Introduction
Rice, Oryza sativa, enriched in starch, lipids and proteins is a staple food for 21% of the global population. About 90% of the total production of rice is consumed in Asia (Sharma and Khanna, Reference Sharma, Khanna, Shah, Khan, Iqbal, Turan and Olgun2019). The commercial value of this crop is fully dependent on its quality across the value chain, and maintaining quality is extremely difficult for domestic and global trade. Seed deterioration due to ageing is a major concern for cereal crops, especially during storage before marketing. Rice seeds are preserved to retain physiological quality over time, whereas grains are stored to reduce losses due to insect and fungal assault (Hay et al., Reference Hay, Rezaei and Buitink2022). The storage conditions and duration appear to limit seed quality in the rice seed industry, and optimizing the storage environment is a priority for improving seed longevity. The future of plant breeding, biodiversity and crop success revolves around the ability of a seed to maintain its vigour and germinability (Arif et al., Reference Arif, Nagel, Neumann, Kobiljski, Lohwasser and Börner2012) during its quiescent stages.
Seeds possess a variety of physical, chemical and genetic traits that contribute to the protection, detoxification and damage repair to enable survival in the dry stage and sustain high vigour throughout storage (Rajjou and Debeaujon, Reference Rajjou and Debeaujon2008). The maintenance of vigour and viability throughout seed storage results in higher rates of germination and seedling establishment and ultimately contributes to higher production (Afzal et al., Reference Afzal, Khalid, Basra, Afzal and Mahmood2019). Seed moisture content (SMC), temperature and the gaseous environment are the most important external factors that influence the storability of seeds (Ellis and Roberts, Reference Ellis and Roberts1981; Schwember and Bradford, Reference Schwember and Bradford2011). Seed ageing is associated with elevated levels of reactive oxygen species (ROS) and the accumulation of oxidative damage to macromolecules, that is, RNA, DNA, lipids and proteins (Kumar et al., Reference Kumar, Prasad, Banerjee and Thammineni2015). DNA oxidation causes mutation and adversely affects seed quality traits (Zhou et al., Reference Zhou, Chen, Luo, Dai, Yang, Zheng and Shu2020). A major factor in rice seed deterioration and the production of off-flavour compounds is rancidification or lipid oxidation (Wang et al., Reference Wang, Zhu, Ramaswamy, Hu and Yu2018). Rancidification occurs in two major ways: hydrolytic and oxidative, both of which are triggered by high temperatures during storage along with high moisture content and oxygen availability (Bhunia et al., Reference Bhunia, Sinha, Kaur, Kaur and Chawla2021). Seed deterioration and ageing during storage have always been of major concern in agriculture and ex situ conservation (Arif et al., Reference Arif, Lohwasser, Nagel and Börner2017). Seed deterioration is frequent when the storage conditions are not ideal. Identifying seed longevity traits in storage is the basis for improving seed quality throughout the supply chain.
Relative humidity (RH) of the storerooms is strongly associated with the SMC at a given temperature (Bradford et al., Reference Bradford, Dahal and Bello2016). Increases in SMC or RH can not only attract microorganisms but also play a part in seed deterioration (Afzal et al., Reference Afzal, Bakhtavar, Ishfaq, Sagheer and Baributsa2017; Bakhtavar and Afzal, Reference Bakhtavar and Afzal2020b). The availability of oxygen and moisture during storage initiates the natural process of respiration in the living parts of the seed and influences its quality (Groot et al., Reference Groot, van Litsenburg, Kodde, Hall, de Vos and Mumm2021). Respiration during storage consumes the available protein, lipid and carbohydrates in the seed causing nutritional loss. Excessive respiration speeds up seed deterioration causing oxidation, which stimulates the peroxidation of lipids and proteins (Zhang et al., Reference Zhang, Zhang, Sun, Meng and Tao2020). The release of volatile compounds by seeds is also associated with lipid peroxidation and alcoholic fermentation during ageing (Colville et al., Reference Colville, Bradley, Lloyd, Pritchard, Castle and Kranner2012).
Increased temperatures, RH and oxygen exposure during storage facilitate mould growth and insect infestation. Even if the SMC is equilibrated to low levels (i.e. 10 and 12%) before storage, it can still undergo the process of deterioration (Groot et al., Reference Groot, de Groot, Surki and Treuren2015). This occurs due to the available amount of oxygen in the storage system that seed can use to respire properly. This respiration causes the seed to age faster and affects its quality. To avoid such issues, scientists are using modified environments where oxygen gas present in the storage system is replaced by carbon dioxide or nitrogen to maintain seed life during storage (Yoon et al., Reference Yoon, Choi and Kang2017). Adding gaseous nitrogen to the storage containers will effectively displace oxygen, thus minimizing oxidation and the growth of microbes that later boost the seeds’ deteriorative events (Jonfia-Essien et al., Reference Jonfia-Essien, Varro and Villers2010). This prevents the seed's energy from immediate utilization in respiration but also maintains the quality of stored seeds. This is common practice in the rice seed industry to avoid the attack of rice weevil in the supply chain, particularly for organic production because chemical fumigation is not allowed.
The objective of this study was to determine the impact of different SMCs on seed longevity of rice following storage in normal atmospheric or hypoxic conditions. Correlations between seed deterioration and the physiological and biochemical attributes of rice seeds were also explored.
Materials and methods
Plant material and initial germination and moisture content testing
The experiment was conducted at the Seed Physiology Lab, Seed Science and Technology Building, University of Agriculture, Faisalabad, Pakistan from June to December 2019. Freshly harvested rice seeds (variety code: 20263957) were procured from Guard Rice Pvt. Ltd., Lahore, Pakistan in March 2019. Initial germination percentage and SMC were determined according to ISTA Protocols (2021). The standard germination test was carried out by using four replicates of 100 seeds each, which were placed between two layers of filter paper, rolled and placed in a polythene bag, which was then placed in an incubator (Sanyo, Japan) at 25°C. For SMC determination (fresh weight basis), 5 g of sample of coarsely ground seeds (using AG-6043 Delux Blender) was placed in an oven (Memmert, Germany) at 103°C for 17 h in three replications. Dried seeds were weighed to find out the actual amount of water lost according to the formula prescribed in ISTA rules.
Seed drying to 10, 12 and 14% moisture content
For this experiment, the SMC levels were selected according to the existing practices of the rice seed industry, that is, 12–14% SMC. After the determination of the initial quality (88% germination and 15.1% SMC), seeds were dried by using seed driers and zeolite beads to set the seed moisture to three different levels, that is, 10.3, 11.8 and 14.0% (fresh weight basis) by keeping the equilibrium RH at 45, 55 and 70%, respectively. The drying beads used to set the SMC contain microscopic pores that are a specific size for water molecules, so only water molecules are taken up, and the drying beads can be recycled endlessly by heating at a temperature of 200°C for 3–4 h. The seeds, after initial drying with the help of a seed drier, were kept in a hermetic bag with a calculated amount of zeolite drying beads (using the bead calculator; http://www.dryingbeads.org) to equilibrate the seed moisture for 7–14 days at ambient conditions. Seed moisture was determined regularly until the desired level (10, 12 and 14%) was reached.
After acquiring the desired moisture levels, the seeds were packed in a hermetic bag and sealed tightly until the experiment was further processed.
