- ATBF
adipose tissue blood flow
- LPL
lipoprotein lipase
The immediate fate of dietary fat is important to health. Indeed, atherogenesis has been described as a postprandial phenomenon(Reference Zilversmit1), and postprandial lipaemia (the rise in plasma TAG concentrations after a meal) is thought to be involved(Reference Karpe2, Reference Goldberg, Kako and Lutz3). Although the exact mechanisms that link postprandial lipaemia remain to be elucidated, small chylomicron remnants have been implicated in the progression of coronary artery disease(Reference Karpe, Steiner and Uffelman4). Postprandial lipaemia has also been shown to be associated with oxidative stress and inflammation as recently reviewed(Reference Paglialunga and Cianflone5). Therefore, it is important to understand factors that influence the duration and magnitude of postprandial lipaemia. A key tissue in the disposal of meal fatty acids is adipose tissue. The importance of adipose tissue in this respect is outlined in the so-called adipose tissue expandability hypothesis(Reference Virtue and Vidal-Puig6). This hypothesis proposes that ‘a failure in the capacity for adipose tissue expansion, rather than obesity per se is the key factor linking positive energy balance and type 2 diabetes’. With increasing adiposity in some individuals, it is proposed that the capacity of adipose tissue to store further TAG is reduced, and lipids begin to accumulate in other tissues. The aim of this paper is to summarise the metabolic studies in human subjects performed by ourselves and others that have helped to understand the way in which adipose tissue metabolises and stores dietary fat.
The single meal model
A typical protocol for studying postprandial lipaemia is to study volunteers after an overnight fast followed by a single meal. While fasting, the role of adipose tissue is to release fatty acids into the systemic plasma in order to supply tissues with a high requirement for fatty acids, such as skeletal muscle and the heart. Thus, fasting plasma NEFA concentrations are high (Fig. 1). After a test meal is given, adipose tissue metabolism is coordinated in order to deal with the nutrient load that is given. A typical metabolic response to a high-fat meal in a healthy non-obese male is shown in Fig. 1. As fat enters the bloodstream in the form of chylomicron-TAG the concentration of plasma TAG increases. The increase in plasma TAG is also partly due to an increase in the concentration of endogenous TAG in VLDL (synthesised in the liver)(Reference Karpe, Steiner and Olivecrona7) and plasma taken after a meal containing fat is often cloudy because these large lipoproteins scatter light. The concentration of plasma TAG starts to fall as it is cleared from the plasma (Fig. 1). This is largely mediated by the action of adipose tissue lipoprotein lipase (LPL)(Reference Fielding and Frayn8), situated at the capillary endothelium. LPL hydrolyses chylomicron-TAG, releasing fatty acids to be taken up by adipose tissue. This pathway is up-regulated by insulin, which increases rapidly in response to the carbohydrate content of the meal (Fig. 1). It has long been known that LPL is inhibited by apoCII but it has recently been shown that LPL is also inhibited physiologically by angiopoietin-like protein-4(Reference Sukonina, Lookene and Olivecrona9). The expression of this protein appears to be decreased in response to food, thus lifting the inhibition and allowing TAG hydrolysis to proceed at a higher rate. Studies from knock-out mice have shown that LPL also appears to require GPIHBP1, a cell-surface glycoprotein synthesised by the endothelium(Reference Beigneux, Davies and Bensadoun10).
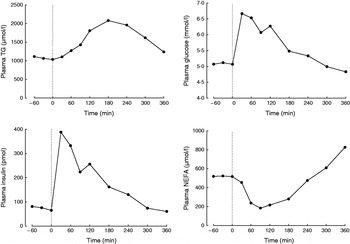
Fig. 1. Typical postprandial concentrations of plasma metabolites in a healthy male after a mixed meal. Data taken from Bickerton et al. (Reference Bickerton, Roberts and Fielding23).
