce a mandatory folic acid fortification programme that would ensure that most bread in Ireland would be fortified(6). The level of folic acid fortification of bread proposed for Ireland (120 μg/100 g bread) would deliver a similar amount of folic acid to women of child-bearing age to that provided to North American women through their mandatory fortification of flour. At the request of the Department of Health and Children the Food Safety Authority of Ireland has led an expert working group to carry out the preparatory work involved in implementing the fortification programme.
Priorities in preparatory work pre-fortification
One of the key lessons learned during the implementation of national folic acid food fortification programmes in the USA and Canada concerns the need for a comprehensive evaluation plan to monitor the effectiveness and safety of the fortification intervention(Reference Rosenberg11). It was recognised that available data in Ireland needed to be up-dated to ensure an accurate pre-fortification assessment of all factors that would be affected by mandatory folic acid fortification(6).
Immediate action was taken to establish comprehensive reliable baseline information on important indicators against which the future effects of mandatory folic acid fortification of most bread in Ireland could be measured. These indicators include:
1. accurate information on the numbers of pregnancies affected by NTD;
2. assessment of the current extent of voluntary folic acid fortification of food on the Irish market and how it affects folic acid intakes;
3. analysis of the current level of voluntary folic acid fortification of bread marketed in Ireland;
4. information on the blood folate status of population subgroups in Ireland.
Preliminary findings from these investigations are discussed later in terms of scientific developments relative to the risk and benefits of folic acid food fortification.
Evaluation of scientific developments relevant to folic acid fortification
Scientific developments relevant to folic acid food fortification are being continuously monitored by the Food Safety Authority of Ireland. Research activity in three areas has come under review in terms of ongoing assessment of the risks and benefits of folic acid food fortification, i.e. cognitive function in older adults, CVD and cancer.
Cognitive function
Cognitive function declines with age, especially cognitive domains related to memory and information processing speed. Folic acid consumption, with or without vitamin B12, may help prevent cognitive impairment in the elderly, possibly through lowering plasma homocysteine levels(Reference Malouf, Grimley and Areosa12, Reference Selhub, Bagley, Miller and Rosenberg13). A 3-year randomised double-blind placebo-controlled trial involving >800 older adults (aged 50–70 years, all vitamin B12 replete) in The Netherlands has found that supplements of 800 μg folic acid over 3 years improve cognitive function(Reference Durga, van Boxtel, Schouten, Kok, Jolles, Katan and Verhoef14). Improvements in this trial were found to involve domains of cognitive function associated with ageing and these changes were shown to be associated with reductions in homocysteine(Reference Durga, van Boxtel, Schouten, Kok, Jolles, Katan and Verhoef14). In a previous similar trial (2-year randomised double-blind placebo-controlled trial in New Zealand involving >276 older adults (≥65 years) with elevated homocysteine levels (≥13 μmol/l) using high-dose supplements of folic acid (1000 μg), vitamin B12 (500 μg) and vitamin B6 (10 mg) as the homocysteine-lowering intervention) no beneficial effects of homocysteine-lowering on cognition were found(Reference McMahon, Green, Skeaff, Knight, Mann and Williams15). The lack of effect found in this earlier trial has been partly attributed to the less-sensitive test used to assess cognitive function (mini-mental state examination) in the New Zealand trial(Reference McMahon, Green, Skeaff, Knight, Mann and Williams15)v. five separate tests of different cognitive domains used in the Dutch trial(Reference Durga, van Boxtel, Schouten, Kok, Jolles, Katan and Verhoef14). However, a recent analysis of the relationship between blood levels of folate and vitamin B12 in older adult participants (>60 years, n 1459) in the 1999–2002 US National Health and Nutrition Examination Survey and measures of cognitive function has found that the protective effects of folate on cognitive function are dependent on adequate vitamin B12 status(Reference Morris, Jacques, Rosenberg and Selhub16). This study suggests that if vitamin B12 status is adequate, then folic acid (even at high intake levels) seems to be protective of cognitive function(Reference Morris, Jacques, Rosenberg and Selhub16, Reference Smith17). However, if vitamin B12 status is not adequate, the associated cognitive impairment seems to be made worse by high intakes of folic acid(Reference Morris, Jacques, Rosenberg and Selhub16, Reference Smith17). These divergent effects of folic acid intake depending on vitamin B12 status need to be confirmed. The two randomised controlled trials described earlier(Reference Durga, van Boxtel, Schouten, Kok, Jolles, Katan and Verhoef14, Reference McMahon, Green, Skeaff, Knight, Mann and Williams15) cannot contribute to this evidence because either a high dose of vitamin B12 was administered with folic acid(Reference McMahon, Green, Skeaff, Knight, Mann and Williams15) or the trial only included individuals who were vitamin B12 replete(Reference Durga, van Boxtel, Schouten, Kok, Jolles, Katan and Verhoef14).