Seed storage under a modified atmosphere
Transparent glass jars with rubber corks were used to store the seeds throughout the experiment in three replications for each treatment for a 6-month storage period. The modified atmosphere in the glass jars before seed storage was created by using a vacuum pump (Diaphragm Vacuum Pump GM-0.33II, China). For each replicate, a transparent glass jar (500 ml capacity) was filled with seeds up to the level of 250 ml. Half of the total experimental units were evacuated using the vacuum pump to eliminate all the air present in the glass jars. The jars were then filled with nitrogen gas to provide modified oxygen conditions throughout 6 months of storage. Jars were stored in controlled conditions in a growth chamber at a temperature of 25°C. Air conditioners and humidifiers were used to keep the conditions constant in the growth chamber. Moreover, thermometers and hygrometers were used for the constant monitoring of RH and temperature within the growth chamber. Each set of 18 glass jars was opened after 3-month intervals to determine seed quality parameters. The SMC was measured as soon as the glass jars were opened to ensure that SMC was maintained at 10, 12 and 14% (Fig. 1).
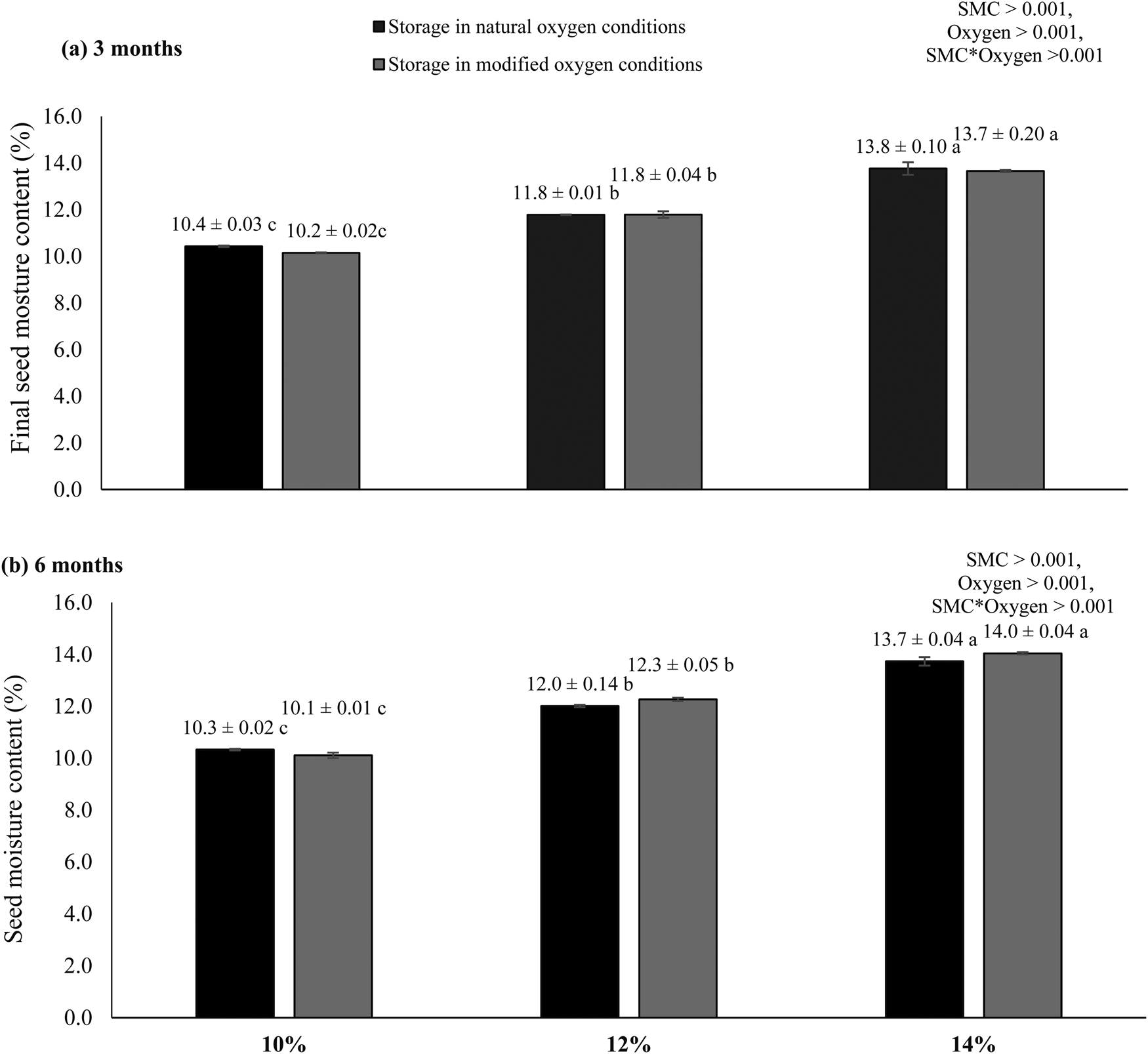
Fig. 1. Final SMCs of rice seeds stored for 3 and 6 months with different initial SMCs (10, 12 and 14%) in natural and modified oxygen environments at constant temperature (25°C). The letters (a–c) indicate that means are not different from each other. Similar letters indicate means with very little or no difference at all. Letter ‘a’ shows the highest values of means, while ‘c’ indicates the lowest means.
Physiological attributes
To determine the germination potential of the normal seedlings of rice samples, 400 seeds were extracted from each treatment and 100 seeds were placed in moistened blotting paper in four replications according to the International Seed Testing Association (ISTA, 2021). The rolled blotting paper was placed in a polythene bag in an incubator (SANYO Japan, MIR-254) at 25 ± 2°C and 60% RH. To prevent the paper towel from drying out, it was sprayed with distilled water at the first count. Seed germination was recorded after radicle protrusion and germination percentage were calculated after 14 d as per the ISTA protocols. Other germination-related parameters, that is, time taken to 50% germination using the equation:
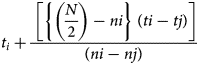 $$t_i + \displaystyle{{\;\left[{\left\{{\left({\displaystyle{N \over 2}} \right)-ni} \right\}\;( {ti-tj} ) } \right]} \over {( {ni-nj} ) }}\;$$
$$t_i + \displaystyle{{\;\left[{\left\{{\left({\displaystyle{N \over 2}} \right)-ni} \right\}\;( {ti-tj} ) } \right]} \over {( {ni-nj} ) }}\;$$according to Coolbear et al. (Reference Coolbear, Francis and Grierson1984), the germination index (GI) as per the equation
mentioned by AOSA (2009), mean germination time with the formula ΣDn/Σn where n is the number of germinated seeds on day D and Dn is the number of counted days before the beginning of germination (Ellis and Roberts, Reference Ellis and Roberts1981), and the vigour index according to the equation: [Root length + Shoot length]*Germination Percentage (Abdul-Baki and Anderson, Reference Abdul-Baki and Anderson1973) were also determined.
Seed viability was determined according to Carvalho et al. (Reference Carvalho, Meneghello, Tunes, Jácome and Soares2017) by immersing 50 seeds in water for 18 h at 18°C. After the imbibition period, the seed embryonic axis was exposed with a longitudinal cut and dipped in a 1% tetrazolium solution for 11–20 h at 40°C. Staining intensity was compared for seeds kept at different storage conditions for the period of 3 and 6 months each.