In contrast to the increase in concentration of plasma TAG after a mixed meal, the concentration of plasma NEFA rapidly decreases, but often rebounds above postabsorptive values at the end of the postprandial period (Fig. 1). The initial decrease is due to the action of plasma insulin on the suppression of intracellular lipases. Thus, insulin is a key mediator of changes in adipose tissue fatty acid trafficking in the transition from fasting to fed states; any meal given with no carbohydrate (e.g. pure fat load) would fail to illicit the metabolic responses that depend on the increase in plasma-insulin concentrations. However, adipose tissue is very sensitive to insulin. Therefore, even a small insulin excursion can reduce plasma insulin concentrations (Fig. 2, after fructose ingestion).

Fig. 2. Origins of plasma NEFA after ingestion of glucose or fructose, 0·75 g sugar/kg body weight plus 500 mg [2H2]palmitate. Data taken from Chong et al. (Reference Jensen45). Data are shown as means and sem, n 12. Systemic plasma NEFA concentrations are shown as filled circles (P<0·05 comparing effect of sugars(Reference Jensen45). Fatty acids estimated to have arisen from adipose tissue intracellular lipolysis are shown as open circles (P=ns comparing effect of sugars). The difference is the estimated concentration of plasma NEFA derived from dietary-TAG ‘spillover’ (see text), calculated as follows: spill over fatty acids (μmol/l)=pNEFA (μmol/l)/(% palmitate in chylomicron-TAG)*100, where pNEFA is the concentration of fatty acids in the plasma NEFA pool derived from chylomicron spillover. pNEFA=concentration of [2H2]palmitate in plasma/chylomicron TTR. TTR=the tracer tracee ratio for [2H2]palmitate. For assumptions see text.
The two-meal model
The single meal model is very useful in understanding the key pathways involved in the metabolism of dietary fatty acids, but in reality, most people on a Western diet would eat a second meal less than 5–8 h later. It became clear that postprandial TAG metabolism became complicated by a second, later meal, giving an unusual plasma TAG profile with more than one peak(Reference Silva, Wright and Williams11). We further investigated plasma TAG concentrations in response to two meals by using naturally occurring fatty acids in foods as tracers of metabolism(Reference Fielding, Callow and Owen12). After an overnight fast, we gave healthy volunteers a high-fat breakfast that was enriched in linoleic acid (18:2n-6). Five hours later, the volunteers consumed a lunch meal that was enriched in oleic acid (18:1n-9). After the second meal, there was a rapid peak in chylomicron-TAG, and the fatty acid composition of this early peak was remarkably similar to that of the breakfast meal. This suggested that the breakfast fat had been residing in a storage pool before being released by a stimulus from lunch. In an elegant study, Robertson et al.(Reference Robertson, Parkes and Warren13) obtained biopsy material from volunteers who had consumed fat 5 h previously, and used electron microscopy to show that the fat was in fact residing in the enterocyte, and that it was released in response to a nutrient stimulus.
Spillover fatty acids
An interesting observation from the two-meal study described above was that the plasma NEFA profile was quite unusual. While we normally expect plasma NEFA concentrations to decrease after a meal, an increase was observed in response to lunch. Moreover, the composition of plasma NEFA after lunch was similar to that of breakfast fat (and the ‘early chylomicron-TAG’ peak). This gave support to the notion that when chylomicron-TAG is hydrolysed by LPL, not all the fatty acids are taken up; some spillover or ‘leak’ into the systemic plasma(Reference Frayn, Kinney and Tucker14–Reference Miles, Park and Walewicz16). ‘Spillover’ fatty acids can be quantified using stable isotope tracers. The assumptions are that LPL does not discriminate between different fatty acids originating from the same meal(Reference Summers, Barnes and Fielding17), and that spillover fatty acids derive solely from chylomicron-TAG, i.e. that within the time course of the study, appearance in the plasma NEFA fraction of meal fatty acids that have arisen via recycling through VLDL-TAG is minimal(Reference Ruge, Hodson and Cheeseman18).