Collectively, these recent reports indicate a beneficial effect of folic acid food fortification on cognitive function of older adults, but this effect may be dependent on adequate vitamin B12 status. Thus, the main implication for folic acid food fortification is to emphasise the importance of ensuring older adults with low vitamin B12 status are identified and treated.
CVD
Folic acid food fortification is associated with a reduced risk of stroke(18–Reference Wang, Qin, Demirtas, Li, Mao, Huo, Sun, Liu and Xu20). While observational data indicate that low folate intake levels are a risk factor for CVD, the evidence from trials is not consistent. Trials carried out to date have been underpowered and so definitive evidence on the possible protective effects against CHD require meta-analysis of the results of recent and ongoing trials(Reference Clarke, Lewington, Sherliker and Armitage21–Reference Wald, Wald, Morris and Law23). The results of such meta-analysis are expected to become available in early 2009.
Cancer
For some time folate has been considered to have a role in cancer prevention. Folate deficiency has been linked to the risk of cancer in human subjects, including cancer of the colon, other parts of the gastrointestinal tract, pancreas and breast (for review, see Kim(Reference Kim24)). Some, but not all, retrospective studies suggest higher dietary intake levels and higher blood markers of folate status are generally associated with a reduced risk of malignant disease, especially involving the colo-rectum(Reference Kim24–Reference Ulrich and Potter26). As a result of these disparate findings a definitive study was undertaken to test the chemopreventive effect of folate on colo-rectal adenomas or polyps (well-established precursors of colo-rectal cancer). This 6-year randomised controlled trial involving individuals with a recent history of these lesions has found that supplementation with a high dose of folic acid (1 mg/d) does not prevent the recurrence of adenomas. Conversely, this study has demonstrated at second follow-up that high-dose folic acid supplementation is associated with a 67% increased risk of advanced lesions (risk ratio 1·67 (95% CI 1·00, 2·80); P=0·05), along with a >2-fold increased risk of having at least three adenomas (risk ratio 2·32 (95% CI 1·23, 4·35); P=0·02)(Reference Cole, Baron and Sandler27). The publication of this study provides support for the theory (arising from animal studies) that folate (folic acid) nutrition may have a dual role in the development of cancer, i.e. a harmful role in addition to the protective role that is well documented in previous studies.
In addition, a recent epidemiological study of time trends for colo-rectal cancer incidence in the USA and Canada speculates that the introduction of folic acid fortification may have been responsible for an observed increase in colo-rectal cancer incidence(Reference Mason, Dickstein, Jacques, Haggarty, Selhub, Dallal and Rosenberg28). This study shows that mandatory fortification of foods with folic acid occurred at about the same time as non-significant increases in colo-rectal cancer incidence(Reference Mason, Dickstein, Jacques, Haggarty, Selhub, Dallal and Rosenberg28). However, it has been noted that if the increased rate of colo-rectal cancer was caused by folic acid fortification, the effect of folic acid on progression of cancer would have had to occur immediately(Reference Bayston, Russell, Wald and Hoffbrand29). Furthermore, the timing of increases in the blood folate status of the American population is also not clearly consistent with changes in colo-rectal cancer incidence outlined in the time trends report(Reference Mason, Dickstein, Jacques, Haggarty, Selhub, Dallal and Rosenberg28).