Electrical conductivity (EC) of the seeds was measured according to ISTA (2021). A seed counter (Contador Pfeuffer, Germany) was used to obtain 50 seeds from each treatment and their weights were recorded by using a digital weighing balance. Seeds were soaked in 250 ml of distilled water for 24 h after determining its initial EC value using an EC meter (HI 99300, Hanna Instruments, Japan). After the soaking period, seed leachates were gently swirled for homogeneity and EC values were recorded by using the same EC meter. The equation used to calculate the EC was
 $${\rm Electrical}\,{\rm conductivity}\,( {{\rm \mu S}\,{\rm c}{\rm m}^{- 1}\,{\rm g}^{- 1}} ) = \displaystyle{{{\rm Final\;}\,{\rm EC}-{\rm Background}\,{\rm EC}} \over {{\rm Weight}\,{\rm of\;}\,50\,{\rm seeds\;}( {\rm g} ) }} \times 100$$
$${\rm Electrical}\,{\rm conductivity}\,( {{\rm \mu S}\,{\rm c}{\rm m}^{- 1}\,{\rm g}^{- 1}} ) = \displaystyle{{{\rm Final\;}\,{\rm EC}-{\rm Background}\,{\rm EC}} \over {{\rm Weight}\,{\rm of\;}\,50\,{\rm seeds\;}( {\rm g} ) }} \times 100$$Biochemical attributes
For detecting the activity of antioxidant enzymes after 3 and 6 months of storage, rice seed samples were ground using a AG-6043 Blender and the seed powder was used in the following assays. For the assay of superoxide dismutase activity, 200 mg of seed powder was extracted in 5 ml of 100 mM potassium phosphate buffer (pH 7.8) using a pestle and mortar. The mixture was centrifuged at 22,000g for 10 min. 100 μl of supernatant liquid and 100 μl of SOD solution containing 50 mM of potassium phosphate buffer, 45 μM of methionine, 20 μM of potassium cyanide, 5.3 μM of riboflavin and 84 μM nitroblue tetrazolium (NBT) were added in ELIZA plates. After 10 min, the absorbance was measured at 600 nm (Misra and Fridovich, Reference Misra and Fridovich1972). The activity of enzyme was expressed on a protein basis. The protein concentration of the extract was measured according to Bradford (Reference Bradford1976).
For the peroxidase (POD) assay, 2 g of the seed sample was homogenized with 10 ml of 0.1 M phosphate buffer (pH 6). The extract was centrifuged at 12,000g for 30 min. The supernatant collected was heated at 65°C for 3 min to inactivate any catalase present in the extract. The peroxidase assay was conducted according to Britton and Mehley's (Reference Britton and Mehley1955) guaiacol oxidation method. The final reaction mixture that was poured into a test tube containing 8 mM of guaiacol, 10 mM of potassium phosphate buffer and 100 μl of enzyme extract together with 2.75 mM of hydrogen peroxidase was added to initiate the reaction. The increase in absorbance at 470 nm was measured using a spectrophotometer.
For the catalase (CAT) assay, 200 mg of ground seeds were homogenized with 5 ml of 50 mM Tris–NaOH (pH 8) containing 0.5% (v/v) Triton X-100, 0.5 mM EDTA and 2% (w/v) PVP. This homogeneous mixture was then centrifuged at 22,000g for 10 min at 4°C. The resultant supernatant was used for the enzyme assay. The CAT assay was conducted according to Aebi (Reference Aebi1984). The reaction in 50 mM potassium phosphate buffer and 250 μl of enzyme extract was started by adding 60 mM hydrogen peroxide. The change in absorbance at 240 nm was measured using a spectrophotometer. The H2O2 decomposition was calculated by using an extinction coefficient of 39.4 mM−1 cm−1. One unit of activity is equivalent to 1 mM of H2O2 degraded per minute and is expressed as unit per milligram of protein.
The DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity was carried out by preparing 1 ml of the sample extract in a test tube along with 2 ml of 1 mM of methanolic DPPH. The solution was mixed thoroughly and incubated at 37°C for 30 min. The blank sample was prepared without adding any standard or sample extract. The change in absorbance was measured at 515 nm, and the percentage of inhibition was calculated (Alhakmani et al., Reference Alhakmani, Kumar and Khan2013).
Hydroxyl radicals (OH⋅) are the by-products of H2O2 decomposed into oxygen and water that initiate lipid peroxidation and damage DNA. To determine the scavenging activity of H2O2, 40 mM of hydrogen peroxide solution was prepared in 50 mM phosphate buffer and the absorbance was measured at 230 nm (Nabavi et al., Reference Nabavi, Ebrahimzadeh, Nabavi, Hamidinia and Bekhradnia2008).
The α-amylase inhibitory activity was measured according to methods described by Kim et al. (Reference Kim, Son, Park, Lee, Lee, Kim and Lee2006) with some modifications. The activity of α-amylase was measured in 0.5 mg of the seed sample extracted in 5 ml of sodium phosphate buffer (pH 6.9). The supernatant (250 μl) was allowed to react with 250 μl of a 1% starch solution. The mixture was incubated at 25°C for 10 min. After that 500 μl of 3,5-dintrisalicylic acid (DNS) solution was added and the mixture was boiled for 5 min at 100°C. After cooling, the absorbance was taken in an Eliza Plate Reader at 540 nm.
Gaseous exchange measurement during storage
Measurements of oxygen and carbon dioxide in the jars were taken after 3 and 6 months of storage using GrainPro O2 and CO2 Analyzers. A disposable hypodermic needle (18-gauge, 5 cm long) was inserted in the rubber caps to collect gas samples from the jars. The needle was attached to a probe connected to the gas analyzer machine and immediately showed the values analysed for gaseous exchange. The analytical range of analyzer was 10–2500 mg oxygen per litre of gas mixture.
Statistical analysis
The experiment was laid out in a completely randomized design with a factorial arrangement in three replications (Steel et al., Reference Steel, Torrie and Dickey1997). Factors included different moisture levels (10, 12 and 14%) and the modified oxygen environment (in the presence of natural and modified oxygen in storage jars). Collected data were analysed by using Statistix 8.1. Analysis of variance (ANOVA) was done to find out the significant difference between the mean values recorded for the parameters mentioned above. Means were compared using the Least Significant Difference (LSD) Test at P ≤ 0.05. Correlation among the parameters was performed in R-Studio (RStudio, 2020) version 2021.09.0+351 for windows.
Results
Final SMCs
To confirm that SMC did not change during the storage experiment, final SMCs were evaluated after 3 and 6 months of storage. The analysis showed no significant difference between initial and final moisture contents of the seeds. The treatments with initial 14% SMC levels were reduced to 13.5 and 13.7%, respectively, for seeds stored in natural and hypoxic conditions after 3 months (Fig. 1). The SMC levels were again constant after 6 months of storage, providing similar values to the initial SMC levels.
Physiological attributes
Highly significant (P ≤ 0.05) differences in the germination of rice seeds stored at various SMC levels were recorded under natural and hypoxic environments (Fig. 2a, b). After 3 and 6 months of storage, seeds performed better for the lower SMC level (10%) with 85 and 77% germination, respectively, which is close to the initial germination (88%), while the germination of seeds with higher SMC levels (12 and 14%) declined. Moreover, these seeds performed better when stored in hypoxic conditions compared to natural oxygen conditions. Germination potential was reduced to almost half of its initial germination ability after 6 months of storage at 14% SMC in natural oxygen conditions (Fig. 2b). For seeds stored at 10% SMC, the germination dropped to 77 and 83% in natural and modified environments, respectively. The GI was the highest for seeds at 10% SMC (Fig. 2c), whereas, at 14% SMC, the GI values were the lowest in both natural environments. After 6 months of storage, seeds showed similar trends as observed after 3 months (Fig. 2d). The results from the GI showed that the rate of germination was better for seeds stored at lower SMC levels (10%), while the seeds with higher SMC levels (14%), which were stored in natural oxygen conditions, demonstrated the lower germination rate.