In the following example, the contribution of whole body ‘spillover fatty acids’ to the systemic plasma NEFA pool has been calculated. The data are from a study in which we investigated the effect of fructose v. glucose on postprandial lipaemia. Two-hundred and fifty milligrams of [2H2]palmitate was given as part of a liquid test meal and was traced into chylomicron-TAG. The appearance of [2H]palmitate in the plasma NEFA fraction was therefore assumed to be due to spillover from LPL-mediated hydrolysis. From this data it was possible to derive the proportion of fatty acids in the total plasma-NEFA pool that had arisen from spillover (Fig. 2). It was found that the higher plasma-NEFA concentration observed after the glucose test meal (compared with fructose) was entirely due to the higher concentration of fatty acids originating from the spillover route (Fig. 2). In other words, spillover fatty acids account for the ‘rebound’ effect whereby plasma-NEFA concentrations are higher at the end of the study period than at the beginning (Fig. 1). The proportion of chylomicron fatty acids that spillover has been found to be the highest (as much as 80%) in the late postprandial period(Reference Evans, Burdge and Wootton19). This may be due to the fact that fatty acids are less easily taken up into adipose tissue against a concentration gradient, i.e. when adipose tissue lipolysis is higher(Reference Evans, Burdge and Wootton19, Reference Miles and Nelson20). This would be in accordance with observations that tissues without a high lipolytic flux such as forearm muscle would appear to have as much as 100% efficiency in terms of the uptake of LPL-derived meal fatty acids(Reference Evans, Burdge and Wootton19).
The technique of arteriovenous difference
Adipose tissue metabolism can be quantified in specific adipose tissue depots by the technique of arteriovenous difference. Blood is sampled simultaneously from an artery (or arterialised vein) and a small vein draining the adipose tissue. The difference in concentration of plasma or blood metabolites between the two sites represents metabolism during one pass through the tissue. We have used this technique to study adipose tissue metabolism of meal fatty acids, initially in human abdominal subcutaneous tissue(Reference Coppack, Fisher and Gibbons21) and more recently in the femoral depot(Reference McQuaid, Manolopoulos and Dennis22). In order to quantify flux through the tissue, adipose tissue blood flow (ATBF) must be measured allowing the net transcapillary flux of fatty acids across the tissue to be calculated. This represents the net balance of different pathways of fatty acid trafficking through the tissue. In the postabsorptive state, there is a large concentration gradient between plasma NEFA entering the tissue (artery) and plasma NEFA leaving the tissue (adipose venous drainage). In a group of healthy men (BMI 23–34), the mean values were 591±41 and 1208±131, respectively(Reference Bickerton, Roberts and Fielding23), and the mean net flux of NEFA from the tissue was 1080 nmol per 100 g tissue per min. Using an average adipose tissue fatty acid composition(Reference Hodson, Skeaff and Fielding24), this equates to approximately 250 μg adipose tissue TAG hydrolysed per 100 g tissue/min. For a person in energy balance with a 20 kg total fat mass this represents approximately 24 g TAG to be replaced (0·12 % of adipose tissue TAG).
Adipose tissue uptake of meal fatty acids
Using the technique of arteriovenous difference and measurements of mass balance, adipose tissue has been found to be a net importer of fatty acids for 5 h following a mixed meal in a number of studies as reviewed(Reference Frayn25). The uptake of meal fatty acids can be studied more specifically using either radioactive(Reference Romanski, Nelson and Jensen26) or stable isotope tracer methodology. With the addition of stable isotope tracers to our protocol of arteriovenous difference described above, we were able to develop a very powerful model to study adipose tissue metabolism in vivo. In this model, [U-13C]palmitic acid is given orally, in order to represent exogenous fatty acids, and [2H2]palmitate is given as a continuous intravenous infusion, in order to label endogenous pools (plasma NEFA and VLDL-TAG), see Fig. 3. Using a specific antibody to apoB100, combined with immunoaffinity chromatography, we are able to separate VLDL from chylomicrons(Reference Heath, Karpe and Milne27). This represents a very important advancement in the study of postprandial fatty acids as there is a large overlap in the size and density of these two lipoprotein classes.