While developments relating to folic acid food fortification in CVD and cognitive function of older adults do not appear to be of major concern, the emerging issues in cancer risk are important. Increased risk of colo-rectal cancer precursors reported in the folate-supplementation trial(Reference Cole, Baron and Sandler27) are associated with folic acid intakes in excess of the upper safe level of 1 mg/d(30), which is many times higher than the increase in folic acid intake that would be delivered by mandatory fortification in Ireland.
The outcome of the folate-supplementation trial(Reference Cole, Baron and Sandler27) raises concerns and many questions, including uncertainty about what constitutes a safe level of folic acid intake and what factors influence this safe level.
Folic acid and the development of cancer
Role of folate in DNA replication and repair
Folate and other B-vitamins (vitamins B6 and B12) function as coenzymes in C1 metabolism, which is critical for the synthesis and methylation of DNA(Reference Scott and Weir31). Folate is required for the conversion of homocysteine into methionine and eventually into S-adenosylmethionine, which is the primary donor of methyl groups for most methylation reactions, including DNA methylation(Reference Scott and Weir31). Folate also assists in the synthesis of purines and thymidylate for DNA synthesis(Reference Scott and Weir31). DNA methylation is central to gene silencing and, potentially, to the suppression of repetitive DNA of viral origin(Reference Ulrich25, Reference Laird32). Deficiency of folate, or the other B-vitamins involved in these C1 reactions, may interfere with DNA methylation and synthesis, leading to aberrant gene expression and DNA instability and the eventual development of birth defects and cancer(Reference Kim24, Reference Davis and Uthus33, Reference Lin, Lee, Cook, Selhub, Manson, Buring and Zhang34). However, the precise influence of folate status on DNA methylation is not well defined; thus, the effects of folate status on cell biology are unclear(Reference Ulrich25).
Dual role of folate in cancer development: the importance of timing
Despite the central function of folate in maintaining DNA integrity and stability, folate nutrition may have dual effects on cancer development(Reference Kim24–Reference Ulrich and Potter26). Several lines of experimental data (studies involving animals and cell cultures) indicate that both the timing and dose of folate supplementation during carcinogenesis are important(Reference Ulrich25, Reference Ulrich and Potter26, Reference Song, Medline, Mason, Gallinger and Kim35, Reference Song, Sohn, Medline, Ash, Gallinger and Kim36). Although increases in folic acid intake before the existence of pre-cancerous lesions (such as adenomas or microscopic adenomas) can prevent tumour development, once pre-cancerous lesions are present supplementation with folic acid may increase tumour growth(Reference Ulrich25). This potential for a dual modulatory role for folate on carcinogenesis has been outlined(Reference Kim24) as follows:
1. cancer prevention: studies of cell biology and studies involving animals provide strong evidence that folate adequacy is protective against cancer through the central role it plays in DNA function and repair. Such studies indicate that where there is folate deficiency, DNA repair and function is upset. The resulting abnormal patterns in DNA function are among the most common identified in the development of cancer;
2. cancer promotion: cancer cells have much higher rates of growth and replication compared with normal healthy cells. These higher growth rates require accelerated DNA synthesis. Thus, the key role that folate plays in DNA synthesis and replication makes folate a potential growth promoter of cancerous cells. Studies of cell biology have shown that cancer cells tend to increase their uptake of folate, which explains why chemical agents that have an anti-folate effect are the basis for chemotherapy for cancer(Reference Farber37, Reference Heinle and Welch38).