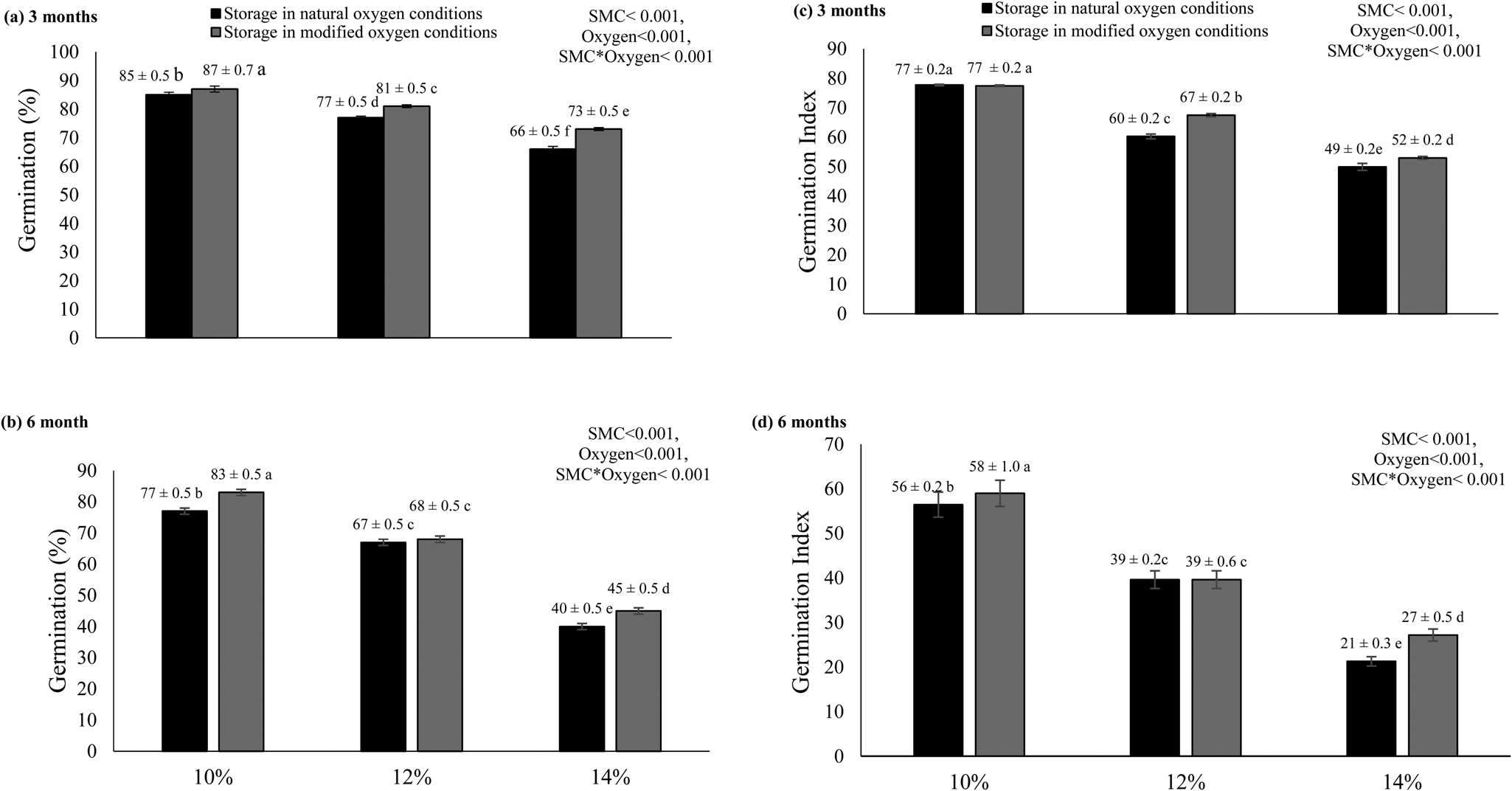
Fig. 2. Germination potential (A,B) and GI (C,D) of rice seeds stored for 3 and 6 months at the initial SMCs of 10, 12 and 14% under natural and modified oxygen environments at constant temperature (25°C). The letters (a, b, etc.) indicate that means are not different from each other. Similar letters indicate means with very little or no difference at all. Letter ‘a’ shows the highest values of means, while ‘f’ indicates the lowest means.
A higher SMC level (14%) negatively affected seed longevity during storage and resulted in delayed germination time and an increase in time to 50% germination (T 50) in natural and hypoxic conditions (Fig. 3). The results also showed that when seeds were stored in the natural oxygen environment, poor performance was visible for the time taken to reach 50% germination and mean germination times in comparison to the treatments stored in modified oxygen conditions.
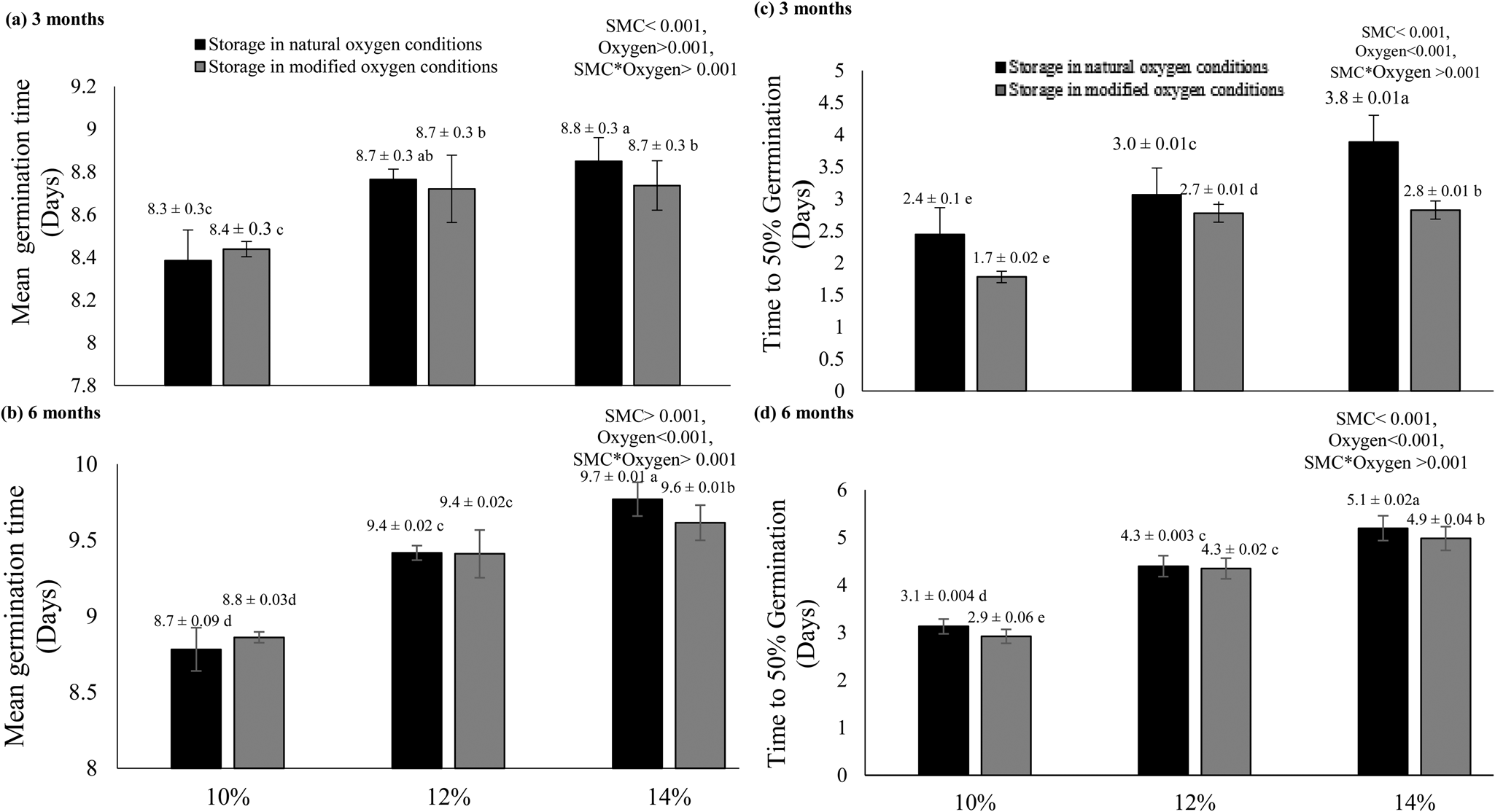
Fig. 3. Mean germination time (a,b) and time to 50% germination (c,d) of rice seeds stored for 3 and 6 months at the initial SMCs of 10, 12 and 14% under natural as well as modified oxygen environments at constant temperature (25°C). The letters (a, b, etc.) indicate that means are not different from each other. Similar letters indicate means with very little or no difference at all. Letter ‘a’ shows the highest values of means, while ‘e’ indicates the lowest means.