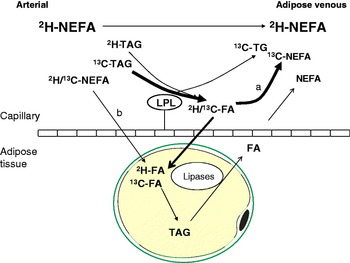
Fig. 3. (Colour online) Pathways of postprandial adipose tissue fatty acid trafficking studied by isotopic labelling of plasma lipid pools. Endogenous pathways are labelled using an intravenous infusion of [2H2]palmitate (K salt) complexed with human albumin. This equilibrates with systemic plasma NEFA and is taken up by the liver where it is esterified to TAG and exported in very VLDL. Exogenous pathways are labelled by the ingestion of [13C]palmitic acid with a test meal. This is incorporated into chylomicron-TAG but soon appears in the plasma NEFA pool via the ‘spillover route (a) after hydrolysis by the enzyme LPL. [13C]palmitic acid also becomes incorporated into VLDL in the liver via plasma NEFA uptake, and through chylomicron remnant uptake. In adipose tissue, such recycled VLDL-TAG fatty acids are not thought to contribute quantitatively to the spillover route. Fatty acids can be taken up directly from the plasma NEFA pool (b).
In the study of healthy men mentioned above(Reference Bickerton, Roberts and Fielding23), we traced 100 mg [U-13C]palmitic acid into the chylomicron fraction where it was measurable at 60 min and peaked at 240 min. We calculated TAG extraction in adipose tissue as (arteriovenous difference of [U-13C]palmitic acid in plasma TAG)×ATBF, and this peaked at 120 min. The time course of fractional TAG extraction is very interesting. At 60 min after the test meal, it is as high as 30% in some individuals but falls to about 10% at 180 min. Presumably, this is because it is only during the early time points that newly released chylomicrons are available for hydrolysis. These newly released chylomicrons would be comparatively large, and therefore a good substrate for LPL(Reference Xiang, Cianflone and Kalant28). In addition, at later time points, plasma TAG-[U-13C]palmitic acid is no longer confined to chylomicrons, and may represent newly synthesised VLDL-TAG. We also calculated the fractional extraction of plasma [2H2]palmitate-TAG, representing the hydrolysis of VLDL-TAG. This was less than the fractional extraction of plasma [U-13C]palmitate-TAG, indicating preferential hydrolysis of chylomicron-TAG rather than VLDL-TAG, in agreement with in vitro findings that LPL binds to chylomicrons with a greater affinity than VLDL(Reference Xiang, Cianflone and Kalant28). Moreover, we demonstrated preferential uptake of fatty acids derived from chylomicron TAG compared with fatty acids taken up directly from the plasma NEFA pool. The latter pathway has only recently been recognised as a significant route of fatty acid uptake into adipose tissue(Reference Koutsari, Dumesic and Patterson29).
Adipose tissue dietary fatty acid metabolism in response to a high-carbohydrate diet
It is well known that a high carbohydrate diet, particularly one that is high in extrinsic sugars, leads to an increase in fasting plasma TAG concentrations(Reference Chong, Fielding and Frayn30). We explored the capacity of subcutaneous adipose tissue to ‘cope’ with clearing meal fatty acids in the context of hypertriacylglycerolaemia promoted by the ingestion of a high carbohydrate, low fat diet for 3 d(Reference Roberts, Bickerton and Fielding31). This rather extreme diet (75% energy from carbohydrate) led to a doubling in fasting plasma TAG from 1·0 to 2·0 mmol/l compared with a diet that was only 45% energy from carbohydrate. In response to a standard test meal, the iAUC (incremental area under the curve) for postprandial lipaemia was similar after both diets. However, after the high carbohydrate diet, postprandial plasma TAG concentrations did not return to baseline by the end of the study (360 min). Fasting and postprandial plasma NEFA concentrations were identical after the two dietary regimens. We traced the meal fat with [13C]palmitic acid and found that plasma [13C]palmitate-TAG extraction and TAG clearance in adipose tissue were unaffected by the background diet. Thus, it would seem that despite an increase in the fasting concentration of plasma TAG after the high-fat diet, the ability of adipose tissue to dispose of dietary fat was unaffected. The metabolism of other tissues was, however, affected; TAG clearance (also measured by arteriovenous difference) was significantly lower across the forearm after the high-carbohydrate diet v. the high-fat diet. Also meal fatty acids tended to be repartitioned away from oxidation, towards esterification in the liver and muscle in response to short-term adaptation to the high carbohydrate diet.