Folic acid and cancer: importance of the amount and form of folate consumed
Two recent studies of post-menopausal breast cancer risk and folate nutrition highlight the importance of the amount of folate consumed in determining safety or risk(Reference Ulrich25, Reference Ericson, Sonestedt, Gullberg, Olsson and Wirfalt39, Reference Stolzenberg-Solomon, Chang, Leitzmann, Johnson, Johnson, Buys, Hoover and Ziegler40). In 2006 the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial in America involving 25 400 women (aged ≥55 years) reported that very high folate intakes, attributed to excessive supplement use (folic acid ≥400 μg/d), may generally be harmful rather than beneficial in breast-cancer development compared with taking no folic acid supplements (adjusted hazard ratio 1·19 (95% CI 1·01, 1·41); P=0·04)(Reference Stolzenberg-Solomon, Chang, Leitzmann, Johnson, Johnson, Buys, Hoover and Ziegler40). Conversely, in a more recent report from the Swedish Malmo Diet and Cancer study (cohort of 11 699 women, aged ≥50 years) women with the highest folate intakes were found to have >40% lower risk of breast cancer compared with women with the lowest folate intakes(Reference Ericson, Sonestedt, Gullberg, Olsson and Wirfalt39). Differences in the amount of folate consumed among these two cohorts of women (mean daily folate intake among women in the highest quintile of intake was 853 μg in the American cohort(Reference Stolzenberg-Solomon, Chang, Leitzmann, Johnson, Johnson, Buys, Hoover and Ziegler40)v. 349 μg in the Swedish cohort(Reference Ericson, Sonestedt, Gullberg, Olsson and Wirfalt39)) may explain the divergent outcomes in breast cancer risk associated with folate intake(Reference Ulrich and Potter26).
However, the form of folate may also explain the different outcomes in these two studies. In the American study increased breast cancer risk was found to be associated with high dose (≥400 μg/d) folic acid supplements(Reference Stolzenberg-Solomon, Chang, Leitzmann, Johnson, Johnson, Buys, Hoover and Ziegler40), while in the Swedish study beneficial effects were found to be associated with total dietary folate intake, i.e. natural folate and folic acid from supplements(Reference Ericson, Sonestedt, Gullberg, Olsson and Wirfalt39). Since only 19% of the Swedish women took folic acid supplements(Reference Ericson, Sonestedt, Gullberg, Olsson and Wirfalt39), the beneficial effects relating to folate intake could be associated with the natural form of the vitamin folate v. the synthetic form folic acid.
The bioavailability of folic acid (monoglutamic form) is much greater than that of natural folate (polyglutamate form). Usually once folic acid is absorbed it is converted to 5-methyltetrahydrofolate and metabolised as natural folate(Reference Scott and Weir31). However, if large doses of folic acid are consumed the mechanisms converting monoglutamates to 5-methyltetrahydrofolate are saturated and ‘free’ (unmetabolised) folic acid appears in plasma(Reference Kelly, McPartlin, Goggins, Weir and Scott41), because the capacity for converting folic acid to 5-methyltetrahydrofolate in the small intestine is limited. If single doses of folic acid exceed 200 μg, they are not metabolised immediately, resulting in ‘free’ folic acid circulating in the blood(Reference Kelly, McPartlin, Goggins, Weir and Scott41); a phenomenon not encountered from consumption of natural folates. Unlike 5-methyltetrahydrofolate, ‘free’ folic acid does not require activation to become involved in DNA synthesis. In a situation in which fast-growing cancer cells require accelerated DNA synthesis the potential of ‘free’ folic acid as a growth promoter of cancerous cells is obvious.
Gene–nutrient interactions
There is a growing body of evidence to link differential cancer risks with the polymorphisms in the folate metabolic pathway (methylenetetrahydrofolate reductase C677T and A1298C). In the case of colo-rectal cancer some research indicates that the two polymorphisms might protect against colo-rectal adenomas developing into cancer(Reference Huang, Han, Li, Mao and Xie42), while other research indicates differential vulnerability to type of colo-rectal tumour(Reference Chang, Lin, Lin, Yang, Wang and Li43, Reference Hubner, Lubbe, Chandler and Houlston44).