Seeds stored at 10% moisture showed a high vigour index in the presence and absence of oxygen after 3 and 6 months of storage (Fig. 4a, b). Overall, the vigour index was better when seeds were stored at low SMC in hypoxic conditions after 3 and 6 months of storage. EC was increased when seeds were stored at 14% SMC compared to seeds with 10% SMC (Fig. 4c, d). High SMC levels in natural oxygen conditions negatively affect the seed quality and produced higher EC values after storage.
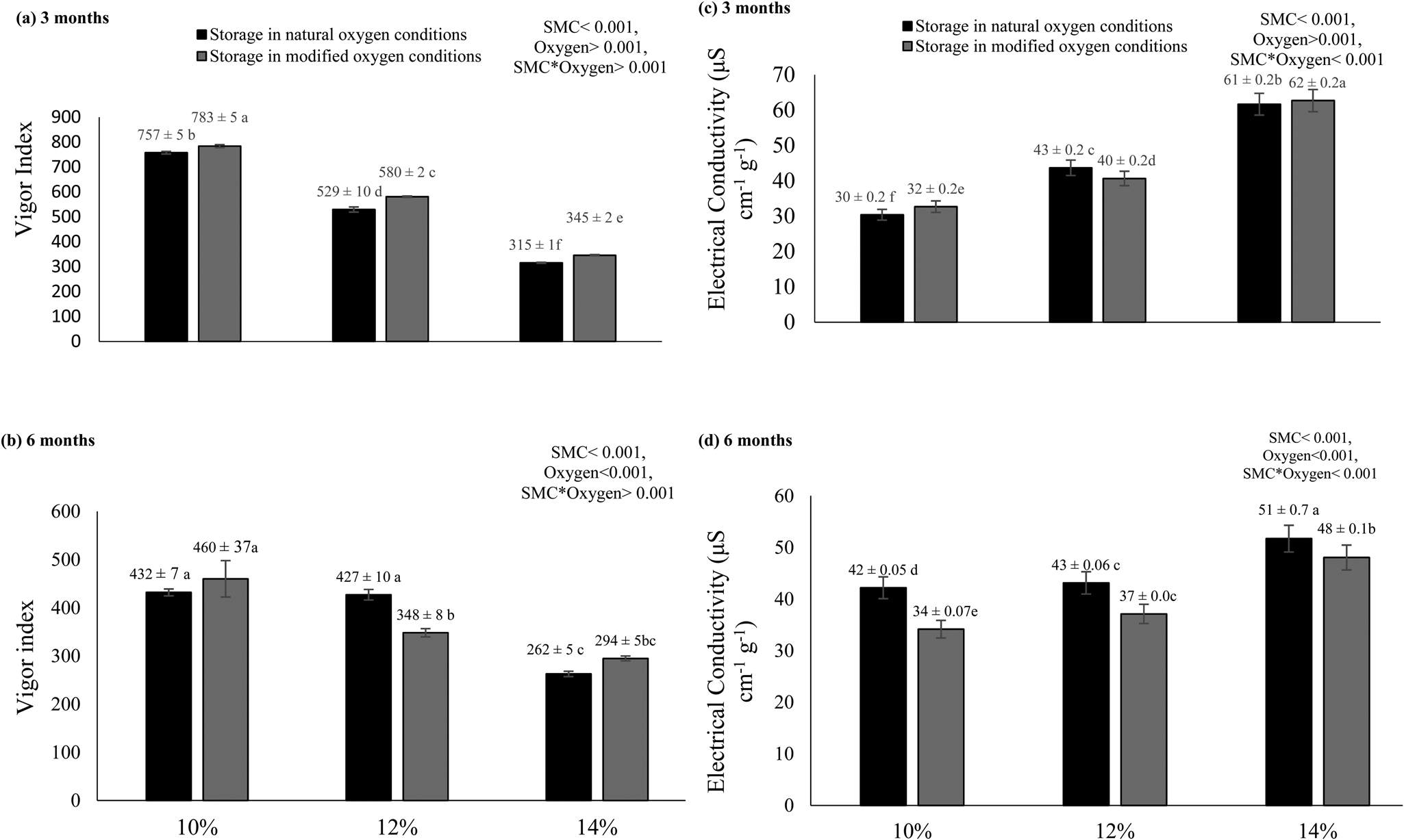
Fig. 4. Vigour index (a,b) and EC (c,d) of rice seeds stored for 3 and 6 months with different initial SMCs (10, 12 and 14%) in natural aas well as modified oxygen environments at constant temperature (25°C). The letters (a, b, etc.) indicate that means are not different from each other. Similar letters indicate means with very little or no difference at all. Letter ‘a’ shows the highest values of means, while ‘f’ indicates the lowest means.
Tetrazolium testing showed that the maximum number of seeds maintained their viability when stored at low SMC levels (10%) compared to seeds stored with high initial SMC levels (14%). The staining pattern was quite visible in seeds stored in modified oxygen environments for all SMC levels. After 6 months of storage, the seeds with 14% initial SMC levels showed the least staining and the minimum number of viable seeds among all of the treatments. Lower SMC levels and the modified oxygen environment during storage helped the seeds to maintain their viability during 6 months of storage.
Biochemical attributes
SOD activity was the lowest when seeds with 10% moisture contents were stored in modified oxygen conditions (Table 1). The maximum SOD activity was observed in seeds stored at 14% SMC followed by seeds with 12% SMC and 10% SMC levels for seeds stored in natural and modified oxygen environments, respectively. When compared to the values obtained after 3 months of storage, enzyme activity increased at a faster rate after 6 months. The findings show that degeneration occurred with time, and it was more severe in seeds stored at high SMC levels. After 3 and 6 months, POD activity was found to be low in seeds with low moisture levels (10%) and high in seeds with 14% SMC levels (Table 1). After storage, this antioxidative enzyme was more active in seeds preserved at high SMC in natural oxygen. Catalase activity increased with an increase in moisture in rice seeds after 3 months (Table 1). Maximum readings were recorded at seeds with 14% moisture in a natural oxygen storage environment. Catalase activity was higher in seeds stored at 10 and 12% SMC compared to seeds stored in hypoxic conditions. A similar trend was observed after 6 months of storage as well (Table 1).
Table 1. Biochemical attributes of rice seeds stored up to 3 and 6 months at initial SMCs of 10, 12 and 14% under natural as well as modified oxygen environments over a constant temperature range (25°C).