Twenty-four hour studies of human adipose tissue metabolism
To follow-on from the arteriovenous difference and two-meal models described above, we investigated postprandial fatty acid metabolism over a 24 h period, in order to study the response to three typical Western-style mixed meals(Reference Ruge, Hodson and Cheeseman18). We used a continuous intravenous infusion of [2H2]palmitate as before, to label systemic fatty acids and used three unique uniformly labelled fatty acid tracers to trace the meal fatty acids. To label the breakfast meal, we used [U-13C]linoleic acid, and to label lunch and dinner, we used [U-13C]oleic acid and [U-13C]palmitic acid, respectively. The uniformly labelled notation indicates that all the carbon atoms are stable isotopes and an advantage of this analytically is that there is minimal background (when using GC-MS) compared with fewer carbons being labelled. Additionally, the high density of labelling offers greater potential for tracing metabolic pathways(Reference Hodson, McQuaid and Humphreys32). The [U-13C]fatty acid tracers appeared in the plasma TAG pool after each respective meal but although the concentration started to fall after about 5 h, there always remained some tracer present. Thus, 24 h after the ingestion of [U-13C]linoleic acid, there was measurable [U-13C]linoleic acid in the plasma-TG pool. This was assumed to represent chylomicron remnants and tracers that had become incorporated into VLDL-TAG via hepatic recycling. Likewise, [U-13C]linoleic acid in the plasma NEFA pool, representing spillover fatty acids was still present at 24 h. Using a radioactive tracer of meal fatty acids, it has been estimated that 0·9% of meal fatty acids remain in the circulation after 24 h(Reference Jensen, Sarr and Dumesic33). In our study, we were able to calculate the total transcapillary flux of fatty acids across subcutaneous abdominal adipose tissue in healthy non-obese men. This calculation represents the total fatty acids, from all pools, crossing in/out of adipose tissue from the plasma. Over the 24 h period, there was net uptake of fatty acids immediately after the first meal, and this continued until approximately 17 h after breakfast, i.e. during the whole of the daytime adipose tissue takes up and stores fatty acids. Although the plasma TAG taken up after a meal was mainly from chylomicrons, a proportion was taken up from VLDL-TAG. The quantitative significance of this pathway increased with each meal and made up one-third of the total of the transcapillary flux after the third meal. Plasma NEFA were also taken up directly, but this remained small. We estimated the amount of fat from the meal that was taken up into adipose tissue at a whole-body level. This increased from 15% of the meal fat after the first meal to 48% after last meal. The calculation is only approximate since it depends on an assumption that regional variation of adipose tissue metabolism is insignificant. However, the increase in stored fat with time is still a valid observation. This observation was dependent on an increase in LPL rate of action during the 24 h period, but even more so by an increase in fatty acid re-esterification (i.e. stepwise formation of TAG via the action of the enzymes monoacylglycerol transferase and diacylglycerol transferase). These lean subjects had a 2-fold increased LPL rate of action over 24 h but a 3-fold increase in net fatty acid uptake.