Folic acid and the development of cancer: summary
The recent data linking folic acid intakes with cancer risk emphasise the importance of ensuring a safe level of folic acid intake across all subgroups of the population. There are many examples in nutritional science to indicate that risk of adverse effects generally occur at the extremes of nutrient intake (e.g. vitamin A, vitamin D, polyunsaturated fats)(30, 45, 46). Considering the evidence discussed earlier, this relationship would also seem to apply to folic acid and the development of cancer, as risk is increased in both folate deficiency and overload (see Fig. 1). In the absence of any folic acid intake, folate deficiency can affect between 5 and 16% of the population, leaving them vulnerable to both NTD and cancer. The big problem is that the optimal level of folic acid intake in relation to cancer risk remains unknown (see Fig. 1). As folate status is partly determined by the genetic characteristics of the population(Reference Molloy, Daly, Mills, Kirke, Whitehead, Ramsbottom, Conley, Weir and Scott7) optimal intake is likely to be influenced by genetic make-up.
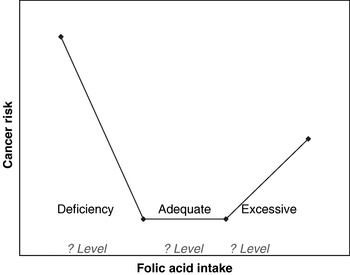
Fig. 1. Folic acid intake and cancer risk. Increased cancer risk is evident at the extremes of deficient and excessive intake levels; however, the criteria defining these extremes are unknown.
Folic acid food fortification: the Irish experience
As outlined earlier, preparatory work for implementation of the mandatory folic acid fortification programme has involved prioritising research to establish reliable baseline indicators of pre-fortification status in Ireland. The remainder of the paper considers the preliminary findings of this research in the context of the scientific developments on risks and benefits associated with folic acid intake.
Pregnancies affected by neural-tube defects in Ireland
The establishment of a national register of congenital birth defects is a long-term process. Thus, for the purposes of getting reliable pre-fortification data on the incidence of pregnancies affected by NTD in Ireland, a special audit of cases occurring from 2005 to 2007 was undertaken. Data on prenatal ultrasound scans and birth records from the twenty-one obstetric units in Ireland was used to estimate prevalence rate for pregnancies affected by NTD. This method is much more complete than previous estimations in Ireland because national coverage was provided and information on all affected pregnancies, including those that do not reach term, was counted. The neonatal outcome of affected pregnancies during this period is shown in Fig. 2, which shows 26% did not reach term and, as such, were unaccounted for in previous reports (S Daly, unpublished results). Despite including these cases, the overall estimation of pregnancies affected by NTD during 2005–7 is lower than previous estimates; preliminary data indicates a rate of 0·92 cases per 1000 pregnancies during 2005–7 v. 1·0–1·3 cases (without including cases not reaching term) per 1000 cases during 1997–2001 (S Daly, unpublished results).
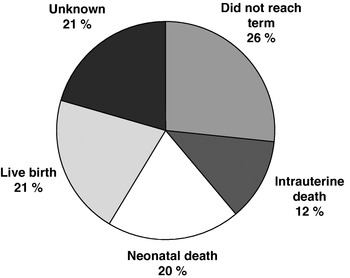
Fig. 2. Neonatal outcome of pregnancies affected by neural-tube defects in Ireland in 2005–7 (S Daly, unpublished results).
Voluntary folic acid food fortification
Folic acid-fortified foods on the Irish market and their impact on folic acid intakes
Surveys of the major supermarket outlets in the Dublin area during the summer of 2007 have identified 211 foods on the Irish market that are voluntarily fortified with folic acid. A summary of food categories fortified, range of fortification levels and market share of fortified products are given in Table 1(Reference McMenamin, Flynn and Burke47). These data show that the range of foods voluntarily fortified with folic acid is extensive and the amounts of folic acid added (according to nutrient content declarations on food labels) between brands is very variable (e.g. fat spreads). This variability is also evident within brands (e.g. different breakfast cereals of the same brand providing 166 μg folic acid/100 g cereal v. 334 μg folic acid/100 g cereal).
Table 1. Voluntary folic acid fortification of food; food categories, fortification levels and market share of fortified foods on Irish market in August 2007 (data from McMenamin et al. (Reference McMenamin, Flynn and Burke47))
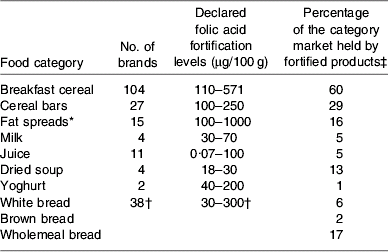
* Follow-up checks in May 2008 identified 50% less folic acid in several brands of fortified fat spreads (August 2007 fortified at 1000 μg/100 g and in May 2008 fortified at 500 μg/100 g).