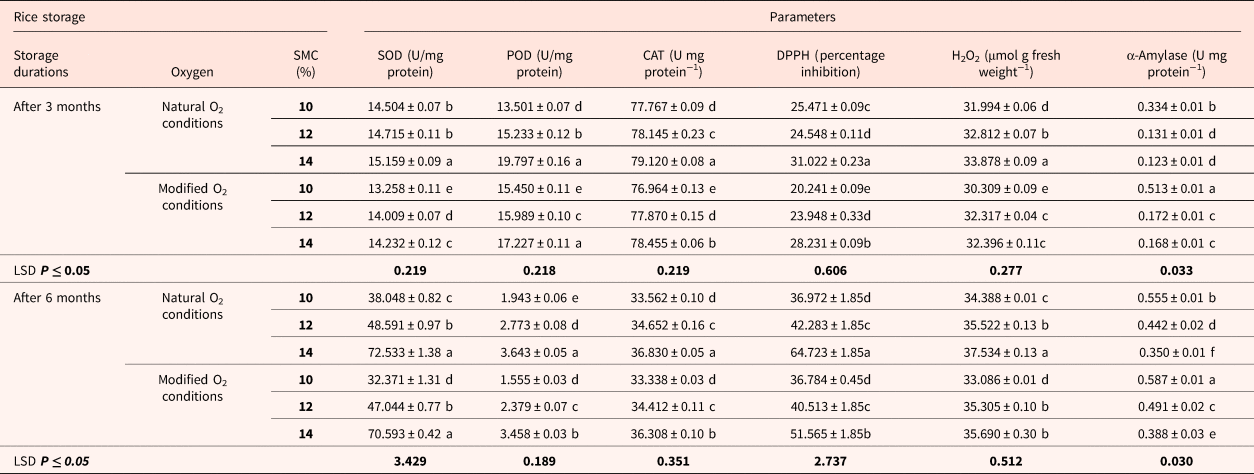
The letters (a, b, etc.) indicate that means are not different from each other. Similar letters indicate means with very little or no difference at all. Letter ‘a’ shows the highest values of means, while ‘f’ indicates the lowest means.
The bold values under ‘SMC' heading shows the initial moisture levels on which the seeds were stored in natural and modified oxygen conditions for 3 and 6 months of storage period. While the ‘LSD' stands for “least square difference’. These values are calculated after statistical tests to understand the significance level of the results obtained.
Scavenging of DPPH radicals showed much the same trends as other antioxidants after 3 and 6 months of storage (Table 1). DPPH scavenging activity for rice seeds after 3 months of storage was higher in treatments stored with oxygen compared to treatments stored in the modified oxygen environment for seeds stored at different moisture contents. Its scavenging activity was high at 14% moisture content in the presence of natural oxygen environment, whereas lower scavenging activity of DPPH radicals was recorded at 10% moisture in hypoxic conditions. An increase in scavenging activity of DPPH was recorded in seeds at each moisture level (10, 12 and 14%) in the presence of oxygen, compared to other treatments. The results showed a similar trend after 6 months of storage. After the fixed storage durations, H2O2 production was the highest in seeds stored with high moisture (14%) along with the availability of oxygen (Table 1). The lowest amount of H2O2 was found in seeds stored at 10% in the modified oxygen conditions after 3 and 6 months of storage duration. Alpha-amylase enzyme activity was the highest in seeds stored in hypoxic conditions at low moisture contents. Seeds with the high moisture contents (12 and 14%) had low α-amylase activity when compared to low moisture seeds (Table 1).
Analysis of gaseous exchange during the storage period
After 3 and 6 months of storage, the concentration of oxygen gas was measured in the airtight glass containers to find out if any respiration activity took place (Fig. 5a, b). The results showed that the oxygen percentage was the highest in seed treatments where moisture was kept the lowest (10%) in both the oxygen conditions. On the other hand, the lowest oxygen concentration was observed in seeds stored at high SMC (14%) in the modified oxygen environment.
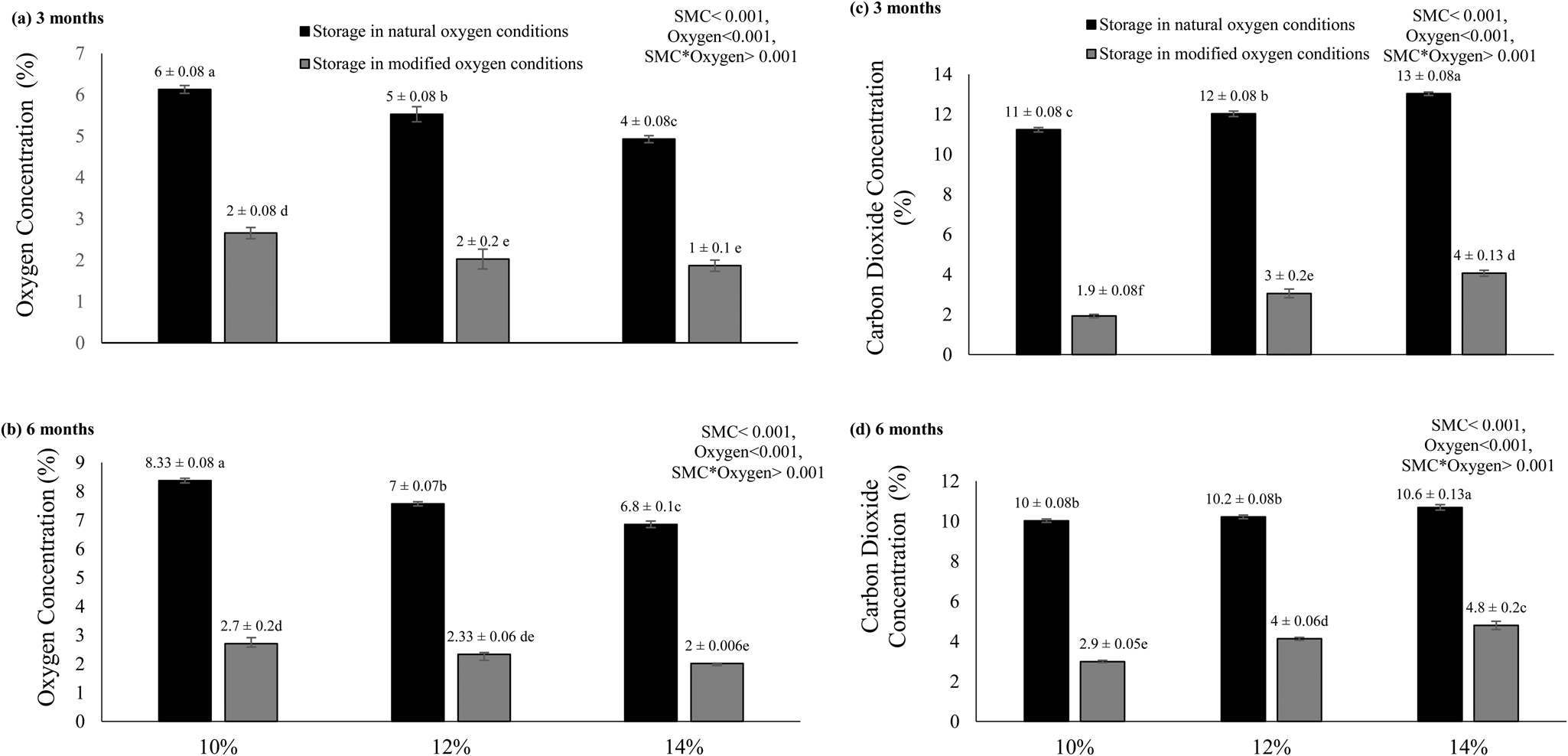
Fig. 5. Final oxygen (a,b) and carbon dioxide (c,d) concentrations of rice seeds stored for 3 and 6 months at initial SMCs of 10, 12 and 14% under natural as well as modified oxygen environments atconstant temperature (25°C). The letters (a, b, etc.) indicate that means are not different from each other. Similar letters indicate means with very little or no difference at all. Letter ‘a’ shows the highest values of means, while ‘f’ indicates the lowest means.