Meal fatty acid handling in insulin resistance/obesity
Fasting plasma TAG concentrations are typically higher in obesity, and this is associated with enhanced hepatic secretion of VLDL(Reference Cummings, Watts and Pal34). This is associated with higher postprandial TAG concentrations due to increased VLDL and chylomicron remnants(Reference Boquist, Hamsten and Karpe35). This is also associated with a prolonged lipaemia, representing delayed clearance of dietary fat. The higher overnight fasting and postprandial concentration of VLDL-TAG in obesity are intimately related to plasma insulin concentrations(Reference Boquist, Hamsten and Karpe35). We recently found that the higher postprandial TAG concentrations in insulin resistant, compared with insulin sensitive men (matched for BMI) was accounted for differences in the contribution of splanchnic sources (from de novo lipogenesis, visceral adipose tissue lipolysis or hepatic TAG) rather than from dietary sources(Reference Hodson, Bickerton and McQuaid36). We found that meal fat clearance, as measured by clearance of [U-13C]palmitate in chylomicron-TAG was lower in the insulin resistant men.
The burden of the higher fasting plasma TAG that often accompanies obesity can be expected to impact upon postprandial adipose tissue metabolism. We used the 24 h model described above to compare the abdominal subcutaneous adipose tissue metabolism of healthy lean and obese men(Reference McQuaid, Hodson and Neville37). Despite higher fasting and postprandial plasma TAG concentrations, plasma NEFA concentrations were very similar throughout the whole of the 24 h period. How plasma NEFA homoeostasis in the postprandial period is maintained in the face of an expanded adipose tissue mass and disturbed TAG metabolism is an interesting question. Using data derived from the intravenous infusion of [2H2]palmitate to calculate rate of appearance of NEFA (RaNEFA), we were able to determine that whole body lipolysis at all time points was considerably down-regulated when expressed in per unit of fat mass. At the tissue level, adipose tissue NEFA release was markedly lower in the obese men. Perhaps even more dramatically, the hydrolysis of meal fatty acids was severely diminished in the obese men; LPL rate of action was very low and failed to be up-regulated during the course of the day (Fig. 4). Total fatty acid trafficking was considerably lower in the obese group. The adipose tissue quiescence seemed to be achieved via two main mechanisms. Firstly, gene expression of key enzymes relating to fatty acid trafficking such as LPL, diacylglycerol acyl transferase 1 and 2 (DGAT1, DGAT2) and hormone sensitive lipase (HSL) were lower in a similar group of obese men(Reference McQuaid, Hodson and Neville37). Secondly, subcutaneous ATBF was markedly lower and very unresponsive for the whole day in the obese group compared with lean group. This is in agreement with findings after a single meal; ATBF is highly associated with insulin sensitivity(Reference Karpe, Fielding and Ilic38). ATBF is integral to adipose tissue function, and an impaired blood flow will lead to a reduced supply of TAG substrate and reduced removal of products of metabolism within the adipocyte or at the capillary endothelium. The difference in adipose tissue handling of meal fatty acids meant that overall, even accounting for differences in adipose tissue mass, the obese group stored a lower proportion of fatty acids from the three meals in adipose tissue. Notably, they did not increase the efficiency of storage over the day, as was found in the lean group. The implications for this are important, an excess of meal fatty acids would lead to increased exposure to other tissues and could lead to the possibility of ectopic fat deposition.
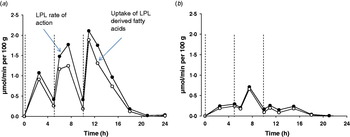
Fig. 4. (Colour online) Meal fatty acid uptake into adipose tissue. Adipose tissue LPL rate of action (assumed to be equivalent to TAG extraction) and uptake of LPL derived fatty acids (TAG extraction minus spillover fatty acids) in nine lean (a) and ten abdominally obese men (b), over a 24 h period, during which three mixed meals were consumed, at t=0, 5 and 10 h (dotted lines). Data are calculated from fatty acid stable isotope tracers, from data in the study of McQuaid et al. (Reference McQuaid, Hodson and Neville37).