† Data for the three types of bread.
‡ Based on volume sales 2007 (data supplied by TNS, Blackrock, Co. Dublin, Republic of Ireland).
The 1999 national survey database of adult food intake has been updated to reflect 2007 levels of voluntary folic acid fortification of food. Probabilistic modelling indicates that folic acid intakes of women aged 18–50 years (target group) based on 2007 compared with 1999 food fortification patterns would be increased by approximately one-third (from 67 μg per individual per d to 90 μg per individual per d; Food Safety Authority of Ireland, unpublished results). While in 1999 more than one-third of women aged 18–50 years (35%; 130 of 369 women) did not consume any folic acid-fortified foods, updating the 1999 data in terms of 2007 folic acid food fortification estimates that this percentage of women would be reduced to <2 (seven of 369 women). Folic acid intakes of all adults in terms of the tolerable upper level for folic acid intake (1000 μg/d(30)) reveal a substantial gap between the potential daily folic acid intakes of the highest folic acid consumers (99th percentile folic acid intakes) at 519 μg v. 1000 μg (Food Safety Authority of Ireland, unpublished results). The principal food categories responsible for the increased estimates of folic acid intake in 2007 were fat spreads (particularly in older men) and bread products, which were not fortified in 1999(Reference McMenamin, Flynn and Burke47) (Food Safety Authority of Ireland, unpublished results). It was not possible to update the food consumption database for changes in food supplement use, or content, and hence it is likely that folic acid exposure is underestimated in this analysis.
An analysis conducted on data from the National Food Consumption Survey of 5–12-year-old children (n 594; data collected in 2003–4) living in Ireland has found that 10% (n 61) had intakes in excess of the tolerable upper level for folic acid(Reference McMenamin, Flynn and Burke47). Supplements made a major contribution to high intakes (100–400 μg in nineteen cases), followed by consumption of foods fortified with folic acid (mainly ready-to-eat breakfast cereals, followed by fortified milks, fortified juices and fortified fat spreads(Reference McMenamin, Flynn and Burke47)). The tolerable upper levels for folic acid intake in children (300 μg for 4–6 year olds; 400 μg for 7–10 year olds; 600 μg for 11–14 year olds) do not relate to adverse health risks but are derived from the tolerable upper level set for adults relating to risk of masking pernicious anaemia(30).
Voluntary folic acid fortification of bread in Ireland: analysis of actual content
Samples of all bread types marketed in Ireland (249 loaves of bread) have been analysed for folic acid content. Approximately one-fifth of bread brands were found to be fortified with folic acid and in general actual levels exceed declared levels. (One sample was found to contain 5096 μg folic acid/100 g bread and this sample was investigated separately. Investigations have revealed a systematic error in the quantity of folic acid added to the flour improver by the improver supplier allegedly following confusion over the agreed specification. This issue was resolved by the manufacturer and validated in subsequent testing by the Food Safety Authority of Ireland.) The difference between actual folic acid content and levels declared on food labels for those fifteen samples that made nutrient declarations is shown in Fig. 3; analysis reveals between 23 and 224% more folic acid than declared on food labels. This level of ‘overage’ is a matter of concern, particularly if it is evident for other voluntarily-fortified foods. These findings also indicate underestimation of dietary folic acid intakes from fortified foods because these intakes are generally assessed in terms of the nutrient content declared on labels. Thus, actual folic acid intakes of population subgroups in Ireland may be greater than those indicated by dietary survey databases, including those updated in terms of current voluntary food fortification levels.
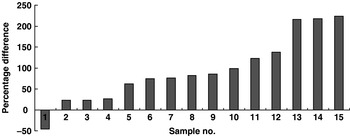
Fig. 3. Difference between folic acid content declared on food labels and actual measured folic acid content of bread voluntarily fortified in Ireland during 2007.