CO2 concentration varied significantly with respect to moisture and oxygen availability during 3 and 6 months of its storage (Fig. 5c, d). The highest level of CO2 was recorded in seeds stored at the high moisture content (14%) in the presence of oxygen. The lowest concentration of CO2 was found in seeds that were stored at 10% SMC in modified oxygen conditions.
Correlation among variables
Germination rate showed a strong correlation (R 2 > 0.95) with α-amylase activity, GI and vigour indices as well as tetrazolium stain scores (Fig. 6). On the other hand, the same traits were strongly negatively correlated (R 2 > −0.80) with all other parameters except O2 and CO2. The correlation between O2 and CO2 was very strong (R 2 > 0.95) and their correlations with other parameters were mostly non-significant except with EC. Mean germination time, peroxidase, superoxide dismutase, CAT, EC, DPPH and hydrogen peroxide were also very strong correlation with each other.
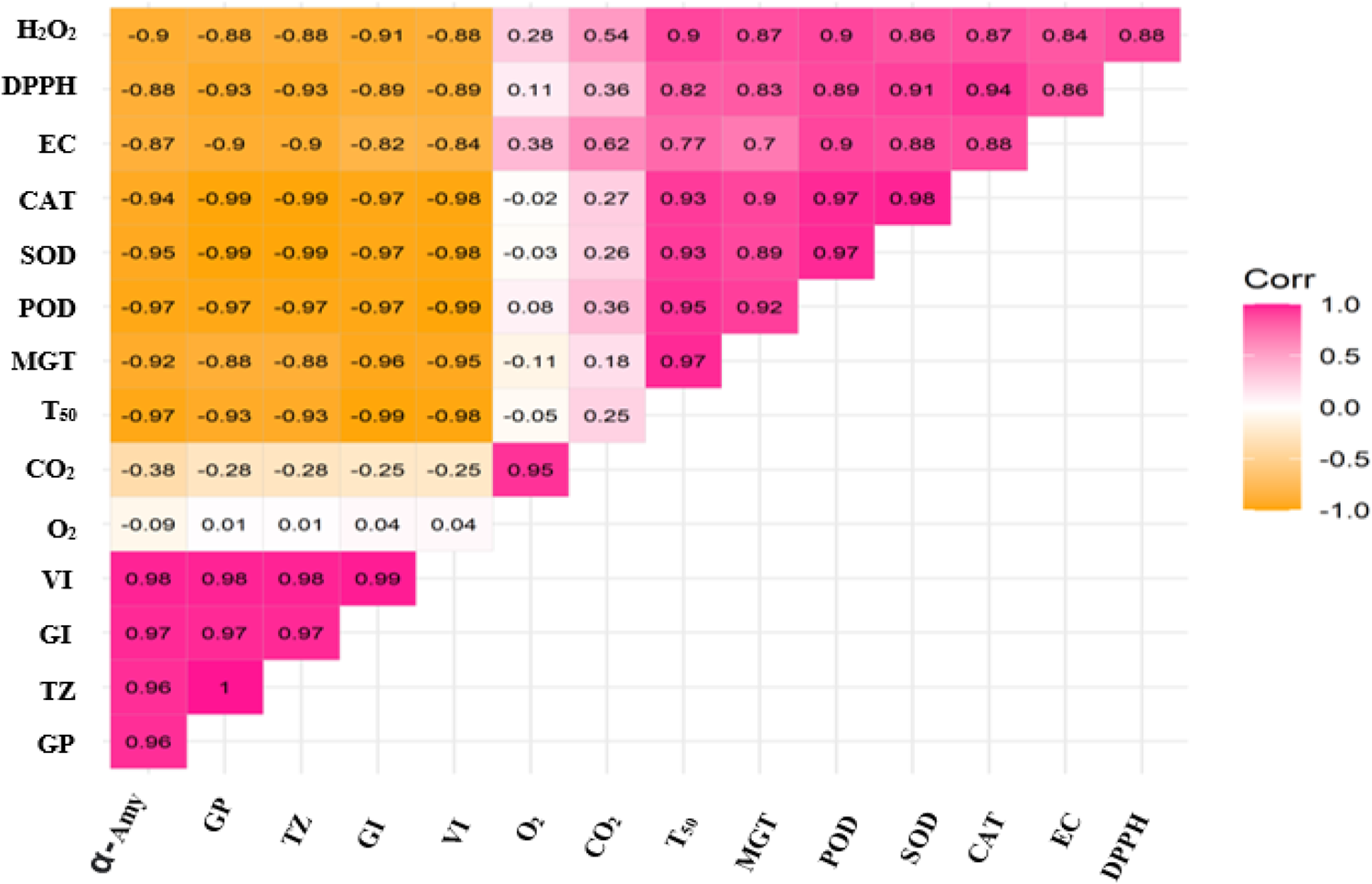
Fig. 6. Schematic representation of correlation among all the biochemical and physiological parameters associated with the experiments performed. Graph shows correlation among germination percentage (GP), germination index (GI), mean germination time (MGT), time to 50% germination (T 50), vigour index (VI), electrical conductivity (EC), tetrazolium test (TZ), superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), 1,1-diphenyl-2-picrylhydrazyl (DPPH), hydrogen peroxide (H2O2), alpha-amylase (α-Amy), oxygen concentration (O2) and carbon dioxide concentration (CO2). Pink and orange colours show positive and negative correlations, respectively; the colour intensity from light to dark determines the degree of strongness of the correlation among the parameters. Furthermore, details are available in the text.
Discussion
The final SMC determination revealed that no exterior interaction of the storage conditions was permitted during the experiment, and the seed SMC levels were maintained at 10, 12, and 14%, which was the core of this investigation. Rice seeds stored at the low moisture level in the hypoxic environment for 3 and 6 months maintained high germination potential, the GI and the vigour index (Figs 2 and 3a, b) compared to seeds stored at high moisture levels (12 and 14%) with oxygen. Time to complete 50% germination, mean germination time and EC of rice seeds at 10% SMC were lower as compared to seeds stored at 14% SMC in both oxygen conditions during 6 months of storage (Figs 2 and 3c, d). If hyper-oxygen conditions are available to the seeds during storage, their quality is jeopardized (Buijs et al., Reference Buijs, Willems, Kodde, Groot and Bentsink2020). Moisture and the natural proportion of oxygen available during storage accelerate the ageing process of the seeds and negatively affect seed quality and germination as explained by De Vitis et al. (Reference De Vitis, Hay, Dickie, Trivedi, Choi and Fiegener2020). Poor germination and deterioration during storage are the reason why the mean germination time and time taken to reach 50% of the germination were higher in seeds with high SMC. The vigour index and EC values of the seeds also indicate that seeds stored in natural oxygen conditions showed signs of deterioration and damage compared to other treatments. Higher EC shows that the seed cell membranes have been damaged, allowing essential ions and amino acids to seep out of the cells, signalling lipid peroxidation (Afzal et al., Reference Afzal, Jaffar, Zahid, Rehman and Basra2020). It has been deduced that these leachates, when discharged from the damaged seeds, had increased the conductivity of the seed surrounding environment at a greater rate than seeds stored at ideal conditions and had intact membranes (Ferreira et al., Reference Ferreira, Fernandes, Aquino, Silva, Nascimento and Leão-Araújo2017). These results are also in line with Afzal et al. (Reference Afzal, Khalid, Basra, Afzal and Mahmood2019) who suggested that moisture is the main factor leading to seed deterioration, compromised vigour, and shorter lifespan during storage.