Metabolism of specific fatty acids
Some of the measurements described above have been made with the assumption that palmitic acid behaves as a typical fatty acid. Differences in the postprandial metabolism of specific fatty acids can be studied in one of two ways. Either different fatty acids are given within the same test meal, and compared, or the response of different meals (containing different fatty acids) is compared.
We have investigated the adipose tissue metabolism of different fatty acid species consumed in a single test meal using the technique of arteriovenous difference, using commercially produced structured TAG or natural fats. Perhaps surprisingly, we found that adipose tissue did not discriminate between the fatty acids that we were testing for either type of test meal. Using structured TAG containing oleic acid plus either stearic or palmitic acid, we found no difference in LPL-mediated hydrolysis of the different fatty acids or in their subsequent uptake by the tissue. The structural position of the individual fatty acids within the TAG molecule was also unimportant. We investigated the metabolism of different fatty acids given in the same test meal (14:0, 16:0, 16:1n-7, 18:0, 18:1n-9, 18:2n-6, 20:5n-3, 22:6n-3). Although the molar proportion of the fatty acids in the meal was not maintained in chylomicrons, net uptake into adipose tissue was entirely proportional to their presence in chylomicrons. Nonetheless, overall, this meant that the storage of meal fatty acids into adipose tissue was in the order n-3 polyunsaturated<saturated<n-6 polyunsaturated<monounsaturated; oleic acid was stored in the greatest amount. Note that these studies did not use stable isotope tracers; therefore represent net uptake. We have recently compared the metabolism of stable isotope tracers of 16:0 ([U-13C]palmitate), 18:1n-9 ([U-13C]oleate) and 18:2n-6 ([U-13C]linoleate; all tracers were given simultaneously in the same test meal. We found that their incorporation into chylomicron TAG was similar. However, there was a tendency for the incorporation of 18:1n-9 to be the highest. This pattern was maintained as the fatty acids became incorporated into plasma NEFA and VLDL-TAG. This was explained by the low partitioning of [U-13C]oleate into plasma cholesteryl ester and phospholipid fractions, and also erythrocyte phospholipid. Meal fatty acids are taken up into adipose tissue mainly via chylomicron TAG, VLDL-TAG and plasma NEFA. Therefore, the isotopic studies have shown that preferential uptake of meal oleate, compared with other meal fatty acids, could occur due to greater availability in these fractions.
Another aspect of comparing the metabolism of different fatty acids is to compare their postprandial metabolism when given separately. In this case, the fatty acids could behave differently due to different physico-chemical properties of the chylomicrons formed from the different test meals. This is difficult to study in human subjects because of the rapidity by which chylomicrons are cleared in the blood. However, animal studies in which the lymph has been accessed have shown that different dietary fatty acids form different sized chylomicrons. For example, linoleic acid forms larger chylomicrons than palmitic acid, and there is some evidence to suggest that this is the case in human subjects too, as reviewed in(Reference Williams, Bateman and Jackson39).
Regional differences in fatty acid metabolism
Not only is the amount of adipose tissue important to health, body fat distribution is also important. The role of visceral fat (upper body fat, associated with a large waist) in relation to the metabolic syndrome has recently been reviewed(Reference Despres, Lemieux and Bergeron40) but the relationship is not clear(Reference Frayn41, Reference Miles and Jensen42). Paradoxically, greater accumulation of fat on the hips is beneficial in terms of risk of myocardial infarction(Reference Yusuf, Hawken and Ounpuu43).
Because of the importance of body fat distribution, studies have attempted to look at mechanisms behind differential accumulation of fat in different depots. Regional meal fat storage has been studied by giving a 3H or 14C fatty acid tracer with a meal and then measuring specific activity in adipose tissue depots 24 h later. An advantage of radioactive tracers in this respect is that they are very sensitive; an important consideration since the tracer is dispersed into a large pool.