Achieving approximately 400 μg folic acid every day using fortified products
In the context of the recent associations between high folic acid intakes and cancer, women may be anxious to avoid excessive folic acid intakes (i.e. intakes >400 μg/d). Considering this issue, the ease of achieving 400 μg folic acid/d using fortified foods on the Irish market has been examined(Reference McMenamin, Flynn and Burke47). Using the folic acid content declared on food labels, numerous daily options were easily devised and the wide range of folic acid-fortified foods available ensured individual food preferences were likely to be accommodated. However, the following challenges to such use of folic acid-fortified foods, in practice, were identified:
1. nutrition labelling in Europe is based on nutrient content/100 g or ml food product rather than an average serving. Estimation of folic acid intake from fortified foods therefore requires previous knowledge of average serving sizes and, in the context of everyday-life, reasonably complex calculations;
2. variability in amounts of folic acid added to different brands of the same food (e.g. bread in Table 1) or between similar foods within the same brand (e.g. cereals) makes it very difficult to become familiar with folic acid content of fortified foods;
3. the ad hoc nature of voluntary fortification, whereby levels of folic acid addition can be discontinued or radically changed, represent further difficulties (e.g. fat spreads in Table 1);
4. ‘overage’ resulting in higher amounts of folic acid in food products relative to the amounts declared on food labels.
Without revision of nutrition labelling regulations (e.g. in favour of using serving size, regulation of tolerance levels around nutrient content declared on labels) practical use of folic acid-fortified foods by women to achieve a daily intake of 400 μg folic acid is extremely difficult.
Blood folate status of population sub-groups in Ireland
Factors such as ‘overage’ (nutrient fortification above level declared), increasing voluntary folic acid food fortification, difficulties in assessing specific food brand intake and underreporting actual food intake collectively suggest that Irish dietary intake data may have under-represented true folic acid intake levels. Biochemical indicators of folate status therefore provide critical assessment of actual exposure to folic acid in the food supply. Erythrocyte folate (RCF) and serum folate have been determined in blood samples of particular subgroups of the Irish population collected during 2006. The population subgroups were purposely recruited to represent age and gender groups of interest and included children <16 years (n 230; 50% female), women of child-bearing age (n 259; 16–40 years) and middle-aged women (n 292; aged 40–60 years), adult men (n 292) and older adults >65 years (n 462; 55% female). Data on multivitamin supplement use were also recorded.
Reflective of the recent proliferation of folic acid-fortified foods on the Irish market, RCF and serum folate levels from samples collected in 2006 generally point to a high folate status, as shown in Tables 2 and 3. Compared with earlier reports from the same laboratory in 1998 showing that 5% of adolescent girls were folate deficient (RCF <150 ng/ml)(Reference Ryan, McPartlin, Gibney and Flynn48), very few (approximately 1%) individuals were found to be folate deficient in 2006. Previously, RCF levels >400 ng/ml in women have been shown to be protective against the development of NTD(Reference Daly, Kirke, Molloy, Weir and Scott49). Samples collected from women of child-bearing age (16–40 years) in 2006, compared with those collected from teenage girls in 1998, indicate that the percentage of women with a RCF status >400 ng/ml has more than doubled in Ireland over recent years (53 in 2006 v. 25 in 1998; Table 2). Dietary intake data in the 1998 study show that folic acid-fortified foods (breakfast cereals, milk and bread) contributed significantly to the variance in RCF levels found among the teenage girls(Reference Ryan, McPartlin, Gibney and Flynn48).