Another important aspect in reducing seed quality during the experiment is the availability of oxygen gas during storage. Groot et al. (Reference Groot, Surki, De Vos and Kodde2012) opined that oxygen molecules, if present, are used up by seeds for vigorous respiration causing compromised quality during storage. High moisture coupled with oxygen can vigorously affect the seed quality as the respiration process starts in the presence of these two abiotic factors (moisture and oxygen) (Afzal et al., Reference Afzal, Bakhtavar, Ishfaq, Sagheer and Baributsa2017; Williams et al., Reference Williams, Murdock and Baributsa2017). Storing seeds with low moisture levels in a hermetic atmosphere with no or very little oxygen gas is responsible for seed quality preservation (Martin and Fitzgerald, Reference Martin and Fitzgerald2002; Tubbs et al., Reference Tubbs, Baributsa and Woloshuk2016; Bakhtavar et al., Reference Bakhtavar, Afzal and Basra2019). It is evident from the literature that membrane disruption occurs in seeds where moisture was high and oxygen was available (Leão et al., Reference Leão, Barbosa, Santos and Vieira2012). Moreover, Murthy et al. (Reference Murthy, Kumar and Sun2003) also explained that the vigour and viability of stored seeds at higher SMCs are compromised. Our results also showed alignment with the previous studies as seed viability was lower in seeds with high SMC levels in hypoxic and hyperoxic conditions of storage.
Excessive accumulation of oxygen and high moisture during storage give rise to the production of ROS such as the hydroxyl radical (OH), superoxide radical (O2−) and hydrogen peroxide (H2O2) that are involved in oxidative damage to mitochondria, proteins, membranes and DNA. Impairment of mitochondria to produce ATP, lipid peroxidation followed by membrane disruption and eventually leading to cell death is the catastrophic effect of excessive ROS production in seeds (Bewley et al., Reference Bewley, Bradford, Hilhorst and Nonogaki2013; Ratajczak et al., Reference Ratajczak, Malecka, Bagniewska-Zadworna and Kalemba2015; Huang et al., Reference Huang, Wu, Zhao, Lu, Wu and Cao2021). Oxidative damages are the main form of destruction caused by ROS production. It initiates lipid peroxidation by attacking membrane phospholipids and storage lipids like tracylglycerols (Weibach et al., Reference Weibach, Nagel, Borner, Altmann and Riewe2019). These exploiting factors can readily affect membrane characteristics such as protein disruption and fluidity. ROS oxidation can also cause strand breaks and mutations (Rajjou and Debeaujon, Reference Rajjou and Debeaujon2008; Groot et al., Reference Groot, de Groot, Surki and Treuren2015). Superoxide dismutase (SOD), peroxidase (POD) and CAT are the antioxidant enzymes which are produced in response to these ROS that are considered as markers for seed deterioration (Sahu et al., Reference Sahu, Sahu, Thomas and Naithani2017). In the current study, SOD, POD and CAT activities increased with an increase in the SMC level, especially in the natural oxygen environment for both 3 and 6 months of storage (Table 1). Similar trends were obtained by Huang et al. (Reference Huang, Wu, Zhao, Lu, Wu and Cao2021) who emphasized that abiotic stressors could result in an increased production of ROS, which lead to the increased production of antioxidant enzymes. Seeds with higher ROS production produce higher antioxidant enzyme activities. At high moisture contents in seeds, the level of H2O2 and DPPH radicles’ scavenging activity was quite high which proves that more deterioration occurred in seeds stored with high initial moisture levels (14%) (Table 1). The natural and hypoxic environments did not produce much difference in regard to ROS and antioxidant activity when the seeds had high moisture, whereas at low moisture the activity of H2O2 and DPPH scavenging was reduced in seeds stored in hypoxic environments. In seeds stored in modified oxygen conditions, the overall ROS and antioxidant enzyme activities were slightly lower than the seeds stored in the presence of the natural proportion of oxygen gas (Bailly, Reference Bailly2004; Wawrzyniak et al., Reference Wawrzyniak, Kalemba, Ratajczak and Chmielarz2020). Furthermore, amylase activity was also assessed, which is closely related to seed germination and seed vigour in rice (Kim et al., Reference Kim, Son, Park, Lee, Lee, Kim and Lee2006). In this study, high moisture contents of the seeds dramatically decreased α-amylase activity during storage (Table 1). Likewise, Wang et al. (Reference Wang, Qi, Zheng, Guo, Gong, Wang, Chen, Huang, Li and Song2017) also found that the high moisture content of seeds during storage significantly reduced α-amylase activities during early germination and inhibited starch hydrolysis processes, resulting in poor seed quality and germination (Huang et al., Reference Huang, Wu, Zhao, Lu, Wu and Cao2021). The biochemical analysis from 3- and 6-month stored seeds showed a drastic increase in the production of antioxidant enzymes and ROS compounds. Increased ROS production can be attributed to high moisture stress with the passage of time. The trend of overall biochemical activity favours Bradford's metronome rule, which states that the clock began ticking as the seeds mature and each tick depicts the inevitable deterioration process (Bello and Bradford, Reference Bello and Bradford2016). However, for increased longevity, the extent of seed deterioration can be delayed by lowering the moisture content to reduce ROS generation (Bakhtavar and Afzal, Reference Bakhtavar and Afzal2020a).
Oxygen plays an important role during storage (Groot et al., Reference Groot, Surki, De Vos and Kodde2012). High moisture (>14%) causes an increase in the respiration rate, which results in increased CO2 production by utilizing the available O2 (Oba et al., Reference Oba, Goneli, Masetto, Hartmann, Michels and Ávila2019). In this study, the oxygen percentage was reduced in seeds stored at high moisture contents compared to seeds stored at low moisture contents. At high moisture (14%), the oxygen level was low. The greatest increase in the CO2 level was also found in seed treatments with high moisture (14%) (Fig. 4). According to the literature, hermetic storage allows no interaction of seeds with external moisture in the environment and reduces O2 permeability to very low levels (Lane and Woloshuk, Reference Lane and Woloshuk2017). With respiration, the internal O2 level decreases and CO2 concentration increases (Navarro, Reference Navarro and Heaps2006). Elevation of CO2 levels by respiration creates anoxia (Jonfia-Essien et al., Reference Jonfia-Essien, Varro and Villers2010). When O2 is restricted in airtight storage, then the available O2 is utilized by the seeds for the process of respiration, releasing CO2 (Walsh et al., Reference Walsh, Baributsa, Remington and Sperling2014). In a recent study, Varsha et al. (Reference Varsha, Kumar and Rathi2022) used soybean seeds to analyse the chemisorption of stored seeds and stated that gaseous diffusion through pores controls the sorption process and 70% CO2 is chemisorbed in seeds. A hypoxic environment is created by replacing oxygen with carbon dioxide gas, which is beneficial for seed lifespan. For maintaining seed viability and vigour of rice, it is important to dry seeds to a safe moisture level (10% SMC), which results in better storage of seeds both in the presence of oxygen as well as in the modified oxygen environment. However, at 12% SMC or above, it is important to store seeds in the absence of oxygen or in the environment with restricted oxygen levels to preserve seed quality.
Conclusion
The availability of oxygen and high SMC (>12%) are major threats to rice seed quality in storage. At low moisture (10%), however, the presence of oxygen was not as damaging as it is for seeds stored at high SMC (14%). Although deterioration took place at all SMC levels, at low moisture (10%), the damage was to a lesser extent than that was observed in seeds stored at high SMC (14%) in natural oxygen conditions.
Author contribution
Ayesha Tahir and Irfan Afzal: Conceptualization; data curation; formal analysis; methodology; writing of original draft; review & editing. Ehsan Khalid and Maryam Razzaq: Methodology and formal analysis. Mian Abdur Rehman Arif: Formal analysis; review & editing.
Conflicts of interest
All authors declared no conflict of interest.