Regional differences in the uptake of meal fatty acids into adipose tissue depots has been comprehensively investigated by Jensen et al. using a [3H]triolein tracer given with a liquid test meal(Reference Jensen, Sarr and Dumesic33). Fatty-acid oxidation was determined by incorporation of the tracer into the plasma-water pool and it was estimated that approximately 50% of the tracer was oxidized over the 24 h. Intra-abdominal fat took up the tracer with the greatest avidity (Table 1) but the upper body depot, being the largest depot, accounted for over half of the uptake of fatty acids not oxidized. After a high-fat, high-energy meal, women store more dietary fatty acids in leg fat than men(Reference Votruba and Jensen44). Moreover, meal fatty acid storage (mg meal fat/g adipose tissue lipid) was greater in women with more leg fat than those with less leg fat. Also, after a high-fat meal, the depots with more fat seemed to take up meal fat less avidly than those with less fat.
Table 1. Uptake of fatty acid tracer into different adipose tissue depots in lean men and women (n 6) (data taken from Jensen et al. (Reference Jensen, Sarr and Dumesic33)). The lower body subcutaneous fat is demarcated as all adipose tissue caudal to the inguinal ligament anteriorly and the ileac crest posteriorly(Reference Jensen45)

* % Taken up/% body fat.
Mcquaid et al. have recently developed a technique to apply the technique of arteriovenous difference to femoral adipose tissue by cannulation of the saphenous vein(Reference McQuaid, Manolopoulos and Dennis22). In accordance with the work of Jensen et al. (Table 1), it was found that net uptake of meal fatty acids was lower in femoral than subcutaneous abdominal tissue. ATBF was also lower in the femoral depot. An interesting finding was that while chylomicron-TAG was the preferred source for postprandial fat deposition in the subcutaneous abdominal depot, the femoral depot discriminated less against VLDL-TAG. It was therefore hypothesized that femoral adipose tissue may accumulate dietary fatty acids that have been recycled as VLDL and NEFA.
Conclusions
Healthy adipose tissue adapts rapidly to the ingestion of a mixed meal. Intracellular lipolysis is immediately suppressed, reducing the flux of fatty acids leaving the tissue. An increase in ATBF facilitates the entry of meal fat into the tissue, thus providing substrate for LPL which is activated by the action of insulin. The fractional extraction of chylomicrons is very efficient, as much as 30% in the early postprandial period. It is possibly higher than this at earlier time points. Not all fatty acids released by the action of LPL are taken up by the tissue; some are released into the plasma, particularly in the late postprandial period where as much as 50% of the plasma NEFA pool is composed of meal fatty acids. While these dietary fatty acids may be taken up by non-adipose tissues, there is the potential to recycle back to adipose tissue and be taken up directly from the plasma NEFA pool. Adipose tissue is a net importer of dietary fat for 5 h following a single test meal and for most of the day during a typical three-meal eating pattern. The action of LPL seems to increase sequentially after meal intake, but uptake of meal fatty acids into adipose tissue increases to a greater extent, suggesting up-regulation of pathways of esterification. Obesity and insulin resistance are associated with higher fasting and postprandial plasma TAG concentrations and reduced efficiency of adipose tissue meal-fat storage. This offers a possible explanation to explain ectopic fat deposition associated with obesity. Hypertriacylglycerolaemia due to a high carbohydrate diet in non-obese individuals does not affect the ability of adipose tissue to clear dietary fat. There is a marked difference in the way that different adipose tissue depots handle dietary fat and this may be related to the metabolic phenotypes associated with different body fat distribution patterns.
Acknowledgements
Financial support is acknowledged from the project ‘Hepatic and adipose tissue and functions in the metabolic syndrome’ (HEPADIP, http://www.hepadip.org/), which is supported by the European Commission as an Integrated Project under the 6th Framework Programme (Contract LSHM-CT-2005-018734). The author declares no conflict of interests. I am indebted to all my work colleagues, past and present, for help, kindness and support. I would like to pay particular thanks to my mentors, Professor Keith Frayn and Fredrik Karpe, and colleagues Sandy Humphreys and Leanne Hodson. Thanks also to Mark Fielding for help with data handling.