Table 2. Percentage of population subgroup cohorts in Ireland according to categories of erythrocyte folate (RCF) levels assessed during 2006 (JM Scott and AM Molloy unpublished results) and 1998(Reference Ryan, McPartlin, Gibney and Flynn48)
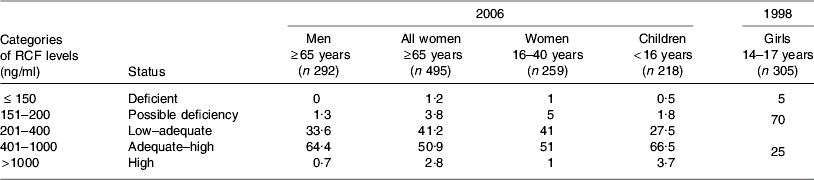
Table 3. Percentage of population subgroup cohorts in Ireland according to categories of serum folate levels assessed during 2006 and 2007 (JM Scott and AM Molloy, unpublished results)
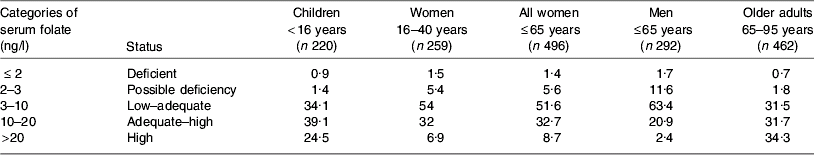
High RCF levels (>1000 ng/ml) are evident in 3–4% of women and children, as compared with <1% of adult men. No RCF data are available for older adults in 2006. However, as shown in Table 3, high serum folate levels are evident in more than one-third (34%) of elderly individuals, and one-quarter (24·5%) of children, compared with 2% of men and <9% of women. All blood samples were collected from non-fasting participants and therefore recent intake of folic acid-fortified food may have distorted the serum folate levels detected. Exclusion of regular consumers of multivitamin supplements (every day or several times weekly) has a minimal effect on 2006 folate status indicators. These data indicate that the increase in folic acid-fortified foods on the Irish market largely explains high levels of folate status indicators among the subgroups examined. In addition, the data on serum folate in particular indicate that children and older adults may be more exposed to fortified foods. In the case of children for whom RCF data exist, these findings for serum folate are consistent with RCF levels.
Folic acid food fortification and the Irish experience: conclusions
The present paper shows that although voluntary food fortification can have beneficial effects on public health, it cannot serve as a reliable public health intervention. Work undertaken by the Food Safety Authority of Ireland in preparation for mandatory fortification has found that voluntary folic acid food fortification has escalated in recent years. Preliminary findings indicate that greater intake of folic acid from such fortified foods in Ireland is a likely explanation for falling rates of pregnancies affected by NTD, but also for high blood folate status indicators, especially among children and the elderly. Recent scientific developments linking excessive folic acid intake with increased risk of colo-rectal cancer emphasise the need to ensure that individuals are not exposed to excessive folic acid levels in the food supply(Reference Cole, Baron and Sandler27–Reference Bayston, Russell, Wald and Hoffbrand29).
The major disadvantage of voluntary folic acid food fortification is that it provides an uneven distribution of folic acid intake across the population (i.e. only consumers of the fortified brands of products are exposed to benefits or risks). This situation is exacerbated by complex nutrition labelling, which prevents judicious use of fortified foods to achieve targeted folic acid intake levels. Finally, given the current range of food products fortified and the variation in amounts of folic acid added between and within different brands, intake levels among consumers are hard to monitor.
These issues collectively indicate an urgent need for regulation of voluntary folic acid food fortification both in terms of amounts of folic acid added and the range of foods in which such fortification is permitted. Furthermore, nutrition labelling of fortified foods should be clear to enable consumers to achieve targeted intake levels. Such legislation is currently being developed at the European level.
Acknowledgements
The work of the Folic Acid Food Fortification Implementation Group, Food Safety Authority of Ireland is gratefully acknowledged. Particular thanks are due to Professor John M. Scott and Dr Anne M. Molloy (Trinity College Dublin, Dublin, Republic of Ireland) and Dr Sean Daly (Coombe Women's Hospital, Dublin, Republic of Ireland) who carried out the baseline monitoring investigative studies, the preliminary findings of which are highlighted in this paper. The work of Una McMenamin and Susan Gallagher, who conducted the supermarket surveys, and Christina Tlustos, who carried out the re-modelling to account for 2007 fortification levels, is gratefully appreciated. Finally, special thanks are due to Aileen Ward who prepared the manuscript.








